Zn–Al Layered Double Hydroxides Synthesized on Aluminum Foams for Fluoride Removal from Water
Abstract
:1. Introduction
2. Materials and Methods
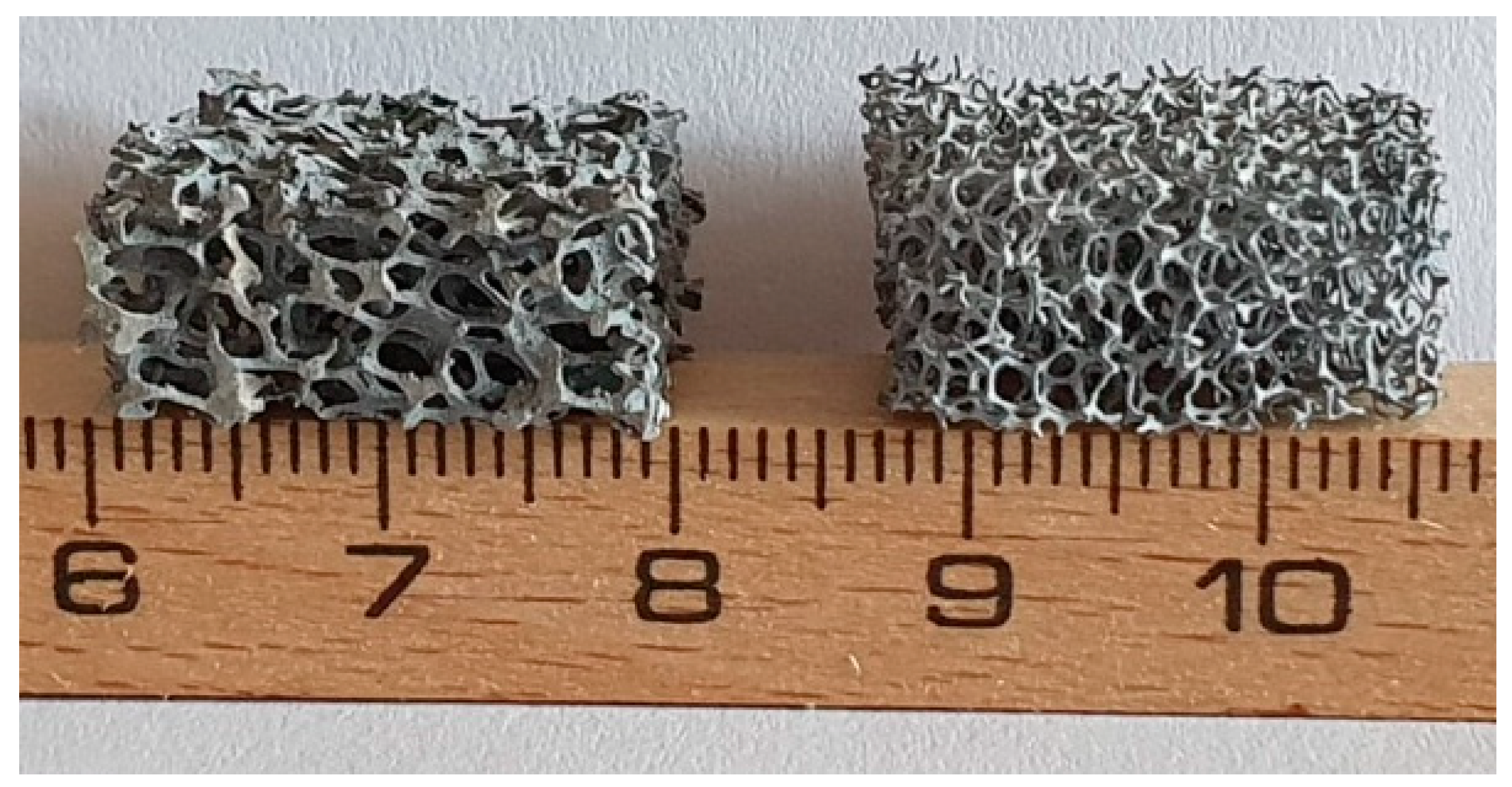
2.1. Materials
2.2. Synthesis of Zn–Al LDH on Aluminum Foams
2.2.1. Single Growth (SG)
2.2.2. Separate Two Growths (STG)
2.2.3. Continuous Two Growth (CTG)
2.3. Characterization
2.4. Fluoride Adsorption Experiments
2.5. Regeneration
3. Results
3.1. Zn–Al LDH Film Synthesis
3.2. Characterization
3.2.1. XRD Characterization
3.2.2. SEM Characterization
3.2.3. XPS Characterization
- (i)
- Anion exchange between the interlayered nitrate and fluoride in solution with the formation of the outer-sphere complex:
- (ii)
- Ligand exchange between surface metal hydroxyl groups (M-OH) of Zn–Al LDH and fluoride in solution with the formation of the inner-sphere complex:
3.3. Fluoride Removal Experiment
3.3.1. Effect of Contact Time
3.3.2. Effect of Initial Concentration
3.3.3. Effect of pH
3.3.4. Effect of the Adsorbent Dosage
3.3.5. Effect of Foam Type and Synthesis Method
3.3.6. Adsorption Kinetics
3.3.7. Regeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gai, W.Z.; Deng, Z.Y. A comprehensive review of adsorbents for fluoride removal from water: Performance, water quality assessment and mechanism. Environ. Sci. Water Res. Technol. 2021, 7, 1362–1386. [Google Scholar] [CrossRef]
- Guiza, S.; Brouers, F.; Bagane, M. Fluoride removal from aqueous solution by montmorillonite clay: Kinetics and equilibrium modeling using new generalized fractal equation. Environ. Technol. Innov. 2021, 21, 101187. [Google Scholar] [CrossRef]
- Tripathy, S.S.; Bersillon, J.L.; Gopal, K. Removal of fluoride from drinking water by adsorption onto alum-impregnated activated alumina. Sep. Purif. Technol. 2006, 50, 310–317. [Google Scholar] [CrossRef]
- Habuda-Stanić, M.; Ravančić, M.; Flanagan, A. A Review on Adsorption of Fluoride from Aqueous Solution. Materials 2014, 7, 6317–6366. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.K.; Gupta, N.; Kumar, V.; Khan, S.A.; Kumar, A. A review of emerging adsorbents and current demand for defluoridation of water: Bright future in water sustainability. Environ. Int. 2018, 111, 80–108. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.; Huang, L.; Yan, J.; Ma, B.; Huang, X.; Luo, Z.; Zhang, H.; Xiao, T. Removal of fluoride from industrial wastewater by using different adsorbents: A review. Sci. Total Environ. 2021, 773, 145535. [Google Scholar] [CrossRef]
- Drouiche, N.; Djouadi-Belkada, F.; Ouslimane, T.; Kefaifi, A.; Fathi, J.; Ahmetovic, E. Photovoltaic solar cells industry wastewater treatment. Desalin. Water Treat. 2013, 51, 5965–5973. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Mageste, A.B.; Dias, A.; Virtuoso, L.S.; Siqueira, K.P.F. Layered double hydroxides for remediation of industrial wastewater containing manganese and fluoride. J. Clean. Prod. 2018, 171, 275–284. [Google Scholar] [CrossRef]
- Rasool, A.; Farooqi, A.; Xiao, T.; Ali, W.; Noor, S.; Abiola, O.; Ali, S.; Nasim, W. A review of global outlook on fluoride contamination in groundwater with prominence on the Pakistan current situation. Environ. Geochem. Health 2017, 40, 1265–1281. [Google Scholar] [CrossRef]
- Maheshwari, R.C.M. Fluoride in drinking water and its removal. J. Hazard. Mater. 2006, 137, 456–463. [Google Scholar] [CrossRef]
- Waghmare, S.S.; Arfin, T. Fluoride removal from water by various techniques. Int. J. Innov. Sci. Eng. Technol. 2015, 2, 560–571. [Google Scholar]
- Grzegorzek, M.; Majewska-Nowak, K.; Ahmed, A.E. Removal of fluoride from multicomponent water solutions with the use of monovalent selective ion-exchange membranes. Sci. Total Environ. 2020, 722, 137681. [Google Scholar] [CrossRef]
- Wei, L.; Li, Z.; Ye, G.; Rietveld, L.C.; van Halem, D. Comparative study of low-cost fluoride removal by layered double hydroxides, geopolymers, softening pellets and struvite. Environ. Technol. 2021. just-accepted: 1–20. [Google Scholar] [CrossRef]
- Pizzoferrato, R.; Richetta, M. Layered double hydroxides (LDHs). Crystals 2020, 10, 1121. [Google Scholar] [CrossRef]
- Daniel, S.; Thomas, S. Layered double hydroxides: Fundamentals to applications. In Layered Double Hydroxide Polymer NanoComposites; Woodhead Publishing: Sawston, UK, 2020; pp. 1–76. ISBN 9780081019030. [Google Scholar]
- Johnston, A.L.; Lester, E.; Williams, O.; Gomes, R.L. Understanding Layered Double Hydroxide properties as sorbent materials for removing organic pollutants from environmental waters. J. Environ. Chem. Eng. 2021, 9, 105197. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Ribeiro, C. [Mg-Al]-LDH and [Zn-Al]-LDH as matrices for removal of high loadings of phosphate. Mater. Res. 2018, 21, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Dass, G. Fluoride Contamination in Drinking Water-A Review. Resour. Environ. 2013, 3, 53–58. [Google Scholar] [CrossRef]
- Li, T.; Miras, H.N.; Song, Y.F. Polyoxometalate (POM)-Layered Double Hydroxides (LDH) composite materials: Design and catalytic applications. Catalysts 2017, 7, 260. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.Z.; Zhang, C.; Zeng, G.M.; Tan, X.F.; Wang, H.; Huang, D.L.; Yang, K.H.; Wei, J.J.; Ma, C.; Nie, K. Design and engineering of layered double hydroxide based catalysts for water depollution by advanced oxidation processes: A review. J. Mater. Chem. A 2020, 8, 4141–4173. [Google Scholar] [CrossRef]
- Pizzoferrato, R.; Ciotta, E.; Ferrari, I.V.; Narducci, R.; Pasquini, L.; Varone, A.; Richetta, M.; Antonaroli, S.; Braglia, M.; Knauth, P.; et al. Layered Double Hydroxides Containing an Ionic Liquid: Ionic Conductivity and Use in Composite Anion Exchange Membranes. ChemElectroChem 2018, 5, 2781–2788. [Google Scholar] [CrossRef]
- Yang, J.; Jing, R.; Wang, P.; Liang, D.R.; Huang, H.; Xia, C.; Zhang, Q.; Liu, A.; Meng, Z.; Liu, Y. Black phosphorus nanosheets and ZnAl-LDH nanocomposite as environmental-friendly photocatalysts for the degradation of Methylene blue under visible light irradiation. Appl. Clay Sci. 2021, 200, 105902. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Casciola, M.; Donnadio, A.; Nocchetti, M.; Pasquini, L.; Narducci, R.; Knauth, P. Anionic conducting composite membranes based on aromatic polymer and layered double hydroxides. Int. J. Hydrogen Energy 2017, 42, 3197–3205. [Google Scholar] [CrossRef]
- Kuthati, Y.; Kankala, R.K.; Lee, C.H. Layered double hydroxide nanoparticles for biomedical applications: Current status and recent prospects. Appl. Clay Sci. 2015, 112–113, 100–116. [Google Scholar] [CrossRef]
- Tan, J.K.E.; Balan, P.; Birbilis, N. Advances in LDH coatings on Mg alloys for biomedical applications: A corrosion perspective. Appl. Clay Sci. 2021, 202, 105948. [Google Scholar] [CrossRef]
- Arrabito, G.; Pezzilli, R.; Prestopino, G.; Medaglia, P.G. Layered double hydroxides in bioinspired nanotechnology. Crystals 2020, 10, 602. [Google Scholar] [CrossRef]
- Chaillot, D.; Bennici, S.; Brendlé, J. Layered double hydroxides and LDH-derived materials in chosen environmental applications: A review. Environ. Sci. Pollut. Res. 2021, 28, 24375–24405. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhao, Y.; Wei, M.; Duan, X. Preparation of oriented layered double hydroxide film using electrophoretic deposition and its application in water treatment. Ind. Eng. Chem. Res. 2011, 50, 2800–2806. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Chetia, M.; Goswamee, R.L.; Banerjee, S.; Chatterjee, S.; Singh, L.; Srivastava, R.B.; Sarma, H.P. Arsenic removal from water using calcined Mg-Al layered double hydroxide. Clean Technol. Environ. Policy 2012, 14, 21–27. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Zimmerman, A.R.; Cao, X. Sorption of arsenic onto Ni/Fe layered double hydroxide (LDH)-biochar composites. RSC Adv. 2016, 6, 17792–17799. [Google Scholar] [CrossRef]
- Zhao, D.; Sheng, G.; Hu, J.; Chen, C.; Wang, X. The adsorption of Pb(II) on Mg2Al layered double hydroxide. Chem. Eng. J. 2011, 171, 167–174. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Li, Z.; Gong, Y. Enhanced removal of trace mercury from surface water using a novel Mg2Al layered double hydroxide supported iron sulfide composite. Chem. Eng. J. 2020, 393, 124635. [Google Scholar] [CrossRef]
- You, Y.; Vance, G.F.; Zhao, H. Selenium adsorption on Mg-Al and Zn-Al layered double hydroxides. Appl. Clay Sci. 2001, 20, 13–25. [Google Scholar] [CrossRef]
- Kaciulis, S.; Bolli, E.; Varone, A.; Richetta, M.; Narducci, R.; Di Vona, M.L.; Ciotta, E.; Pizzoferrato, R. Adsorption of heavy metals by layered double hydroxides grown in situ on Al foam. Surf. Interface Anal. 2020, 52, 996–999. [Google Scholar] [CrossRef]
- Li, Y.; Bi, H.Y.; Jin, Y.S. Facile preparation of rhamnolipid-layered double hydroxide nanocomposite for simultaneous adsorption of p-cresol and copper ions from water. Chem. Eng. J. 2017, 308, 78–88. [Google Scholar] [CrossRef]
- Gao, H.; Cao, R.; Xu, X.; Xue, J.; Zhang, S.; Hayat, T.; Alharbi, N.S.; Li, J. Surface Area- and Structure-Dependent Effects of LDH for Highly Efficient Dye Removal. ACS Sustain. Chem. Eng. 2019, 7, 905–915. [Google Scholar] [CrossRef]
- Barghouthi, Z.; Amereih, S. Spectrophotometric determination of fluoride in drinking water using aluminium complexes of triphenylmethane dyes. Water SA 2012, 38, 543–548. [Google Scholar] [CrossRef]
- Guo, X.; Yin, P.; Yang, H. Superb adsorption of organic dyes from aqueous solution on hierarchically porous composites constructed by ZnAl-LDH/Al(OH)3 nanosheets. Microporous Mesoporous Mater. 2018, 259, 123–133. [Google Scholar] [CrossRef]
- Shan, R.R.; Yan, L.G.; Yang, Y.M.; Yang, K.; Yu, S.J.; Yu, H.Q.; Zhu, B.C.; Du, B. Highly efficient removal of three red dyes by adsorption onto Mg-Al-layered double hydroxide. J. Ind. Eng. Chem. 2015, 21, 561–568. [Google Scholar] [CrossRef]
- Halajnia, A.; Oustan, S.; Najafi, N.; Khataee, A.R.; Lakzian, A. Adsorption-desorption characteristics of nitrate, phosphate and sulfate on Mg-Al layered double hydroxide. Appl. Clay Sci. 2013, 80–81, 305–312. [Google Scholar] [CrossRef]
- Lundehøj, L.; Cellier, J.; Forano, C.; Nielsen, U.G. Atomic Level Understanding of Orthophosphate Adsorption by Magnesium Aluminum-Layered Double Hydroxides—A Multitechnique Study. J. Phys. Chem. C 2019, 123, 24039–24050. [Google Scholar] [CrossRef]
- Li, F.; Jin, J.; Shen, Z.; Ji, H.; Yang, M.; Yin, Y. Removal and recovery of phosphate and fluoride from water with reusable mesoporous Fe3O4@mSiO2@mLDH composites as sorbents. J. Hazard. Mater. 2020, 388, 121734. [Google Scholar] [CrossRef]
- Pizzoferrato, R.; Ciotta, E.; Ferrari, I.V.; Braglia, M.; Medaglia, P.G.; Mattoccia, A.; Di Giamberardino, L.; Richetta, M.; Knauth, P.; Di Vona, M.L. Ionic conductivity of ZnAl layered double hydroxide films grown on aluminum substrate. Solid State Ionics 2018, 314, 30–35. [Google Scholar] [CrossRef]
- He, S.; Zhao, Y.; Wei, M.; Evans, D.G.; Duan, X. Fabrication of hierarchical layered double hydroxide framework on aluminum foam as a structured adsorbent for water treatment. Ind. Eng. Chem. Res. 2012, 51, 285–291. [Google Scholar] [CrossRef]
- Scarpellini, D.; Falconi, C.; Gaudio, P.; Mattoccia, A.; Medaglia, P.G.; Orsini, A.; Pizzoferrato, R.; Richetta, M. Morphology of Zn/Al layered double hydroxide nanosheets grown onto aluminum thin films. Microelectron. Eng. 2014, 126, 129–133. [Google Scholar] [CrossRef]
- Sasai, R.; Sato, H.; Sugata, M.; Fujimura, T.; Ishihara, S.; Deguchi, K.; Ohki, S.; Tansho, M.; Shimizu, T.; Oita, N.; et al. Why Do Carbonate Anions Have Extremely High Stability in the Interlayer Space of Layered Double Hydroxides? Case Study of Layered Double Hydroxide Consisting of Mg and Al (Mg/Al = 2). Inorg. Chem. 2019, 58, 10928–10935. [Google Scholar] [CrossRef] [PubMed]
- Iyi, N.; Okamoto, K.; Kaneko, Y.; Matsumoto, T. Effects of anion species on deintercalation of carbonate ions from hydrotalcite-like compounds. Chem. Lett. 2005, 34, 932–933. [Google Scholar] [CrossRef]
- Dhillona, A.; Nair, M.; Kumar, D. Analytical methods for determination and sensing of fluoride in biotic and abiotic sources: A review. RSC 2016, 8, 5338–5352. [Google Scholar] [CrossRef]
- Marques, T.L.; Coelho, N.M.M. Proposed flow system for spectrophotometric determination of fluoride in natural waters. Talanta 2013, 105, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Haj-Hussein, A.T.; Al-Momani, I.F. Indirect spectrophotometric determination of fluoride in water with zirconium-spadns by flow injection analysis. Anal. Lett. 1989, 22, 1581–1599. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Li, Y.; Sulieman, K.M.; He, X.; Sun, F. Facile and large-scale production of ZnO/Zn-Al layered double hydroxide hierarchical heterostructures. J. Phys. Chem. B 2006, 110, 21865–21872. [Google Scholar] [CrossRef] [PubMed]
- Babakhani, S.; Talib, Z.A.; Hussein, M.Z.; Ahmed, A.A.A. Optical and thermal properties of Zn/Al-layered double hydroxide nanocomposite intercalated with sodium dodecyl sulfate. J. Spectrosc. 2014, 2014, 467064. [Google Scholar] [CrossRef] [Green Version]
- Kiriarachchi, H.D.; Abouzeid, K.M.; Bo, L.; El-Shall, M.S. Growth mechanism of sea urchin zno nanostructures in aqueous solutions and their photocatalytic activity for the degradation of organic dyes. ACS Omega 2019, 4, 14013–14020. [Google Scholar] [CrossRef] [Green Version]
- Sugapriya, S.; Lakshmi, S.; Chandarshekar, B.; Ranjithkumar, R. ZnO Needle-like structures: Synthesis and characterization. Kongunadu Res. J. 2015, 2, 13–15. [Google Scholar] [CrossRef]
- Malevu, T.D.; Ocaya, R.O.; Malevu, T.D.; Ocaya, R.O. Effect of Annealing Temperature on Structural, Morphology and Optical Properties of ZnO Nano-Needles Prepared by Zinc-Air Cell System Method. Artic. Int. J. Electrochem. Sci. 2015, 10, 1752–1761. [Google Scholar]
- Cai, J.; Zhao, X.; Zhang, Y.; Zhang, Q.; Pan, B. Enhanced fluoride removal by La-doped Li/Al layered double hydroxides. J. Colloid Interface Sci. 2018, 509, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, X.; Abudula, A.; Zhang, Z.; Ma, X.; Tang, K.; Hao, X.; Guan, G. Highly efficient defluoridation using a porous MWCNT@NiMn-LDH composites based on ion transport of EDL coupled with ligand exchange mechanism. Sep. Purif. Technol. 2019, 223, 154–161. [Google Scholar] [CrossRef]
- Kameda, T.; Oba, J.; Yoshioka, T. Recyclable Mg-Al layered double hydroxides for fluoride removal: Kinetic and equilibrium studies. J. Hazard. Mater. 2015, 300, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Bazrafshan, E.; Balarak, D.; Panahi, A.H.; Kamani, H.; Mahvi, A.H. Fluoride removal from aqueous solutions by cupricoxide nanoparticles. Fluoride 2016, 49, 233. [Google Scholar]
- Das, D.P.; Das, J.; Parida, K. Physicochemical characterization and adsorption behavior of calcined Zn/Al hydrotalcite-like compound (HTlc) towards removal of fluoride from aqueous solution. J. Colloid Interface Sci. 2003, 261, 213–220. [Google Scholar] [CrossRef]
- Mandal, S.; Mayadevi, S. Adsorption of fluoride ions by Zn-Al layered double hydroxides. Appl. Clay Sci. 2008, 40, 54–62. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Xiao, H.; Lu, H.; Zhou, Y. Synthesis of Li-Al layered double hydroxides (LDHs) for efficient fluoride removal. Ind. Eng. Chem. Res. 2012, 51, 11490–11498. [Google Scholar] [CrossRef]
- Suchorski, Y.; Rupprechter, G. Heterogeneous Surfaces as Structure and Particle Size Libraries of Model Catalysts. Catal. Lett. 2018, 148, 2947–2956. [Google Scholar] [CrossRef] [PubMed] [Green Version]














| Samples | LDH [g] | LDH/Al [%] | |
|---|---|---|---|
| Type I | I-SG | 0.0059 | 0.83 |
| I-STG | 0.0114 | 1.97 | |
| I-CTG | 0.0166 | 2.32 | |
| Type II | II-SG | 0.0070 | 1.25 |
| II-STG | 0.0147 | 2.51 | |
| II-CTG | 0.0099 | 1.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Narducci, R.; Varone, A.; Kaciulis, S.; Bolli, E.; Pizzoferrato, R. Zn–Al Layered Double Hydroxides Synthesized on Aluminum Foams for Fluoride Removal from Water. Processes 2021, 9, 2109. https://doi.org/10.3390/pr9122109
Li Y, Narducci R, Varone A, Kaciulis S, Bolli E, Pizzoferrato R. Zn–Al Layered Double Hydroxides Synthesized on Aluminum Foams for Fluoride Removal from Water. Processes. 2021; 9(12):2109. https://doi.org/10.3390/pr9122109
Chicago/Turabian StyleLi, Yuliu, Riccardo Narducci, Alessandra Varone, Saulius Kaciulis, Eleonora Bolli, and Roberto Pizzoferrato. 2021. "Zn–Al Layered Double Hydroxides Synthesized on Aluminum Foams for Fluoride Removal from Water" Processes 9, no. 12: 2109. https://doi.org/10.3390/pr9122109
APA StyleLi, Y., Narducci, R., Varone, A., Kaciulis, S., Bolli, E., & Pizzoferrato, R. (2021). Zn–Al Layered Double Hydroxides Synthesized on Aluminum Foams for Fluoride Removal from Water. Processes, 9(12), 2109. https://doi.org/10.3390/pr9122109










