1. Introduction
Recently, intensive research has been carried out in the field of natural polymers to meet the specific requirements of biomedical applications, such as scaffolds for tissue engineering, and drug delivery, wound dressings, and medical implants.
In the last 20 years, cellulose materials with well-defined nanostructures have come into focus of scientific research. Having at least one nanoscaled dimension, the family of nanocelluloses is represented by cellulose nanocrystals, cellulose nanofibers, and biocellulose (also called bacterial nanocellulose, biotech nanocellulose, and microbial cellulose). These nanocelluloses are mainly different in origin, method of production, and product characteristics [
1].
Biocellulose is synthesized by aerobic microorganisms such as acetic acid bacteria of the genus Komagateibacter as a flat biofilm at the interface between nutrient-containing aqueous media and air. In this process, the biocellulose is formed as a nanofiber network with high purity (natively composed of 1% cellulose and 99% water) and high mechanical strength. Particularly important properties of biocellulose are also the high biocompatibility and the interconnecting microporous structure, which are stabilized by the high crystallinity of the nanofiber network as a requirement for the diffusion of liquids, ions, and small molecules and definable gas permeability. Depending on the application, the shape (e.g., flat or tubular hydrogel) and the dimension as well as the properties of biocellulose materials can be adapted. This includes synthesis conditions (type of synthesis technology, bacterial strain, and nutrient sources), incorporation of additives during or after synthesis, and post processing steps such as drying methods, leading to different pore sizes and nanofiber network structures [
2,
3,
4,
5,
6]. Thereby, biocellulose products are promising candidates for numerous medical and also technical applications [
7]. The latter includes electronic devices [
8], food and food packaging [
9,
10], papermaking [
11], and barrier membranes [
12,
13].
This review highlights the preparation and characterization of tubular biocellulose implants as well as their application with a special focus on abdominal surgery. It should be emphasized that this contribution is a result of a strong scientific interaction of polymer scientists and surgeons. Based on this interdisciplinary approach, we describe a critical and detailed assessment of the application of biocellulose materials in modern abdominal oncologic surgery. Key features are incidence malignancy and surgical interventions, complication rates, and current studies with biocellulose, respectively the potential of application for biocellulose. Using the example of the very first data from large animal experiments on the use of biocellulose tubes for the regeneration of the bile duct, attention will be drawn to the performance of this natural polymer as a tubular implant material. The data, although promising, may only indicate biocellulose as a potential candidate for the repair or replacement of abdominal hollow organs.
2. Preparation and Characterization of Tubular Biocellulose Implants
The design of three-dimensional shaped biocellulose hydrogels with specific characteristics is of particular interest in the area of medical implants. To date, it remains a great challenge to promote the regeneration of damaged organs or to completely replace them. Due to its high purity and biocompatibility, a nanofiber network with high mechanical strength, the ease of sterilization, and good surgical handling, biocellulose materials gain more and more attention as a future medical implant. A lot of research has been carried out regarding the production of tubular biocellulose for the replacement of small lumen blood vessels (<6 mm) [
7,
14]. The latest research is characterized by investigations regarding the application of biocellulose tubes (non-autologous implants) for other hollow organs in regenerative medicine; see also
Section 3.
There are two different approaches for the preparation of tubular biocellulose implants: a static cultivation of biocellulose hydrogels and a dynamic one. The first method can further be subdivided into the processing of flat biocellulose hydrogels and the direct formation of the tubes during the biotechnological synthesis.
2.1. Static Preparation of Tubular Biocellulose Materials
For the first approach, a flat biocellulose hydrogel in desired thickness was produced, which was followed by rolling this hydrogel into a multilayered tubular shape. To preserve the shape, the rolled biocellulose was dried [
15] or treated with concentrated alkali solution [
16] in a structure-determining mold. Li et al. combined rolling biocellulose hydrogels into multilayered tubes, lyophilization for creating tubes with a shape memory effect, and cell-laden biocellulose by microfluidics to imitate blood vessels in vitro [
17]. This strategy could also be used to mimic the structures of other hollow organs.
The second approach is the direct synthesis of tubular-shaped biocellulose. In 2001, the preparation of short-length tubes (length up to 10 mm, inner diameter: 0.6–6.0 mm) was described. The culture broth was filled in the space between a glass rod placed inside a hollow glass cylinder, resulting in tubes that are especially suitable for microsurgery [
18]. Probably, the most described technology for the production of tubes with increased length is the biosynthesis of biocellulose in/on oxygen permeable tubes with different dimensions. Bodin et al. utilized a silicone tube that was fixed inside a hollow glass cylinder. The space in between was filled with culture broth. Biocellulose was synthesized on top of the silicone, and it was characterized by a layered structure, an even inner side, and a more porous outer side. Oxygen in different concentrations was blown through the silicone tube and its influence on burst pressure, Young’s modulus, elongation break, and morphology was investigated. It could be shown that with the increasing oxygen ratio, the burst pressure increased, the elongation break decreased, the amount of layers increased, and the yield of cellulose increased, while the Young’s modulus was unaffected [
19]. There are several variants of this technology to create biocellulose tubes with different properties. It is possible to replace the outer glass tube with a second silicone tube or to place a glass rod inside a silicone tube. The materials used are variable as long as sufficient oxygen supply to the bacteria can be ensured. Bao et al. investigated the differences of these three variants (single silicone tube inside hollow glass cylinder, double silicone tube, and glass rod inside a silicone tube) on the structure, mechanical properties, and hemocompatibility. The space between the two materials was filled with culture broth and placed inside a container filled with oxygen for cultivation. It turned out that the biocellulose tubes, formed inside the hollow glass cylinder on top of the silicone, were characterized by a laminated structure, the lowest biocellulose content, and the weakest mechanical properties. In contrast, the double silicone tubes possessed an unstratified structure with a dense network, the greatest biocellulose content, and the strongest mechanical properties. The biocellulose tubes, which were formed inside a silicone tube containing a glass rod, had a looser network with the smoothest luminal surface and greater hemocompatibilty [
20]. In addition to the type of technology, the tube properties can be further modified by the cultivation conditions such as the cultivation time and type/concentration of the carbon source. The use of fructose led to a higher biocellulose production and stronger mechanical properties compared with glucose [
21].
Tubes can also be prepared by integrating biocellulose into a microfibrous support, resulting in a biocellulose composite. This method is particularly suitable for blood vessel implants, but it can also be transferred to other applications by selecting different support materials to generate the desired properties of the tube composite. The principle is based on electrospinning a fibrous tubular support, which is exemplary made of cellulose acetate for improved endothelialization and hemocompatibility, followed by a step-by-step in situ biosynthesis of biocellulose [
22,
23].
2.2. Dynamic Preparation of Tubular Biocellulose Materials
The methods described so far are mainly based on conventional static biocellulose production. For the replacement of hollow abdominal organs such as ureter, esophagus, and bile duct, it is essential to be able to manufacture biocellulose in tubular form—but especially the biotechnological design of variable inner diameters, nanofiber network structures, and surface properties is a key feature of the future implants. An approach that offers these opportunities is the Mobile Matrix Reservoir Technology (MMR Tech) developed by Klemm et al. Inspired by nature (biocellulose formation at the surface of liquid films on templates), this dynamic biotechnology allows a process-controlled design of the function-determining biomaterial properties. For producing biocellulose tubes, a cylindrical template was moved dynamically between the aqueous culture medium and the air space of a bioreactor. When dipping, the surface of the template was wetted with the culture medium. The biocellulose was formed in the air space on the template surface. This process can be repeated until the desired thickness is achieved. After finishing biosynthesis, the biocellulose was detached from the template and purified in aqueous alkali solution. The tubes are characterized by a variable inner diameter (2.5 up to 30 mm), a burst pressure >1100 mbar, and a suture retention strength >5 N. In case of MMR Tech, the biocellulose formation rate is not limited by hindered diffusion processes of nutrients and oxygen. During every immersion step, the newly synthesized biocellulose layer was covered with bacteria and culture medium. It could be shown that the multilayered structure is a specific result of the MMR Tech independent of the bacterial strain. The multilayered structure does not delaminate, even at surgical handling and implantation. Using MMR Tech, the shape of the template is not limited. Klemm et al. used sheets of different dimensions, cylindrical templates, hollow bodies, and branched constructions. The type of template materials ranges from different types of wood, glass, ceramics, as well as metals to different polymers, and even the coating of textiles and paper is possible. For the use as a template, the stability of the material under sterilization conditions as well as the wettability of its surface by the aqueous culture medium and the non-toxicity for the bacteria are of high importance [
7,
24,
25].
2.3. Selected Property and Structural Studies of Tubular Biocellulose
The suitability of biocellulose tubes produced according to MMR Tech was examined in our own works in vitro and in vivo regarding the regeneration of damaged bile ducts. Up to now, only little is known about the interaction of biocellulose and bile in the literature. For this reason, first laboratory studies were carried out in preparation of the described bile duct regeneration in
Section 3.1. The stability of biocellulose to fresh sheep bile and vice versa should be clarified, as well as the permeability of tube surfaces to bile. Four variants of the biocellulose tubes with a length of 100 mm were used. Variant 1 (V1) was a never-dried biocellulose tube formed on a cylindrical bamboo template with a diameter of 5 mm. Based on this variant, three others were produced. For variant 2 (V2), V1 was dried in the air stream to test whether this compressed micropore system is permeable to bile. Variant 3 (V3) was produced by inverting V1 (
Figure 1) so that the inner surface was directed outward and the outer surface was directed inward. The idea behind tube inversion was that the dense surface of the tube, which faces the air during biosynthesis, faces the bile in the later implant. The inversion was performed with the aim of designing the pore and network structure on both the inner and outer surface of the potential implant in such a way that improved colonization by cells can be achieved. Variant 4 (V4) was produced in the same way as V3 and dried in the air stream [
26].
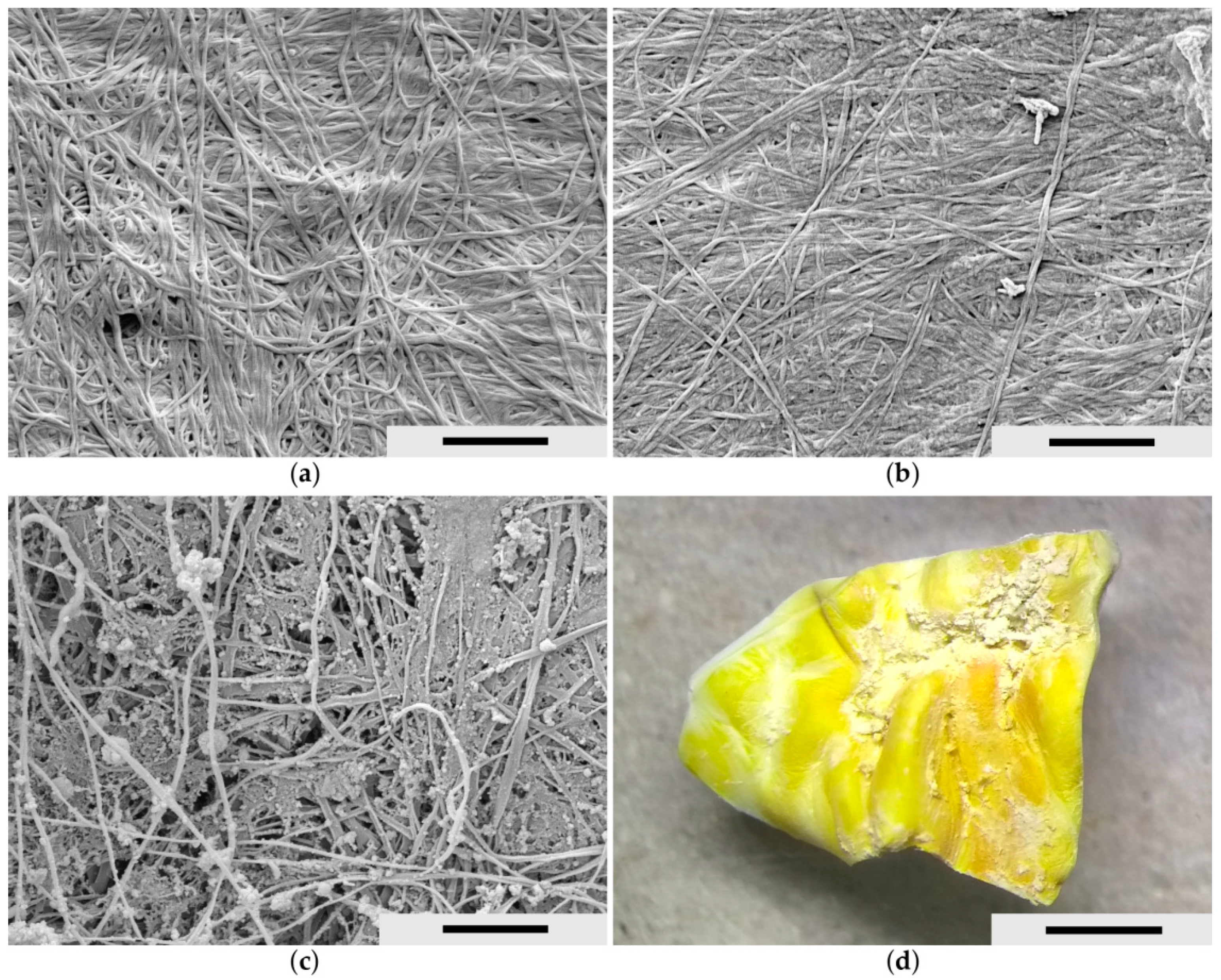
Figure 2 shows the scanning electron microscopic (SEM) images of the biocellulose tubes after synthesis and purification in a never-dried state before bile treatment experiments. For this purpose, the never-dried biocellulose tubes were freeze-dried. The surfaces of the variants V1 and the inverted V3 are illustrated. The originally denser outer surface of V1 (
Figure 2a) became more porous during inversion due to the shear forces acting on it (
Figure 2d) [
26].
The tube variants were incubated in fresh sheep bile for five days and examined using scanning electron microscopy to investigate whether the interaction of biocellulose with bile leads to structural changes in the biocellulose network. In addition to samples from the laboratory studies, an explant from large animal studies in pigs was also included (
Figure 3c,d); see also
Section 3.1. In order to prepare wet biocellulose materials for electron microscopy, the samples were freeze-dried to remove the water without altering the ultrastructure. On the other hand side, explanted samples of animals were chemically fixed with a mixture of 4% (
w/
v) paraformaldehyde and 2.5% (
v/
v) glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) for 24 h at room temperature. Afterwards, the explantation samples were dehydrated in ascending ethanol concentrations (30, 50, 70, 90, and 100%) for 15 min each and critical-point dried using liquid CO
2. Then, both types of samples were sputter coated with gold using a CCU-010 sputter coater (safematic GmbH, Zizers, Switzerland) and investigated with a field emission SEM LEO-1530 Gemini (Carl Zeiss NTS GmbH, Oberkochen, Germany) at magnification of 10,000× (
Figure 3) [
26].
Figure 3 shows the ultrastructure of the luminal surface of biocellulose tubes. To prove resistance to bile, the fiber structure of untreated material was compared to material exposed to bile in vitro showing no differences. Furthermore, material obtained by explantation after animal experiments where biocellulose tubes were successfully tested as a support for bile duct regeneration is shown in
Figure 3c. In addition to some organic debris, no structural change or damage of the biocellulose fibers could be found.
To investigate whether there are changes in bile due to incubation with biocellulose, UV/VIS spectra were recorded over a wavelength range of 360–600 nm. Bile that was not in contact with biocellulose over the incubation period was used as a control. The bile spectrum shows a characteristic maximum at approximately 420 nm. Bile incubated with biocellulose also shows this characteristic spectrum. This allows the conclusion that the composition of the bile does not change due to the biocellulose. Another preliminary investigation was to study the permeability of biocellulose tube variants V1–V4 filled with bile in direct contact with liquid physiological saline and in a humidity chamber created by saline. The biocellulose tubes functioned as a form-giving scaffold for the bile but did not completely separate it from the environment. The bile could penetrate the microporous network of the biocellulose and thus still allowed interaction with the environment without flowing out. The diffusion rate depended on the tube variant. In the case of the dried biocellulose tubes, the diffusion rate was lower than that of the never-dried tubes [
26]. These initial results, obtained in laboratory studies, are not negatively opposed to in vivo studies. The animal studies (pig) described in
Section 3.1 were performed using biocellulose tube V3.
3. Tubular Biocellulose in Oncologic Abdominal Surgery—A Critical Assessment
In modern abdominal oncologic surgery, the field for potential application of biomaterials is broad. One has to distinguish between parenchymal organs, e.g., the liver, and hollow organs. It should be emphasized that biocellulose can partially or completely fulfill the requirements for organ-specific implant materials and specific procedures due to its chemical and physical characteristics—as mentioned before. The following paragraph provides a critical assessment of the use of tubular biocellulose, especially tendered for selected hollow organs (
Table 1). To date, definitive studies assessing the performances of biocellulose-based devices in surgery are indispensable.
3.1. Bile Duct
3.1.1. Incidence of Relevant Biliary Diseases
Only 3% of tumors of the intestinal tract account for biliary cancer [
27]. However, the application of biocellulose is not only confined to oncologic surgery. From our point of view, there are two other fields: (1) bile duct necrosis after liver transplantation, which is most commonly associated with a preservation problem and (2) an incidental damage of the bile duct during removal of the gallbladder. The rate of bile duct problems after liver transplantation ranges between 15 and 25% [
28]. The damage of a bile duct during cholecystectomy ranges from 0.1 to 1.5% [
29].
3.1.2. Surgery
In most cases, a substantial defect of the bile duct cannot be reconstructed anatomically. In the vast majority of cases, the biliary drainage is realized by a so-called bilio-enteric anastomosis where a part of the small bowel is anastomosed directly with the bile duct. This often goes along with strictures of the anastomosis requiring intervention or with ascending infections to the biliary tree, since the sphincter mechanism in the duodenum is bypassed through the shortcut between bile duct and small intestine.
3.1.3. Potential Application for Biocellulose
In a large animal study, 3 cm of the extrahepatic common bile duct was resected and interposed with a biocellulose tube (steam sterilized). After twelve weeks, the bile duct has been completely regenerated, and the graft served as a leading structure for this regeneration process (
Figure 4). This approach could be used in all of the above-mentioned conditions (in oncologic surgery, for biliary complications after liver transplantation and in bile duct injuries), where actually, a bilio-enteric anastomosis is state-of-the art. The main advantage is in its use as a temporary graft, which can be removed at the end of the regeneration process [
26,
30,
31,
32].
De Abreu [
33] used a biocellulose film for the repair of a longitudinal incision. This large elliptical defect was treated with biocellulose. Even in a long-term follow-up after 330 days, the defect showed a complete healing process and biliary flow continuity.
3.2. Esophagus
3.2.1. Incidence Malignancy and Surgical Interventions
There are two main types of esophageal cancer, squamous cell cancer and adenocarcinoma, which differ in populations and have completely distinct biological characteristics, geographical distributions, and risk factors. The highest incidence rate of esophageal cancer can be found in Eastern Asia, with an age-standardized incidence rate of 12.3 per 100,000 in 2020 [
34]. In addition to a definite radiation therapy, the surgical resection of the tumor is the main part of the curative treatment. In most cases, this is performed as a thoraco-abdominal approach with the reconstruction of the gastrointestinal passage via an esophago-gastrostomy after the establishment of a gastric sleeve (a new connection between esophagus and the remaining stomach).
3.2.2. Complication Rate
After a resection of the esophagus, the main complication is the anastomotic leakage with a subsequent and severely dreaded mediastinitis (severe inflammation of the space between the right and left lung). The rate of leakages ranges from 3 to 21%, with a subsequent mortality rate from 0 to 35% [
35,
36]. The medical need for biocellulose derives from the prevention of leakage: it is essential to decrease this rate either through sheathing the anastomosis or through an internal splinting of the anastomotic region.
3.2.3. Current Studies with Biocellulose
Actually, there are no clinical studies using a biocellulose tube for circular esophageal replacement. The only study dealing with biocellulose is the evaluation of a biocellulose patch for the treatment of a semicircular defect in the esophagus wall published by Zhu et al. [
37]. The used patch was kombucha-synthesized biocellulose (KBC)—immersed in glycerol—compared to a cohort treated with autologous cervical fascia. The macroscopic evaluation after six months showed that KBC patches in the reconstructed artificial esophagus had exfoliated completely. Interestingly, the inner surface of grafts areas showed normal mucosal tissue. The control group showed similar results; however, there was a mild stricture in the region of interest. The microscopic evaluation showed a nearly normal histological architecture. The authors concluded that the used patch was suitable for esophageal repair. The limitation of this experimental study is clearly that the treated esophageal wall defect did not comprise the whole circumference of the esophagus.
A complete other approach was published by Lua et al. [
38]: the authors used carboxymethyl cellulose containing patches for the prevention of esophageal strictures after endoscopic submucosal dissection (endoscopic removal of parts of a hollow organ without the need of an operation). Even if the rate of strictures was high (57%), the authors assume the cellulose patches as a safe and effective prophylactic treatment for esophageal stricture especially after an extended endoscopic submucosal dissection.
3.3. Stomach
3.3.1. Incidence Malignancy and Surgical Interventions
Gastric cancer is estimated to be the fourth most common cancer in both sexes. This means it is the fourth leading malignancy-associated cause of death in 2020. There are strong regional distribution differences, since Eastern Asia contributes nearly 65% of new global gastric cancer cases each year. Central and Eastern Europe have the second highest incidence rate of gastric cancer worldwide. The incidence is significantly lower in Northern and Western Europe, similar to the USA [
34].
The surgical therapy is chosen depending on the localization of the tumor and the histological subtype. Especially in the diffuse type according to the Lauren classification, a complete removal of the stomach, a gastrectomy, has to be performed.
3.3.2. Complication Rate
The rate of anastomotic leakage after gastrectomy is reported to be between 2.1 and 14.6% with a subsequent mortality between 0 and 50% [
39]. Especially cases where a re-operation is needed increase the economic burden of a hospital significantly [
40].
3.3.3. Potential Application for Biocellulose
To the best of our knowledge, there were no experimental or clinical studies evaluating biocellulose in gastric surgery. Potential applications are, from the clinician’s point of view, again the sheathing or interposition of an esophageal–jejunal anastomosis after a gastrectomy (new connection between esophagus and the small intestine after removal of the stomach) or the use of a biocellulose patch after a pyloroplastic (technique for widening the sphincter at the end of the stomach). However, there is a need for experimental studies in this field, since the medical need is apparent in this area.
3.4. Small Intestine
3.4.1. Incidence Malignoma
Malignant tumors of the small intestine are very rare and account for only 2% of gastrointestinal cancer. The four most common types are adenocarcinoma, neuroendocrine tumors, sarcoma, and lymphoma [
41].
3.4.2. Surgery
The complete removal of the tumor offers the best chance for long-term survival [
41]. Depending on the localization of the tumor and a possible growth in the vessel-supplying mesenterial root, the tumor resection goes along with a more or less extended loss of small intestine. In normal cases, only a segment of the small intestine has to be removed.
3.4.3. Complication Rate
The rate of anastomotic leakage is low after a routine small bowel resection. However, especially in non-malignant but inflammatory bowel disease, fistula can occur quite often, which leads to the need of re-operation in the majority of cases.
3.4.4. Potential Application for Biocellulose
Since a loss of functional small intestine after a routine resection can be compensated easily by the human body, the need of tubular biocellulose is rare. It might be an option to treat patients with a short bowel syndrome after an extensive small bowel resection with a tubular interposition. However, it is up to now unclear whether the regenerative potential of biocellulose also triggers a small bowel regeneration restoring the function of the small intestine including motility and resorption surface. A similar approach was used by Grikscheit et al. in 2004 [
42], who applied tissue-engineered small intestine by the transplantation of organoid units on a polymer scaffold into the omentum of Lewis rats and implanted this generated material into the small intestine of rats.
Furthermore, it could be an option to sheath a critical anastomosis or to apply a patch of biocellulose for avoiding a recurrent fistula situation.
3.5. Large Intestine
3.5.1. Incidence Malignoma
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy in men and the second most common in women. For both genders, colorectal cancer is the second leading cause of malignancy-associated death [
34]. In addition to chemotherapy or radiation, the surgical removal of the tumor is an important column in the curative therapy of CRC.
3.5.2. Surgery
Depending on the localization of the tumor, a removal of the affected colon section has to be performed. The spectrum ranges from routine removals of the right hemicolon (with a low complication rate) to complex resections of the lower rectum.
3.5.3. Complication Rate
After resection of CRC and reconstruction of the intestinal continuity with an anastomosis, the leakage of the anastomosis is the most important major complication. The incidence ranges from 2 to 7% depending especially on the localization of the primary tumor and the complexity of the reconstruction [
43].
3.5.4. Potential Application for Biocellulose
As stated above in the section about the small intestine, there are no current studies evaluating biocellulose in colonic surgery. Again, a sheathing of a critical anastomosis could be a good option. As an interposition graft, it is actually unclear if the muscle layer of the intestinal regenerates completely after an interposition of biocellulose. This could go along with an impairment of the intestinal peristaltic wave, which leads to immense problems in intestinal motility. This issue is a major point for upcoming clinical applications.
3.6. Pancreas
3.6.1. Incidence Malignoma
Pancreatic cancer is the seventh leading cancer-associated cause of death worldwide with a steadily increasing incidence especially in highly developed countries [
44].
3.6.2. Surgery
Depending on the localization of the tumor, surgery is performed: this ranges from pancreatic tail resection without the need of an anastomosis to pancreatic head resections to complete pancreatectomy. The most complex with a potential demand for the use of biocellulose is the resection of the pancreatic head with a critical anastomosis between the small intestine and the pancreatic tail.
3.6.3. Complication Rate
Especially after a pancreatic head resection, the occurrence of a pancreatic fistula significantly influences the morbidity and mortality. The rate of relevant pancreatic fistula goes up to 20%, even in experienced high-volume centers [
45].
3.6.4. Potential Application for Biocellulose
There are two main types of anastomoses after a pancreatic head resection: a pancreaticojejunostomy, where the pancreatic tail is anastomosed with the small intestine and a pancreaticogastrostomy, where the pancreatic tail is anastomosed directly in the back wall of the stomach. For anatomical reasons, the use of biocellulose would be an option after the performance of a pancreaticojejunostomy, since this anastomosis could be easily sheathed with cellulose. Even the use of a larger biocellulose tube for strengthening the pancreatic could be a matter of debate to decrease the actual high rate of pancreatic fistula. However, there are no current studies, neither clinical nor experimental, in this issue.
3.7. Ureter/Urethra
3.7.1. Incidence Malignoma
Not only malignant diseases can cause symptomatic strictures within the urinary tract. In addition to postoperative conditions causing strictures, there might also be a tumor compression, an ureteral necrosis after kidney transplantation, or a radiation treatment which can cause ureteral stenosis [
46]. Within the urethra, the etiology of strictures is subdivided in four main categories: idiopathic, iatrogenic, inflammatory, and traumatic. The prevalence of urethral strictures is 200/100,000 in younger men and up to >600/100,000 in men above 65 years [
47].
3.7.2. Surgery
The therapeutic spectrum, exemplary for ureteral strictures, ranges from Psoas hitch operation to Boari flaps to renal autotransplantations with pyelovesical anastomosis. Therefore, the therapeutic options are broad with good functional long-term results and low treatment-associated morbidity [
46].
3.7.3. Complication Rate
Depending on the chosen treatment, the field of potential complications is broad, but the recurrence of a treated stricture is the most frightened complication.
3.7.4. Potential Application for Biocellulose
There are some studies dealing with biocellulose scaffolds in this field. Bodin et al. [
48] seeded a biocellulose polymer with urine-derived stem cells. The authors could show a potential application for tissue-engineered urinary conduits. Using a biocellulose/gelatin composite scaffold seeded with keratinocytes and muscle cells, the creation of a patent urethra was observed by Lv et al. [
49]. Further experiments of this group combining biocellulose and bladder acellular matrix led to an enhanced angiogenesis and epithelialization during the induction of a successful rabbit urethral repair [
50].
4. Conclusions
This contribution aims to show that tubular hydrogel bodies made of biocellulose are a promising implant material for the regeneration or replacement of soft tissue hollow organs. Both static and dynamic technologies have been developed to produce such hydrogels.
The medical need for the application of biocellulose in modern abdominal surgery is high. There are several organ systems that could benefit significantly from the chemical and physical characteristics of this natural polymer. Consisting of 99% water and 1% cellulose, this hydrogel is distinguished by a high purity and biocompatibility and functional hydroxyl groups. The microporous nanofibernetwork enables mechanical stability, a suitability for all suture procedures commonly used in surgery, and can be colonized with cells. The implant materials can be sterilized and therefore also show long-term storage stability. The use of the material is especially tendered for the reconstructive part after the resection of a certain part of an organ. Depending on the type of implantation (e.g., interposition or sheathing), it could be possible to use the biocellulose as temporary graft allowing the particular organ to regenerate along the graft as leading structure. This would enable removing the biocellulose after a certain time. The other approach is the sheathing of a critical region, e.g., a complex anastomosis, with biocellulose. In most cases, the removal of the graft is impossible, so the incorporation of the graft would be a necessary requirement.
For the development and manufacture of these implants, it is essential to tailor the function-determining properties to the characteristic features of the respective organ. These include the shape, dimensions, surface structures, and nanofiber network architecture. Furthermore, it is necessary to adapt the surface and network properties of the interconnecting microporous system to the individual characteristics of the hollow organs by incorporating additives (composite formation) and post-processing (e.g., targeted drying for the modification of the pore system). The technologies used for this purpose must be designed in such a way that they allow a scale-up, reproducibility of process-controlled work steps, and automation. It should be possible to protect the development stages achieved in this process under patent law and to attract investors.
Author Contributions
Conceptualization, D.O.K., F.R., U.S., V.R., K.P.-W.; software, S.N., F.R., V.R.; formal analysis, C.R., F.K.; investigation, D.O.K., V.R., K.P.-W., F.K., C.R., S.N., U.S., F.R.; data curation, D.O.K., V.R., K.P.-W., F.K., C.R., S.N., U.S., F.R.; writing—original draft preparation, V.R., F.R., S.N., F.K.; writing—review and editing, D.O.K., V.R., K.P.-W., F.K., C.R., S.N., U.S., F.R.; visualization, V.R., K.P.-W., F.R.; supervision, D.O.K., U.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal protocols used in this work were evaluated and approved by the Thuringian State Office of Food Safety and Consumer Protection (Protocol-Number: 08-001/13). They are in accordance with FELASA guidelines and the German law.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Thomas Richter for the technical development of the bioreactor for Mobile Matrix Reservoir Technology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Klemm, D.; Lindström, T.; Abitbol, T.; Kralisch, D. The nanocellulose family. In Nanocellulose Based Composites for Electronics, 1st ed.; Thomas, S., Pottathara, Y.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–14. ISBN 9780128223550. [Google Scholar]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef] [Green Version]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. Engl. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Gama, M.; Gatenholm, P.; Klemm, D. Bacterial Nanocellulose: A Sophisticated Multifunctional Material; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-138-07316-6. [Google Scholar]
- Pértile, R.A.N.; Moreira, S.; Gil da Costa, R.M.; Correia, A.; Guãrdao, L.; Gartner, F.; Vilanova, M.; Gama, M. Bacterial cellulose: Long-term biocompatibility studies. J. Biomater. Sci. Polym. Ed. 2012, 23, 1339–1354. [Google Scholar] [CrossRef] [Green Version]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef]
- Klemm, D.; Petzold-Welcke, K.; Kramer, F.; Richter, T.; Raddatz, V.; Fried, W.; Nietzsche, S.; Bellmann, T.; Fischer, D. Biotech nanocellulose: A review on progress in product design and today’s state of technical and medical applications. Carbohydr. Polym. 2021, 254, 117313. [Google Scholar] [CrossRef] [PubMed]
- Nizam, P.A.; Gopakumar, D.A.; Pottathara, Y.B.; Pasquini, D.; Nzihou, A.; Thomas, S. Nanocellulose-based composites: Fundamentals and applications in electronics. In Nanocellulose Based Composites for Electronics, 1st ed.; Thomas, S., Pottathara, Y.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 15–29. ISBN 9780128223550. [Google Scholar]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial cellulose as a raw material for food and food packaging applications. Front. Sustain. Food Syst. 2019, 3, 362. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Liu, Z.; Shen, R.; Chen, S.; Yang, X. Bacterial cellulose in food industry: Current research and future prospects. Int. J. Biol. Macromol. 2020, 158, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Skočaj, M. Bacterial nanocellulose in papermaking. Cellulose 2019, 26, 6477–6488. [Google Scholar] [CrossRef]
- Tomé, L.C.; Brandão, L.; Mendes, A.M.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Freire, C.S.R.; Marrucho, I.M. Preparation and characterization of bacterial cellulose membranes with tailored surface and barrier properties. Cellulose 2010, 17, 1203–1211. [Google Scholar] [CrossRef]
- Urbina, L.; Corcuera, M.Á.; Eceiza, A.; Retegi, A. Stiff all-bacterial cellulose nanopaper with enhanced mechanical and barrier properties. Mater. Lett. 2019, 246, 67–70. [Google Scholar] [CrossRef]
- Wacker, M.; Kießwetter, V.; Slottosch, I.; Awad, G.; Paunel-Görgülü, A.; Varghese, S.; Klopfleisch, M.; Kupitz, D.; Klemm, D.; Nietzsche, S.; et al. In vitro hemo- and cytocompatibility of bacterial nanocelluose small diameter vascular grafts: Impact of fabrication and surface characteristics. PLoS ONE 2020, 15, e0235168. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Guan, Q.; Han, Z.; Yang, H.; Ling, Z. Preparation Method of Bacterial Cellulose Composite Board with Tubular Structure. CN Patent 112092419 A, 18 June 2019. [Google Scholar]
- Hong, F.; Bao, L.; Tang, J.; Ji, C.; Chen, L.; Hu, G. Bacterial Nano Cellulose Base Pipe with Inner Surface Texture Modification and Preparation Method and Application. CN Patent 109912828 A, 30 January 2019. [Google Scholar]
- Li, Y.; Jiang, K.; Feng, J.; Liu, J.; Huang, R.; Chen, Z.; Yang, J.; Dai, Z.; Chen, Y.; Wang, N.; et al. Construction of small-diameter vascular graft by shape-memory and self-rolling bacterial cellulose membrane. Adv. Healthc. Mater. 2017, 6, 1601343. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Bodin, A.; Bäckdahl, H.; Fink, H.; Gustafsson, L.; Risberg, B.; Gatenholm, P. Influence of cultivation conditions on mechanical and morphological properties of bacterial cellulose tubes. Biotechnol. Bioeng. 2007, 97, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Tang, J.; Hong, F.F.; Lu, X.; Chen, L. Physicochemical properties and in vitro biocompatibility of three bacterial nanocellulose conduits for blood vessel applications. Carbohydr. Polym. 2020, 239, 116246. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, X.; Bao, L.; Chen, L.; Hong, F.F. Comparison of two types of bioreactors for synthesis of bacterial nanocellulose tubes as potential medical prostheses including artificial blood vessels. J. Chem. Technol. Biotechnol. 2017, 92, 1218–1228. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, S.; Peng, M.; Gama, M.; Yang, Z.; Deng, X.; Zhou, J.; Ouyang, C.; Luo, H. Controllable synthesis of biomimetic nano/submicro-fibrous tubes for potential small-diameter vascular grafts. J. Mater. Chem. B 2020, 8, 5694–5706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, S.; Zhu, X.; Luo, H.; Gama, M.; Peng, M.; Deng, X.; Wan, Y. Heparinization and hybridization of electrospun tubular graft for improved endothelialization and anticoagulation. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111861. [Google Scholar] [CrossRef] [PubMed]
- Fried, W.; Klemm, D.; Kopsch, V.; Koth, D.; Kramer, F.; Moritz, S.; Richter, T.; Schumann, D.; Udhardt, U. Process for Producing Hollow Bodies from Microbial Cellulose. International Patent WO2013113675A1, 29 January 2013. [Google Scholar]
- Klemm, D.; Kopsch, V.; Koth, D.; Kramer, F.; Moritz, S.; Richter, T.; Udhart, U.; Fried, W. Vorrichtung zur Herstellung von Hohlkörpern aus Mikrobiellem Polymer. German Patent DE102012201272B4, 13 January 2012. [Google Scholar]
- Klemm, D.; Rauchfuß, F.; Kramer, F.; Petzold-Welcke, K.; Richter, T.; Ruhe, C.; Tannapfel, A. Design of biocellulose implants for first successful bile duct regeneration. In Proceedings of the 251st ACS National Meeting & Exposition, San Diego, CA, USA, 13–17 March 2016. [Google Scholar]
- Bergquist, A.; von Seth, E. Epidemiology of cholangiocarcinoma. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Boeva, I.; Karagyozov, P.I.; Tishkov, I. Post-liver transplant biliary complications: Current knowledge and therapeutic advances. World J. Hepatol. 2021, 13, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Khadra, H.; Johnson, H.; Crowther, J.; McClaren, P.; Darden, M.; Parker, G.; Buell, J.F. Bile duct injury repairs: Progressive outcomes in a tertiary referral center. Surgery 2019, 166, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Rauchfuß, F.; Kramer, F.; Fried, W.; Tannapfel, A.; Petzold-Welcke, K.; Klemm, D.; Settmacher, U. Biocellulosis induces regeneration of the extrahepatic bile duct. HPB 2019, 21, 540–541. [Google Scholar] [CrossRef] [Green Version]
- Rauchfuß, F. Biocellulosis as scaffold for tissue regeneration in modern surgery. In Proceedings of the 225th ACS National Meeting & Exposition, New Orleans, MI, USA, 18–22 March 2018. [Google Scholar]
- Rauchfuß, F.; Settmacher, U.; Klemm, D.; Fried, W.; Richter, T.; Petzold-Welcke, K.; Ruhe, C. Medical Implant Based on Nanocellulose. International Patent WO2017021468A1, 9 February 2017. [Google Scholar]
- Abreu, G.F.; de Batista, L.L.; Adeodato, D.C.; Albuquerque, A.V.; de Ferraz-Carvalho, R.S.; Lima, R.P.; de Souza, V.S.; de Carvalho, G.L.; de La Aguiar, J. Use of bacterial cellulose film for repair of bile duct injury in pigs. J. Biomater. Appl. 2020, 35, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shi, L.; He, X.; Luo, Y. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol. Rep. 2021, 9, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jiang, H. The assessment of intraoperative technique-related risk factors and the treatment of anastomotic leakage after esophagectomy: A narrative review. J. Gastrointest. Oncol. 2021, 12, 207–215. [Google Scholar] [CrossRef]
- Low, D.E. Update on staging and surgical treatment options for esophageal cancer. J. Gastrointest. Surg. 2011, 15, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, F.; Qian, W.; Wang, Y.; You, Q.; Zhang, T.; Li, F. Esophageal replacement by hydroxylated bacterial cellulose patch in a rabbit model. Turk. J. Med. Sci. 2015, 45, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Lua, G.W.; Tang, J.; Liu, F.; Li, Z.S. Prevention of esophageal strictures after endoscopic submucosal dissection: A promising therapy using carboxymethyl cellulose sheets. Dig. Dis. Sci. 2016, 61, 1763–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makuuchi, R.; Irino, T.; Tanizawa, Y.; Bando, E.; Kawamura, T.; Terashima, M. Esophagojejunal anastomotic leakage following gastrectomy for gastric cancer. Surg. Today 2019, 49, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.K.; Chen, M.-M.; Yan, M.; Zhu, Z.-G. Reoperation for early postoperative complications after gastric cancer surgery in a Chinese hospital. World J. Gastroenterol. 2010, 16, 98–103. [Google Scholar] [CrossRef]
- Reynolds, I.; Healy, P.; Mcnamara, D.A. Malignant tumours of the small intestine. Surgeon 2014, 12, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Grikscheit, T.C.; Siddique, A.; Ochoa, E.R.; Srinivasan, A.; Alsberg, E.; Hodin, R.A.; Vacanti, J.P. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann. Surg. 2004, 240, 748–754. [Google Scholar] [CrossRef]
- Dhindsa, B.S.; Naga, Y.; Saghir, S.M.; Daid, S.G.S.; Chandan, S.; Mashiana, H.; Dhaliwal, A.; Sidhu, A.; Sayles, H.; Ramai, D.; et al. Endo-sponge in management of anastomotic colorectal leaks: A systematic review and meta-analysis. Endosc. Int. Open 2021, 9, E1342–E1349. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Yelamanchi, R. Pancreatic adenocarcinoma: A review of recent paradigms and advances in epidemiology, clinical diagnosis and management. World J. Gastroenterol. 2021, 27, 3158–3181. [Google Scholar] [CrossRef]
- Horvath, P.; Beckert, S.; Nadalin, S.; Königsrainer, A.; Königsrainer, I. Pancreas-preserving surgical management of grade-C pancreatic fistulas after pancreatic head resection by external wirsungostomy. Langenbeck’s Arch. Surg. 2016, 401, 457–462. [Google Scholar] [CrossRef]
- Paffenholz, P.; Heidenreich, A. Modern surgical strategies in the management of complex ureteral strictures. Curr. Opin. Urol. 2021, 31, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.G. Current management of urethral stricture disease. Indian J. Urol. 2016, 32, 27–33. [Google Scholar] [CrossRef]
- Bodin, A.; Bharadwaj, S.; Wu, S.; Gatenholm, P.; Atala, A.; Zhang, Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials 2010, 31, 8889–8901. [Google Scholar] [CrossRef]
- Lv, X.; Yang, J.; Feng, C.; Li, Z.; Chen, S.; Xie, M.; Huang, J.; Li, H.; Wang, H.; Xu, Y. Bacterial cellulose-based biomimetic nanofibrous scaffold with muscle cells for hollow organ tissue engineering. ACS Biomater. Sci. Eng. 2016, 2, 19–29. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.; Li, Z.; Zhang, M.; Yao, J.; Sheng, N.; Lu, M.; Wang, H.; Chen, S. Urethra-inspired biomimetic scaffold: A therapeutic strategy to promote angiogenesis for urethral regeneration in a rabbit model. Acta Biomater. 2020, 102, 247–258. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).