Effects of CoMo/γ-Al2O3 Catalysts on Product Hydrocarbon and Phenol Distribution during Hydrodeoxygenation of Oxidized Bio-Oil in a Batch Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bio-Oil Production and Oxidation
2.3. CoMo/γ-Al2O3 Catalyst Reduction and Sulfidation
2.4. Hydrodeoxygenation of Oxidized Bio-Oil
2.5. Characterization
2.5.1. Physical Properties
2.5.2. Gas Chromatography-Mass Spectrometry
2.5.3. Gas Chromatography
3. Results and Discussion
3.1. Physical Properties of Raw Bio-Oil, Oxidized Bio-Oil, and Organic Liquids
3.2. GC/MS Analysis of Raw Bio-Oil, Oxidized Bio-Oil, and Organic Liquids
3.3. GC Analysis of the Exit Gas Component
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Lu, Z.H.; Luo, Y.; Zou, A.H.; Yao, Q.L.; Chen, X.S. Mesoporous Carbon Nitride Supported Pd and Pd-Ni Nanoparticles as Highly Efficient Catalyst for Catalytic Hydrolysis of NH3BH3. Chemcatchem 2018, 10, 1620–1626. [Google Scholar] [CrossRef]
- Chen, J.M.; Lu, Z.H.; Yao, Q.L.; Gang, F.; Luo, Y. Complete Dehydrogenation of N2H4BH3 with NiM-Cr2O3 (M = Pt, Rh, Ir) Hybrid Nanoparticles. J. Mater. Chem. A 2018, 6, 20746–20752. [Google Scholar] [CrossRef]
- Fang, J.; Meng, Z.W.; Li, J.S.; Du, Y.H.; Qin, Y.; Jiang, Y.; Bai, W.L.; Chase, G.G. The Effect of Operating Parameters on Regeneration Characteristics and Particulate Emission Characteristics of Diesel Particulate Filters. Appl. Therm. Eng. 2019, 148, 860–867. [Google Scholar] [CrossRef]
- Zhang, S.L.; Yao, Q.L.; Li, Q.Y.; Feng, G.; Lu, Z.H. Complete Hydrogen Production from Hydrazine Borane over Raney Ni Catalyst at Room Temperature. Energy Technol. 2019, 7, 3. [Google Scholar] [CrossRef]
- Li, J.H.; Yan, Q.G.; Zhang, X.F.; Zhang, J.L.; Cai, Z.Y. Efficient Conversion of Lignin Waste to High Value Bio-Graphene Oxide Nanomaterials. Polymers 2019, 11, 623. [Google Scholar] [CrossRef] [Green Version]
- Navarathna, C.M.; Bombuwala Dewage, N.; Keeton, C.; Pennisson, J.; Henderson, R.; Lashley, B.; Zhang, X.; Hassan, E.B.; Perez, F.; Mohan, D.; et al. Biochar adsorbents with enhanced hydrophobicity for oil spill removal. ACS Appl. Mater. Interfaces 2020, 12, 9248–9260. [Google Scholar] [CrossRef] [PubMed]
- Samaraweera, H.; Sharp, A.; Edwards, J.; Pittman, C.U., Jr.; Zhang, X.; Hassan, E.B.; Thirumalai, R.; Warren, S.; Reid, C.; Mlsna, T. Lignite, Thermally-Modified and Ca/Mg-Modified Lignite for Phosphate Remediation. Sci. Total Environ. 2021, 773, 145631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Elsayed, I.; Navarathna, C.; Schueneman, G.T.; Hassan, E.B. Biohybrid hydrogel and aerogel from self-assembled nanocellulose and nanochitin as a high-efficiency adsorbent for water purification. ACS Appl. Mater. Interfaces 2019, 11, 46714–46725. [Google Scholar] [CrossRef]
- Fang, J.; Shi, R.; Meng, Z.W.; Jiang, Y.; Qin, Z.H.; Zhang, Q.; Qin, Y.; Tan, J.; Bai, W.L. The interaction effect of catalyst and ash on diesel soot oxidation by thermogravimetric analysis. Fuel 2019, 258, 116151. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Q.; Meng, Z.W.; Luo, Y.; Ou, J.; Du, Y.H.; Zhang, Z.L. Effects of ash composition and ash stack heights on soot deposition and oxidation processes in catalytic diesel particulate filter. J. Energy Inst. 2020, 93, 1942–1950. [Google Scholar] [CrossRef]
- Hu, J.L.; Wildfire, C.; Stiegman, A.E.; Dagle, R.A.; Shekhawat, D.; Abdelsayed, V.; Bai, X.W.; Tian, H.J.; Bogle, M.B.; Hsu, C.R.; et al. Microwave-driven heterogeneous catalysis for activation of dinitrogen to ammonia under atmospheric pressure. Chem. Eng. J. 2020, 397, 125388. [Google Scholar] [CrossRef]
- Zou, H.T.; Zhang, S.L.; Hong, X.L.; Yao, Q.L.; Luo, Y.; Lu, Z.H. Immobilization of Ni-Pt nanoparticles on MIL-101/rGO composite for hydrogen evolution from hydrous hydrazine and hydrazine borane. J. Alloys Compd. 2020, 835, 155426. [Google Scholar] [CrossRef]
- Zhang, X.; Jeremic, D.; Kim, Y.; Street, J.; Shmulsky, R. Effects of surface functionalization of lignin on synthesis and properties of rigid bio-based polyurethanes foams. Polymers 2018, 10, 706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.F.; Kim, Y.; Islam, E.; Taylor, M.; Eberhardt, T.L.; Hassan, E.; Shmulsky, R. Rigid polyurethane foams containing lignin oxyalkylated with ethylene carbonate and polyethylene glycol. Ind. Crop. Prod. 2019, 141, 706. [Google Scholar] [CrossRef]
- Zhang, X.F.; Kim, Y.S.; Eberhardt, T.L.; Shmulsky, R. Lab-scale structural insulated panels with lignin-incorporated rigid polyurethane foams as core. Ind. Crop. Prod. 2019, 132, 292–300. [Google Scholar] [CrossRef]
- Anis, S.; Zainal, Z.A. Tar reduction in biomass producer gas via mechanical, catalytic, and thermal methods: A review. Renew. Sustain. Energy Rev. 2011, 15, 2355–2377. [Google Scholar] [CrossRef]
- Zhang, L.B.; Luo, Y.; Wijayapala, R.; Walters, K.B. Alcohol stabilization of low water content pyrolysis oil during high temperature treatment. Energy Fuels 2017, 31, 13666–13674. [Google Scholar] [CrossRef]
- Luo, Y.; Guda, V.K.; Hassan, E.; Steele, P.H.; Mitchell, B.; Yu, F. Hydrodeoxygenation of oxidized distilled bio-oil for the production of gasoline fuel type. Energy Convers. Manag. 2016, 112, 319–327. [Google Scholar] [CrossRef]
- Luo, Y.; Hassan, E.; Guda, V.; Wijayapala, R.; Steele, P.H. Upgrading of syngas hydrotreated fractionated oxidized bio-oil to transportation grade hydrocarbons. Energy Convers. Manag. 2016, 115, 159–166. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 12. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 9. [Google Scholar] [CrossRef] [Green Version]
- Czernik, S.; Bridgwater, A.V. Overview of Applications of Biomass Fast Pyrolysis Oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-Oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Neuenschwander, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Solantausta, Y. Catalytic hydroprocessing of fast pyrolysis bio-oil from pine sawdust. Energy Fuels 2012, 26, 3891–3896. [Google Scholar] [CrossRef]
- Diebold, J.P. A Review of the chemical and physical mechanisms of the storage stability of fast pyrolysis bio-oils. Natl. Renew. Energy Lab. 2000, 2, 1. [Google Scholar]
- Hu, X.; Wang, Y.; Mourant, D.; Gunawan, R.; Lievens, C.; Chaiwat, W.; Gholizadeh, M.; Wu, L.; Li, X.; Li, C.Z. Polymerization upon heating up of bio-oil: A model compound study. AIChE J. 2013, 59, 888–900. [Google Scholar] [CrossRef]
- Li, X.; Gunawan, R.; Wang, Y.; Chaiwat, W.; Hu, X.; Gholizadeh, M.; Mourant, D.; Bromly, J.; Li, C.Z. Upgrading of bio-oil into advanced biofuels and chemicals. Part III. Changes in aromatic structure and coke forming propensity during the catalytic hydrotreatment of a fast pyrolysis bio-oil with Pd/C catalyst. Fuel 2014, 116, 642–649. [Google Scholar] [CrossRef]
- Tanneru, S.K.; Steele, P.H. Pretreating bio-oil to increase yield and reduce char during hydrodeoxygenation to produce hydrocarbons. Fuel 2014, 133, 326–331. [Google Scholar] [CrossRef]
- Parapati, D.R.; Guda, V.K.; Penmetsa, V.K.; Steele, P.H.; Tanneru, S.K. Comparison of reduced and sulfided CoMo/-Al2O3 catalyst on hydroprocessing of pretreated bio-oil in a continuous packed-bed reactor. Environ. Prog. Sustain. Energy 2014, 33, 726–731. [Google Scholar] [CrossRef]
- Ardiyanti, A.R.; Khromova, S.A.; Venderbosch, R.H.; Yakovlev, V.A.; Heeres, H.J. Catalytic hydrotreatment of fast-pyrolysis oil using non-sulfided bimetallic Ni-Cu catalysts on a δ-Al2O3 support. Appl. Catal. B Environ. 2012, 117–118, 105–117. [Google Scholar] [CrossRef]
- Lee, C.L.; Ollis, D.F. Interactions between catalytic hydrodeoxygenation of benzofuran and hydrodesulfurization of dibenzothiophene. Acad. Press 1984, 87, 325–331. [Google Scholar] [CrossRef]
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nat. Int. Wkly. J. Sci. 2002, 418, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Shao, S.; Huang, Q.; Liang, X.; Jiang, L.; Li, Y. Review of catalytic pyrolysis of biomass for bio-oil. In Proceedings of the 2011 International Conference on Materials for Renewable Energy & Environment (ICMREE 2011), Shanghai, China, 20–22 May 2011; Institute of Electrical and Electronics Engineers: Piscataway Township, NJ, USA, 2011; pp. 366–370. [Google Scholar]
- Cui, H.Y.; Wang, J.H.; Zhuo, S.P.; Li, Z.H.; Wang, L.H.; Yi, W.M. Upgrading bio-oil by esterification under supercritical CO2 conditions. J. Fuel Chem. Technol. 2010, 38, 673–678. [Google Scholar] [CrossRef]
- Li, W.; Pan, C.; Zhang, Q.; Liu, Z.; Peng, J.; Chen, P.; Lou, H.; Zheng, X. Upgrading of low-boiling fraction of bio-oil in supercritical methanol and reaction network. Bioresour. Technol. 2011, 102, 4884–4889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luo, Z.; Dang, Q.; Wang, J.; Chen, W. Upgrading of bio-oil over bifunctional catalysts in supercritical monoalcohols. Energy Fuels 2012, 26, 2990–2995. [Google Scholar] [CrossRef]
- Elliott, D.C. Historical developments in hydroprocessing bio-oils. Energy Fuels 2007, 21, 1792–1815. [Google Scholar] [CrossRef]
- Xu, H.; Fan, X.; Li, G.S.; Xu, Y.Y.; Mo, W.L.; Kuznetsov, P.N.; Ma, F.Y.; Wei, X.Y. Preparation of Co-Mo/gamma-Al2O3 catalyst and the catalytic hydrogenation effects on coal-related model compounds. J. Energy Inst. 2021, 96, 52–60. [Google Scholar] [CrossRef]
- Mederos-Nieto, F.S.; Santoyo-Lopez, A.O.; Hernandez-Altamirano, R.; Mena-Cervantes, V.Y.; Trejo-Zarraga, F.; Centeno-Nolasco, G.; Ramirez-Jimenez, E. Renewable fuels production from the hydrotreating over NiMo/gamma-Al2O3 catalyst of castor oil methyl esters obtained by reactive extraction. Fuel 2021, 285, 119168. [Google Scholar] [CrossRef]
- Luo, Y.; Street, J.; Steele, P.; Entsminger, E.; Guda, V. Activated carbon derived from pyrolyzed pinewood char using elevated temperature, KOH, H3PO4, and H2O2. Bioresources 2016, 11, 10433–10447. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Guda, V.K.; Steele, P.H.; Wan, H. Hydrodeoxygenation of oxidized and hydrotreated bio-oils to hydrocarbons in fixed-bed continuous reactor. Bioresources 2016, 11, 4415–4431. [Google Scholar] [CrossRef]
- Luo, Y.; Hassan, E.; Miao, P.; Xu, Q.; Steele, P.H. Effects of single-stage syngas hydrotreating on the physical and chemical properties of oxidized fractionated bio-oil. Fuel 2017, 209, 634–642. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, X.; Pu, H.; Pan, H.L.; Feng, X.; Ding, W.H.; Ding, C.F.; Dang, Y.H. Single stage catalytic hydrodeoxygenation of pretreated bio-oil. Bioresources 2021, 16, 2747–2755. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, K.; Fei, L. The Effects of Activation conditions on physical properties of activated carbon. Bioresources 2020, 15, 7640–7647. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, A. Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil: A review. Renew. Sustain. Energy Rev. 2016, 58, 1293–1307. [Google Scholar] [CrossRef]
- Elliott, D.C.; Neuenschwander, G.G. Liquid fuels by low-severity hydrotreating of biocrude. Fuel Energy Abstr. 1998, 39, 259. [Google Scholar]
- Jones, S.B.; Valkenburg, C.; Walton, C.W.; Elliott, D.C.; Holladay, J.E.; Stevens, D.J.; Kinchin, C.; Czernik, S. Production of Gasoline and Diesel from Biomass via Fast Pyrolysis, Hydrotreating and Hydrocracking: A Design Case; Pacific Northwest National Laboratory: Richland, WA, USA, 2009. [Google Scholar]
| Properties | Raw Bio-Oil | Oxidized Bio-Oil | Organic Liquids | ||||
|---|---|---|---|---|---|---|---|
| / | / | a RC-10 | b SC-5 | b SC-8 | b SC-10 | b SC-15 | |
| HHV, MJ/kg | 14.75 | Misfire | 40 | 33.83 | 37.27 | 43.31 | 44.65 |
| Total acid number, mg KOH/g | 95.2 | 179.5 | 0.55 | 5.54 | 3.25 | 0.55 | 0.55 |
| Water content, vol.% | 28.4 | 30.9 | 5.06 | 2.62 | 2.71 | 1.64 | 0.53 |
| Oil phase weight, g | / | / | 18.2 | 17.65 | 15.3 | 14.25 | 13.1 |
| Water phase weight, g | / | / | 50.5 | 53.35 | 50.6 | 40.45 | 56.1 |
| Yield, wt% | / | / | 20 | 19 | 17 | 16 | 14 |
| Carbon, wt% | 43.6 | 31.8 | 80.81 | 85.38 | 83.14 | 85.29 | 87.25 |
| Hydrogen, wt% | 7.8 | 7.8 | 10.27 | 11.08 | 11.18 | 11.75 | 12.35 |
| Nitrogen, wt% | 0.20 | 0.02 | 1.06 | 0.13 | 0.16 | 0.85 | 0.12 |
| Oxygen, wt% | 48.4 | 60.64 | 7.85 | 3.41 | 5.52 | 2.10 | 0.28 |
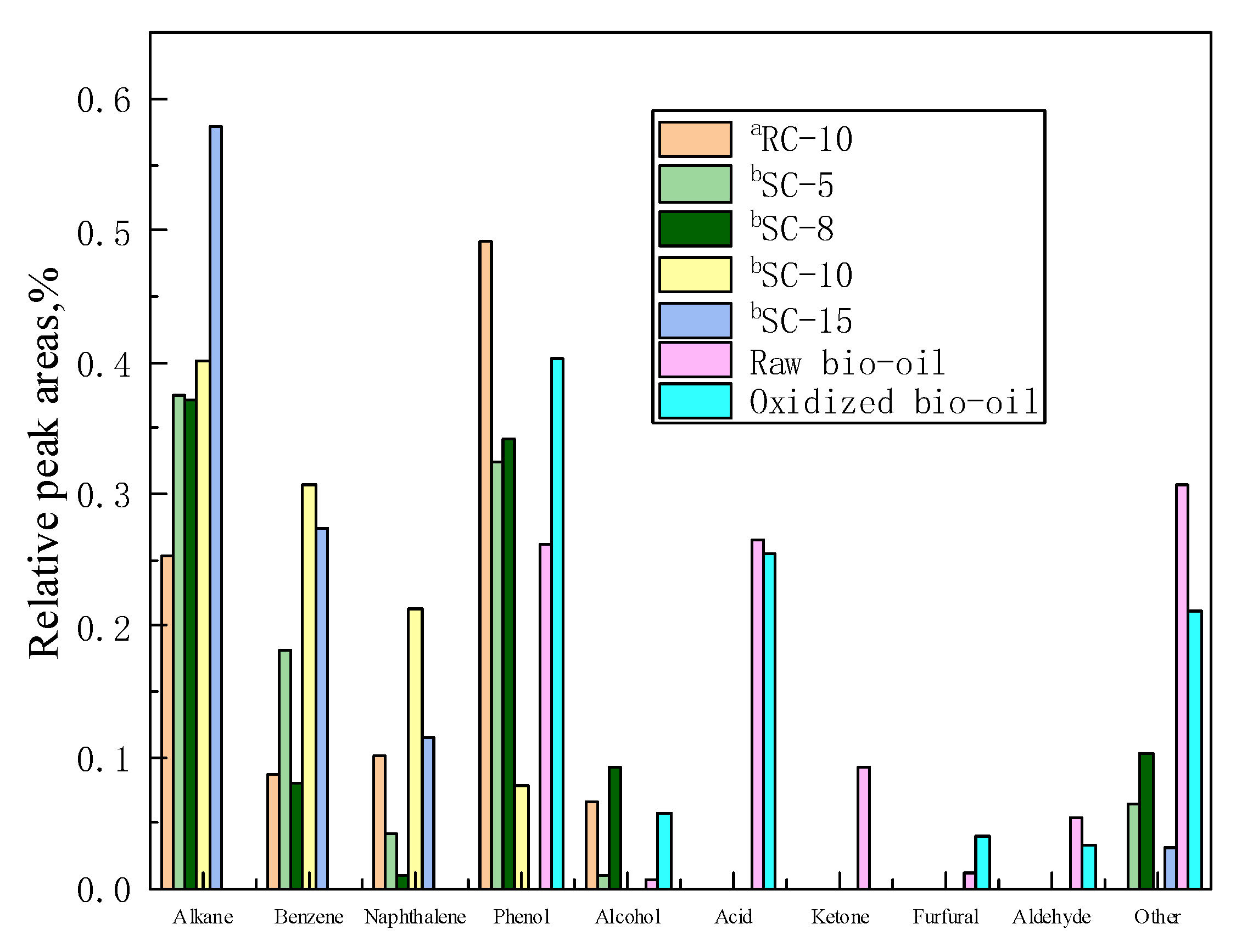
| Types | Raw Bio-Oil | Oxidized Bio-Oil | a RC-10 (Organic Liquid) | b SC-5 (Organic Liquid) | b SC-8 (Organic Liquid) | b SC-10 (Organic Liquid) | b SC-15 (Organic Liquid) |
|---|---|---|---|---|---|---|---|
| Alkane | / | / | 25 | 37 | 37 | 40 | 58 |
| Benzene | / | / | 9 | 18 | 8 | 31 | 27 |
| Naphthalene | / | / | 10 | 4 | 1 | 21 | 11 |
| Phenol | 26 | 40 | 49 | 33 | 34 | 8 | / |
| Alcohol | 1 | 6 | 7 | 1 | 9 | / | / |
| Acid | 27 | 26 | / | / | / | / | / |
| Ketone | 9 | / | / | / | / | / | / |
| Furfural | 1 | 4 | / | / | / | / | / |
| Aldehyde | 5 | 3 | / | / | / | / | / |
| Other | 31 | 21 | / | 7 | 10 | / | 3 |
| Catalysts | a RC-10 | b SC-5 | b SC-8 | b SC-10 | b SC-15 |
|---|---|---|---|---|---|
| H2/% | 52.84 | 57.09 | 58.28 | 53.82 | 58.06 |
| CH4/% | 1.57 | 1.99 | 1.13 | 2.1 | 2.82 |
| CO/% | 1.00 | 0.71 | 0.59 | 0.52 | 0.65 |
| CO2/% | 10.48 | 8.65 | 7.16 | 7.72 | 8.33 |
| C2H6/% | 0.04 | 0.01 | 0.03 | 2.17 | 2.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Pan, H.; Zhou, X.; Du, Z.; Li, G.; Wu, J.; Zhang, X.; Zhang, C. Effects of CoMo/γ-Al2O3 Catalysts on Product Hydrocarbon and Phenol Distribution during Hydrodeoxygenation of Oxidized Bio-Oil in a Batch Reactor. Processes 2021, 9, 2138. https://doi.org/10.3390/pr9122138
Luo Y, Pan H, Zhou X, Du Z, Li G, Wu J, Zhang X, Zhang C. Effects of CoMo/γ-Al2O3 Catalysts on Product Hydrocarbon and Phenol Distribution during Hydrodeoxygenation of Oxidized Bio-Oil in a Batch Reactor. Processes. 2021; 9(12):2138. https://doi.org/10.3390/pr9122138
Chicago/Turabian StyleLuo, Yan, Hongling Pan, Xuan Zhou, Zhicai Du, Guotao Li, Juan Wu, Xuefeng Zhang, and Chunquan Zhang. 2021. "Effects of CoMo/γ-Al2O3 Catalysts on Product Hydrocarbon and Phenol Distribution during Hydrodeoxygenation of Oxidized Bio-Oil in a Batch Reactor" Processes 9, no. 12: 2138. https://doi.org/10.3390/pr9122138
APA StyleLuo, Y., Pan, H., Zhou, X., Du, Z., Li, G., Wu, J., Zhang, X., & Zhang, C. (2021). Effects of CoMo/γ-Al2O3 Catalysts on Product Hydrocarbon and Phenol Distribution during Hydrodeoxygenation of Oxidized Bio-Oil in a Batch Reactor. Processes, 9(12), 2138. https://doi.org/10.3390/pr9122138








