Biocompatible Osmium Telluride-Polypyrrole Nanocomposite Material: Application in Prostate Specific Antigen Immunosensing
Abstract
:1. Introduction
2. Experimental Details
2.1. Chemicals and Sample Preparation
2.2. Instrumentation
2.3. Synthesis of TGA Capped OsTe2QD
2.4. Immunosensor Fabrication
3. Results and Discussion
3.1. Optical Properties of TGA-OsTe2QD and PPy
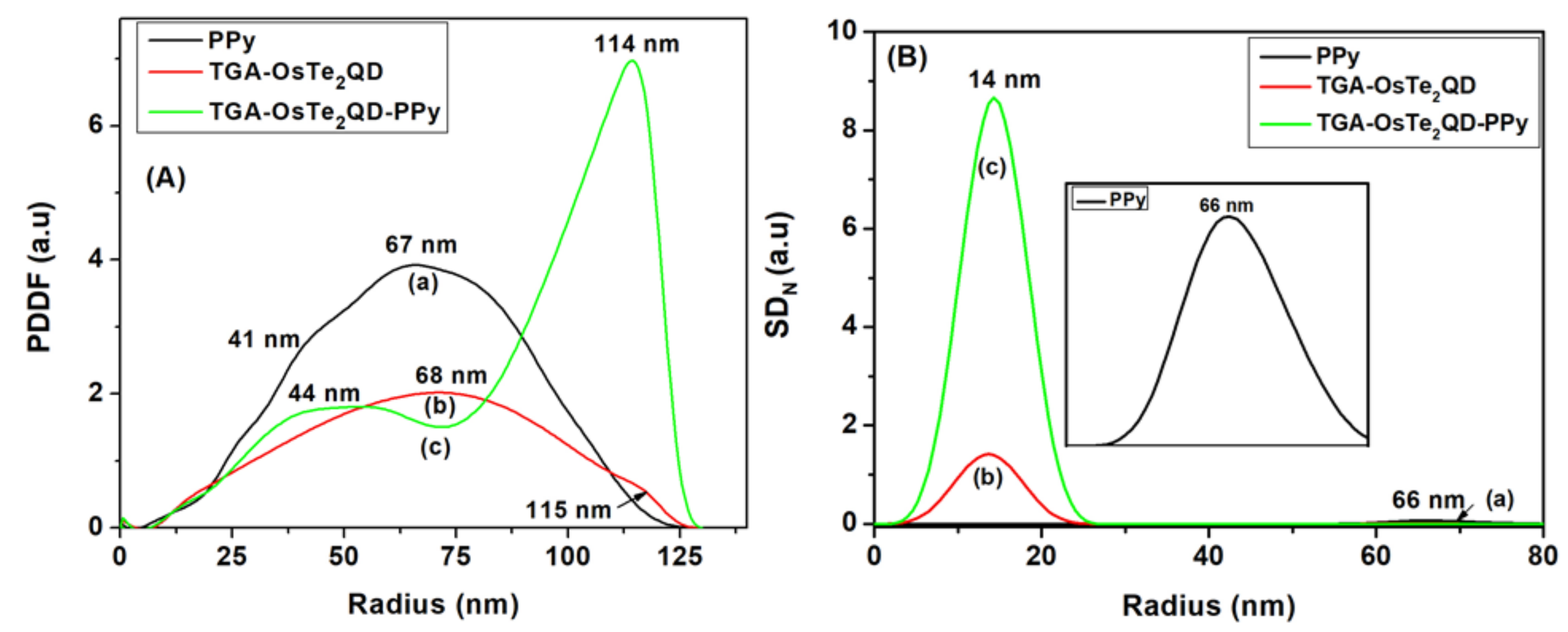
3.2. Internal Structure of TGA-OsTe2QD and PPy
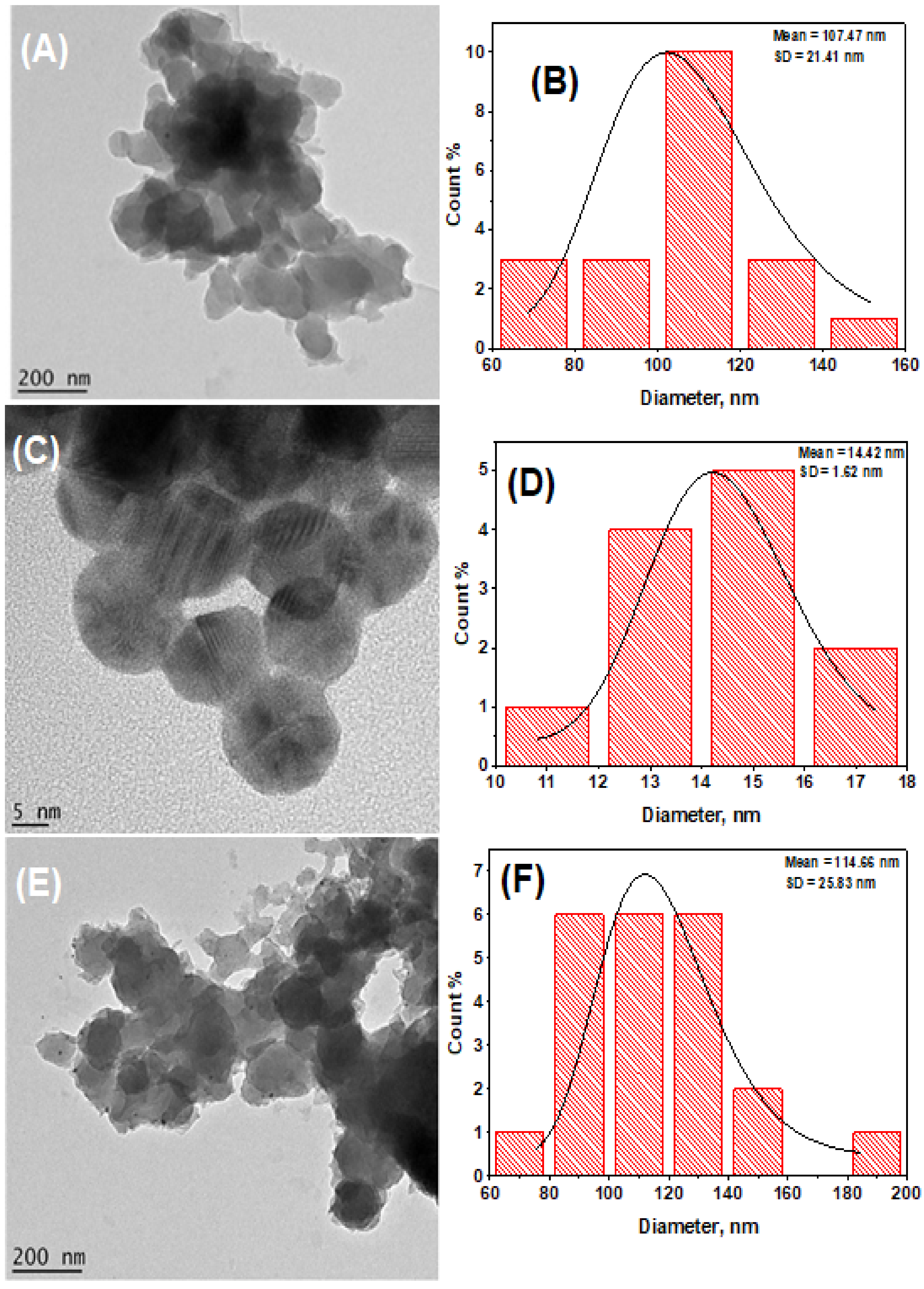
3.3. Morphological Properties of TGA-OsTe2QD and PPy
3.4. Electroanalysis of Immunosensor Platform
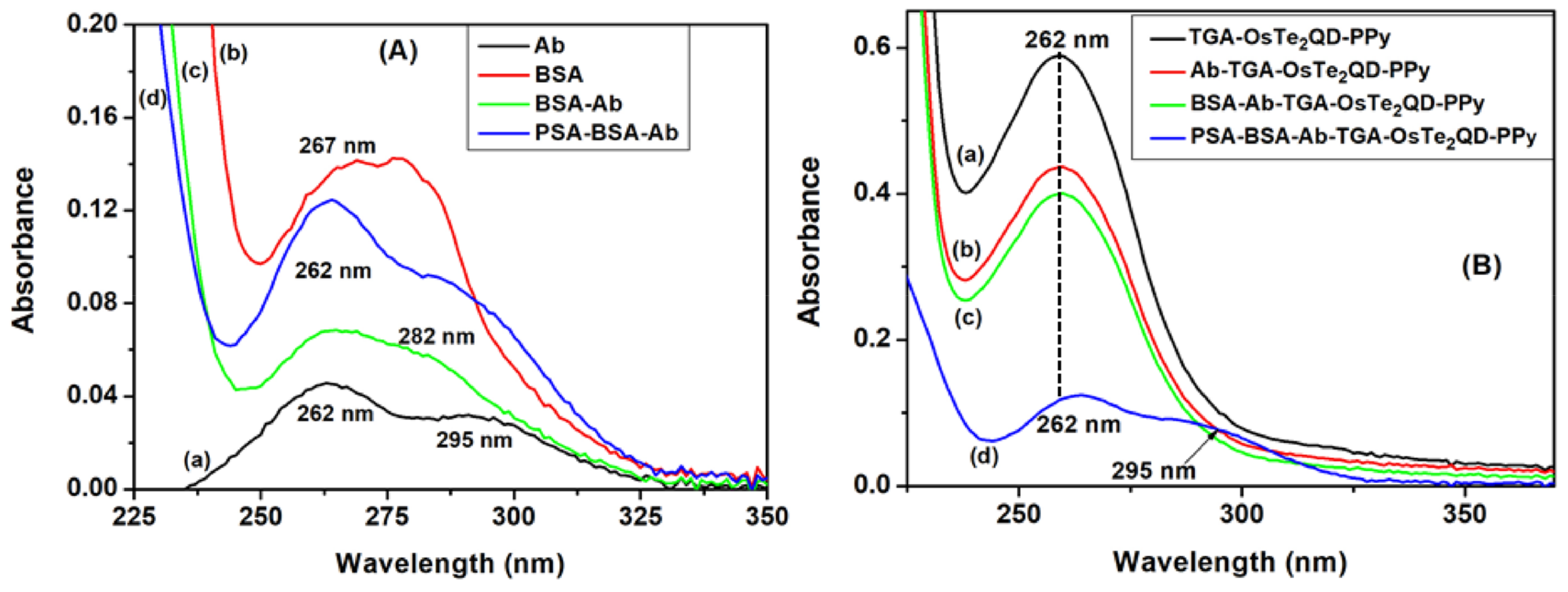
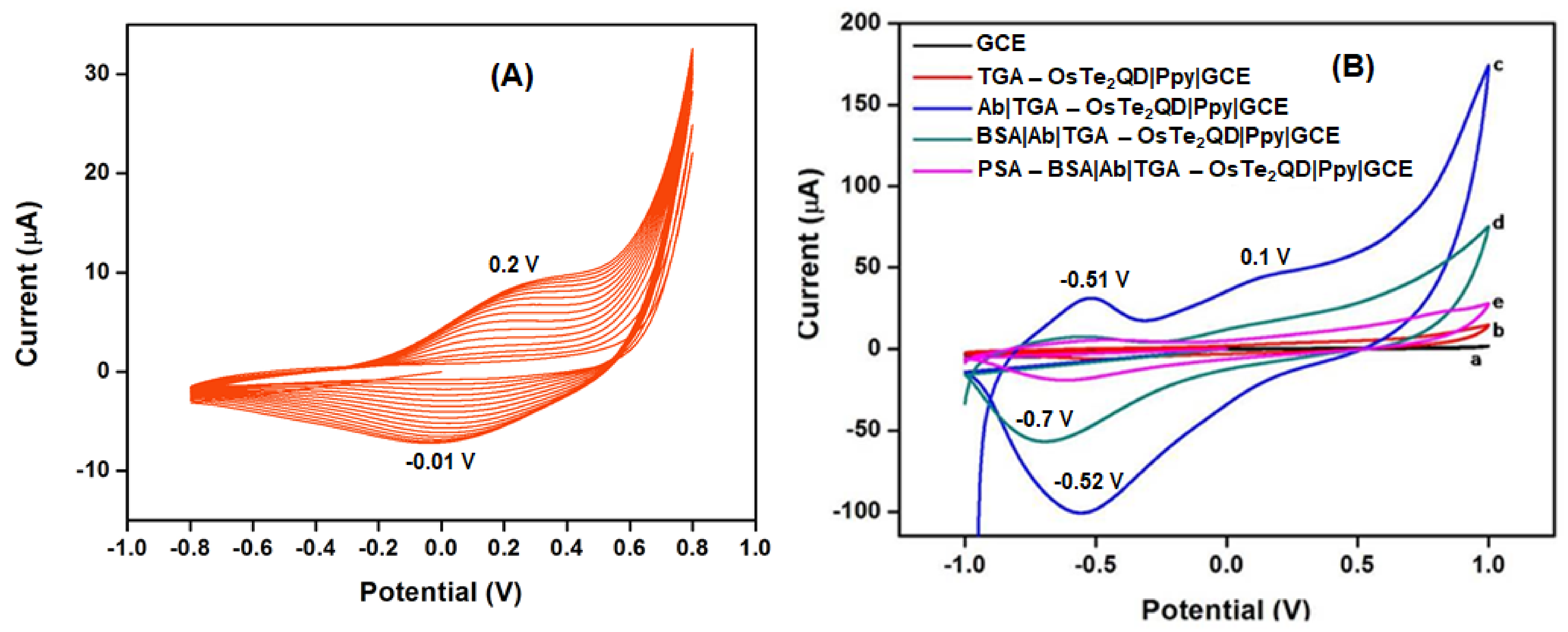
3.4.1. Analysis of Biosensor Platform
3.4.2. Electrochemistry of the Immunosensor Platform
3.4.3. Electrochemical Responses for PSA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quintero-Jaime, A.F.; Berenguer-Murcia, Á.; Cazorla-Amorós, D.; Morallón, E. Carbon nanotubes modified with Au for electrochemical detection of prostate specific antigen: Effect of Au nanoparticle size distribution. Front. Chem. 2019, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.Y.; Wang, J.; Liu, G.; Wu, H.; Wai, C.M.; Lin, Y. A nanoparticle label/immunochromatographic electrochemical biosensor for rapid and sensitive detection of prostate-specific antigen. Biosens. Bioelectron. 2008, 23, 1659–1665. [Google Scholar] [CrossRef]
- Kanyong, P.; Rawlinson, S.; Davis, J. Immunochemical assays and nucleic-acid detection techniques for clinical diagnosis of prostate cancer. J. Cancer 2016, 7, 523–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, B.J.; Jeun, M.; Jang, G.H.; Song, S.H.; Jeong, I.G.; Kim, C.S.; Searson, P.C.; Lee, K.H. Diagnosis of prostate cancer via nanotechnological approach. Int. J. Nanomed. 2015, 10, 6555–6569. [Google Scholar] [CrossRef] [Green Version]
- Schröder, F.H.; van der Maas, P.; Beemsterboer, P.; Kruger, A.B.; Hoedemaeker, R.; Rietbergen, J.; Kranse, R. Evaluation of the digital rectal examination as a screening test for prostate cancer. J. Natl. Cancer Inst. 1998, 90, 1817–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S. Imaging and intervention in prostate cancer: Current perspectives and future trends. Indian J. Radiol. Imaging 2014, 24, 139–148. [Google Scholar] [CrossRef]
- Li, J.; Guan, X.; Fan, Z.; Ching, L.M.; Li, Y.; Wang, X.; Cao, W.M.; Liu, D.X. Non-invasive biomarkers for early detection of breast cancer. Cancers 2020, 12, 2767. [Google Scholar] [CrossRef]
- Prensner, J.R.; Rubin, M.A.; Wei, J.T.; Chinnaiyan, A.M. Beyond PSA: The next generation of prostate cancer biomarkers. Sci. Transl. Med. 2012, 4, 127rv3. [Google Scholar] [CrossRef] [Green Version]
- Nayak, S.; Blumenfeld, N.R.; Laksanasopin, T.; Sia, S.K. POC Diagnostics: Recent Devs in a Connected Age. Physiol. Behav. 2018, 176, 139–148. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, Z.; Zhou, H.S. Review—Measurement and Analysis of Cancer Biomarkers Based on Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037525. [Google Scholar] [CrossRef]
- Bilkey, G.A.; Burns, B.L.; Coles, E.P.; Bowman, F.L.; Beilby, J.P.; Pachter, N.S.; Baynam, G.; Hugh, H.J.; Nowak, K.J.; Weeramanthri, T.S. Genomic testing for human health and disease across the life cycle: Applications and ethical, legal, and social challenges. Front. Public Health 2019, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, J.; Zhou, B.; Ian, H.; Shao, H. Advanced sensitivity amplification strategies for voltammetric immunosensors of tumor marker: State of the art. Biosens. Bioelectron. 2021, 178, 113021. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Falcão, I.R.d.A.; Souza, J.E.d.S.; Rocha, T.G.; de Sousa, I.G.; Cavalcante, A.L.G.; de Oliveira, A.L.B.; de Sousa, M.C.M.; Santos, J.C.S.d. Designing of Nanomaterials-Based Enzymatic Biosensors: Synthesis, Properties, and Applications. Electrochem 2021, 2, 149–184. [Google Scholar] [CrossRef]
- Adeyoju, O.; Iwuoha, E.I.; Smyth, M.R. Reactivities of amperometric organic phase peroxidase-modified electrodes in the presence and absence of thiourea and ethylenethiourea as inhibitors. Anal. Chim. Acta 1995, 305, 57–64. [Google Scholar] [CrossRef]
- El-Said, W.A.; Abdelshakour, M.; Choi, J.H.; Choi, J.W. Application of conducting polymer nanostructures to electrochemical biosensors. Molecules 2020, 25, 307. [Google Scholar] [CrossRef] [Green Version]
- Ramanavicius, S.; Ramanavicius, A. Conducting polymers in the design of biosensors and biofuel cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Gómez, I.J.; Sulleiro, M.V.; Mantione, D.; Alegret, N. Carbon nanomaterials embedded in conductive polymers: A state of the art. Polymers 2021, 13, 745. [Google Scholar] [CrossRef]
- Cheng, Y.; Da Ling, S.; Geng, Y.; Wang, Y.; Xu, J. Microfluidic synthesis of quantum dots and their applications in bio-sensing and bio-imaging. Nanoscale Adv. 2021, 3, 2180–2195. [Google Scholar] [CrossRef]
- Shrikrushna, S.; Kher, J.A.; Kulkarni, M.V. Influence of Dodecylbenzene Sulfonic Acid Doping on Structural, Morphological, Electrical and Optical Properties on Polypyrrole/3C-SiC Nanocomposites. J. Nanomed. Nanotechnol. 2015, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Attar, A.R.; Blumling, D.E.; Knappenberger, K.L. Photodissociation of thioglycolic acid studied by femtosecond time-resolved transient absorption spectroscopy. J. Chem. Phys. 2011, 134, 024514. [Google Scholar] [CrossRef]
- Anantharaj, S.; Nithiyanantham, U.; Ede, S.R.; Ayyappan, E.; Kundu, S. π-stacking intercalation and reductant assisted stabilization of osmium organosol for catalysis and SERS applications. RSC Adv. 2015, 5, 11850–11860. [Google Scholar] [CrossRef]
- Strabler, C.M.; Sinn, S.; Pehn, R.; Pann, J.; Dutzler, J.; Viertl, W.; Prock, J.; Ehrmann, K.; Weninger, A.; Kopacka, H.; et al. Stabilisation effects of phosphane ligands in the homogeneous approach of sunlight induced hydrogen production. Faraday Discuss. 2017, 198, 211–233. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Karabanovas, V.; Ramanaviciene, A.; Rotomskis, R. Stabilization of (CdSe)ZnS quantum dots with polypyrrole formed by UV/VIS irradiationinitiated polymerization. J. Nanosci. Nanotechnol. 2009, 9, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Elmahdy, M.M.; Sarhan, A.; Abdel Hamid, M.I.; Ahmed, M.T. Structure and dynamics of polypyrrole/chitosan nanocomposites. Polym. Int. 2018, 67, 1615–1628. [Google Scholar] [CrossRef]
- Zogg, H.; Arnold, M.; Felder, F.; Rahim, M.; Fill, M.; Boye, D. Epitaxial lead-chalcogenides on Si and BaF2 for mid-IR detectors and emitters including cavities. In Proceedings of the SPIE 7082, Infrared Spaceborne Remote Sensing and Instrumentation XVI, 70820H, San Diego, CA, USA, 10–14 August 2008. [Google Scholar] [CrossRef]
- Dutta, K. Green synthesis and transport properties of ZnS-PPy hybrid nanocomposites. Int. J. Sci. Eng. Res. 2018, 9, 249–264. [Google Scholar]
- Kaur Sidhu, G.; Kumar, R. Study the Structural and Optical behaviour of Conducting Polymer based nanocomposites: ZrO2-Polypyrrole Nanocomposites. In IOP Conference Series: Materials Science and Engineering, Proceedings of the Second International Conference on Materials Science and Technology (ICMST 2016), Kerala, India, 5–8 June 2016; IOP Publishing, Ltd.: Bristol, UK, 2018; Volume 360. [Google Scholar] [CrossRef]
- Gholamrezaei, S.; Salavati-Niasari, M.; Ghanbari, D.; Bagheri, S. Hydrothermal preparation of silver telluride nanostructures and photo-catalytic investigation in degradation of toxic dyes. Sci. Rep. 2016, 6, 20060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, K.J.; Ahmed, T.; Gautam, P.; Sadhu, A.; Lien, D.-H.; Chen, S.-C.; Chueh, Y.-L.; Kuo, H.-C. Recent Advances in Two-Dimensional Quantum Dots and Their Applications. Nanomaterials 2021, 11, 1549. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Rashid, S.A.; Noor, A.S.M.; Kharazmi, A.; Lim, H.N.; Mahdi, M.A. Optical Band Gap and Thermal Diffusivity of Polypyrrole-Nanoparticles Decorated Reduced Graphene Oxide Nanocomposite Layer. J. Nanomater. 2016, 2016, 1949042. [Google Scholar] [CrossRef]
- Guerrero-Gonzalez, R.; Orona, F.A.; Saucedo-Flores, E.; Ruelas, R.; Pelayo-Ceja, J.E.; Lopez-Delgado, R.; Cordova-Rubio, A.; Álvarez-Ramos, M.E.; Ayon, A. Synthesis of Si and CdTe quantum dots and their combined use as down-shifting photoluminescent centers in Si solar cells. Mater. Renew. Sustain. Energy 2019, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Gaponik, N.P.; Talapin, D.V.; Rogach, A.L.; Eychmuller, A. Electrochemical synthesis of CdTe nanocrystal/polypyrrole composites for optoelectronic applications. J. Mater. Chem. 2000, 10, 2163–2166. [Google Scholar] [CrossRef]
- Ruppert, C.; Aslan, O.B.; Heinz, T.F. Optical Properties and Band Gap of Single- and Few-Layer MoTe2 Crystals. Nano Lett. 2014, 14, 6231–6236. [Google Scholar] [CrossRef] [PubMed]
- Abdi, M.M.; Ekramul Mahmud, H.N.M.; Abdullah, L.C.; Kassim, A.; Rahman, M.Z.A.; Chyi, J.L.Y. Optical band gap and conductivity measurements of polypyrrole-chitosan composite thin films. Chin. J. Polym. Sci. 2012, 30, 93–100. [Google Scholar] [CrossRef]
- Feleni, U.; Sidwaba, U.; Makelane, H.; Iwuoha, E. Core–Shell Palladium Telluride Quantum Biosensor for Detecting Indinavir Drug. J. Nanosci. Nanotechnol. 2019, 19, 7974–7981. [Google Scholar] [CrossRef]
- Bilibana, M.P.; Feleni, U.; Williams, A.R.; Iwuoha, E. Impedimetric microcystin-LR aptasensor prepared with sulfonated poly(2,5-dimethoxyaniline)–silver nanocomposite. Processes 2021, 9, 179. [Google Scholar] [CrossRef]
- Oh, J.; Lee, J.S.; Jang, J. Ruthenium decorated polypyrrole nanoparticles for highly sensitive hydrogen gas sensors using component ratio and protonation control. Polymers 2020, 12, 1427. [Google Scholar] [CrossRef]
- Pacoste, L.C.; Jijana, A.N.; Feleni, U.; Iwuoha, E. Mercaptoalkanoic Acid-Induced Band Gap Attenuation of Copper Selenide Quantum Dot. ChemistrySelect 2020, 5, 4994–5005. [Google Scholar] [CrossRef]
- Maruthamuthu, S.; Chandrasekaran, J.; Manoharan, D.; Magesh, R. Conductivity and dielectric analysis of Nanocolloidal polypyrrole particles functionalized with Higher weight percentage of poly(styrene sulfonate) using the dispersion polymerization method. J. Polym. Eng. 2017, 37, 481–492. [Google Scholar] [CrossRef]
- Memela, M.; Feleni, U.; Mdluli, S.; Ramoroka, M.E.; Ekwere, P.; Douman, S.; Iwuoha, E. Electro-photovoltaics of Polymer-stabilized Copper–Indium Selenide Quantum Dot. Electroanalysis 2020, 32, 3086–3097. [Google Scholar] [CrossRef]
- Antosiewicz, J.M.; Shugar, D. UV–Vis spectroscopy of tyrosine side-groups in studies of protein structure. Part 2: Selected applications. Biophys. Rev. 2016, 8, 163–177. [Google Scholar] [CrossRef] [Green Version]
- Antosiewicz, J.M.; Shugar, D. UV–Vis spectroscopy of tyrosine side-groups in studies of protein structure. Part 1: Basic principles and properties of tyrosine chromophore. Biophys. Rev. 2016, 8, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Bertozo, L.d.C.; Neto, E.T.; de Oliveira, L.C.; Ximenes, V.F. Oxidative alteration of Trp-214 and Lys-199 in human serum albumin increases binding affinity with phenylbutazone: A combined experimental and computational investigation. Int. J. Mol. Sci. 2018, 19, 2868. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; He, Y.; Liu, D.; He, Y.; Hou, X.; Cheng, Y.; Liu, J. Characterization of interaction between scoparone and bovine serum albumin: Spectroscopic and molecular docking methods. RSC Adv. 2018, 8, 25519–25525. [Google Scholar] [CrossRef] [Green Version]
- Farschi, F.; Saadati, A.; Hasanzadeh, M. A novel immunosensor for the monitoring of PSA using binding of biotinylated antibody to the prostate specific antigen based on nano-ink modified flexible paper substrate: Efficient method for diagnosis of cancer using biosensing technology. Heliyon 2020, 6, e04327. [Google Scholar] [CrossRef]
- Hayashi, M.; Tournilhac, F. Thermal stability enhancement of hydrogen bonded semicrystalline thermoplastics achieved by combination of aramide chemistry and supramolecular chemistry. Polym. Chem. 2017, 8, 461–471. [Google Scholar] [CrossRef]
- Hefnawy, A.; Mostafa, D.; Ebrahim, S.; Soliman, M. Micro composite multi structural formable steel: Optimization of electrodeposition parameters and anti-corrosion properties of polypyrrole coatings. Biointerface Res. Appl. Chem. 2021, 11, 13402–13411. [Google Scholar] [CrossRef]
- Kim, S.; Jang, L.K.; Park, H.S.; Lee, J.Y. Electrochemical deposition of conductive and adhesive polypyrrole-dopamine films. Sci. Rep. 2016, 6, 30475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, E.H.; Waryo, T.; Sadik, O.A.; Iwuoha, E.I.; Baker, P.G.L. Constitution of novel polyamic acid/polypyrrole composite films by in-situ electropolymerization. Electrochim. Acta 2014, 128, 439–447. [Google Scholar] [CrossRef]
- Muñoz, R.; Santos, E.M.; Galan-Vidal, C.A.; Miranda, J.M.; Lopez-Santamarina, A.; Rodriguez, J.A. Ternary quantum dots in chemical analysis. Synthesis and detection mechanisms. Molecules 2021, 26, 2764. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Kausaite-Minkstimiene, A.; Ramanaviciene, A. Metal nanoparticle and quantum dot tags for signal amplification in electrochemical immunosensors for biomarker detection. Chemosensors 2021, 9, 85. [Google Scholar] [CrossRef]
- Upan, J.; Youngvises, N.; Tuantranont, A.; Karuwan, C.; Banet, P.; Aubert, P.H.; Jakmunee, J. A simple label-free electrochemical sensor for sensitive detection of alpha-fetoprotein based on specific aptamer immobilized platinum nanoparticles/carboxylated-graphene oxide. Sci. Rep. 2021, 11, 13969. [Google Scholar] [CrossRef]
- Oliveira, N.; Costa-Rama, E.; Viswanathan, S.; Delerue-Matos, C.; Pereira, L.; Morais, S. Label-free Voltammetric Immunosensor for Prostate Specific Antigen Detection. Electroanalysis 2018, 30, 2604–2611. [Google Scholar] [CrossRef]
- Jonous Akbari, Z.; Shayeh, J.S.; Yazdian, F.; Yadegari, A.; Hashemi, M.; Omidi, M. An electrochemical biosensor for prostate cancer biomarker detection using graphene oxide–gold nanostructures. Eng. Life Sci. 2019, 19, 206–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Y.; Wang, H.; Ma, H.; Wu, D.; Du, B.; Wei, Q. Sandwich-type electrochemical immunosensor for ultrasensitive detection of prostate-specific antigen using palladium-doped cuprous oxide nanoparticles. RSC Adv. 2016, 6, 84698–84704. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Y.; Wang, Y.; Hu, L.; Ma, H.; Wang, G.; Wei, Q. Label-free Electrochemiluminescent Immunosensor for Detection of Prostate Specific Antigen based on Aminated Graphene Quantum Dots and Carboxyl Graphene Quantum Dots. Sci. Rep. 2016, 6, 20511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, A.; Kaushik, A.; Arya, S.K.; Bhansali, S. Mediator free highly sensitive polyaniline-gold hybrid nanocomposite based immunosensor for prostate-specific antigen (PSA) detection. J. Mater. Chem. 2012, 22, 14763–14772. [Google Scholar] [CrossRef]
- Rafique, S.; Bin, W.; Bhatti, A.S. Electrochemical immunosensor for prostate-specific antigens using a label-free second antibody based on silica nanoparticles and polymer brush. Bioelectrochemistry 2015, 101, 75–83. [Google Scholar] [CrossRef]
- Mandal, N.; Pakira, V.; Samanta, N.; Das, N.; Chakraborty, S.; Pramanick, B.; RoyChaudhuri, C. PSA detection using label free graphene FET with coplanar electrodes based microfluidic point of care diagnostic device. Talanta 2021, 222, 121581. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huo, Y.; Bai, J.; Ning, B.; Peng, Y.; Li, S.; Han, D.; Kang, W.; Gao, Z. Rapid and sensitive detection of prostate-specific antigen via label-free frequency shift Raman of sensing graphene. Biosens. Bioelectron. 2020, 158, 112184. [Google Scholar] [CrossRef]

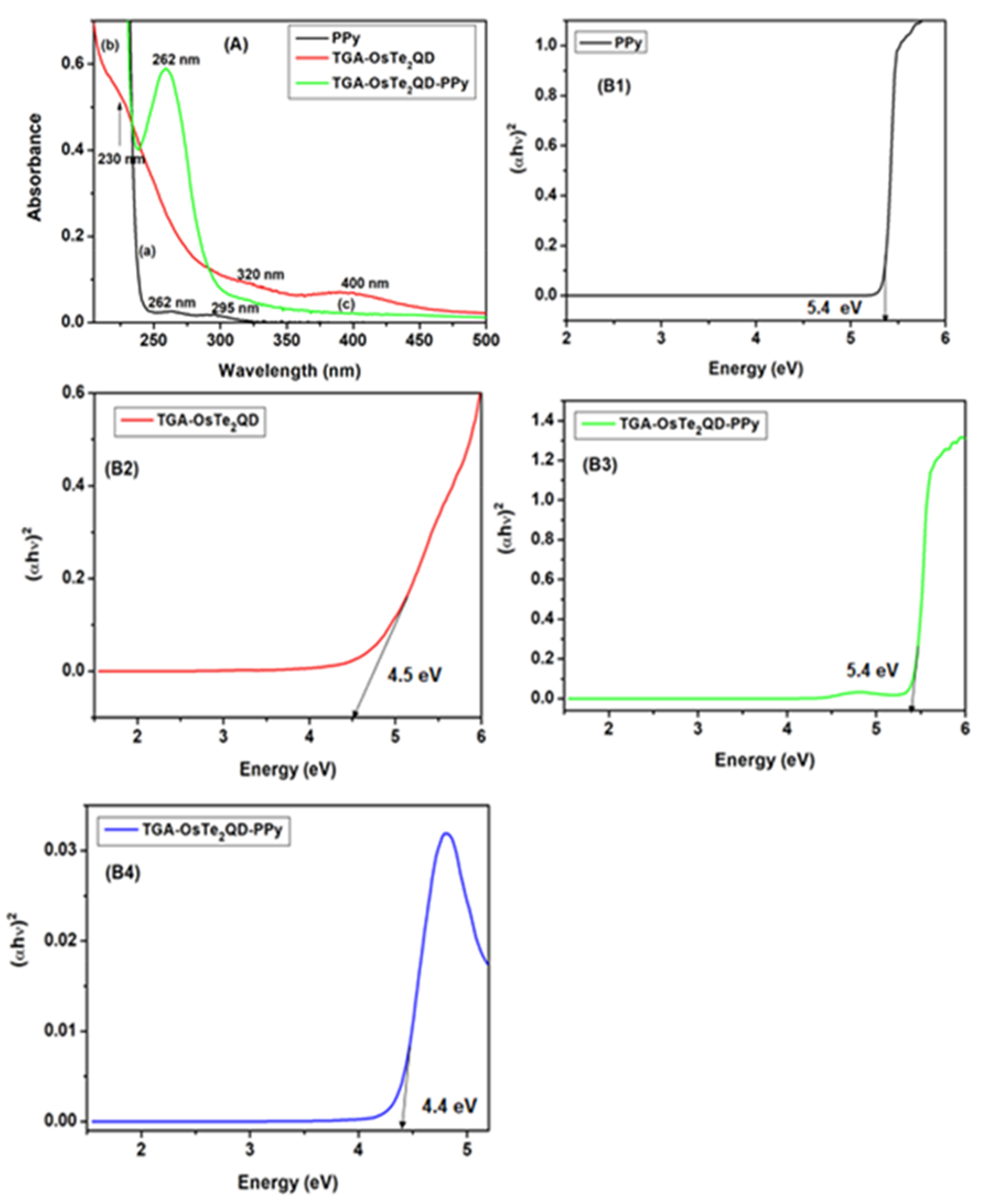

| Metal Telluride Chalcogenides | Band Gap Energy (eV) | Reference | Metal–Polymer Composites | Band Gap Energy (eV) | Reference |
|---|---|---|---|---|---|
| PbEuTe PbSrTe | 4.5 4.7 | [26] | ZnS-PPy | 4.26–4.7 | [27] |
| Ag2Te | 3.64 | [29] | ZrO2-PPy | 5.07 | [28] |
| WTe2 | 0.2 | [30] | NPs/rGO-PPy | 3.58–3.85 | [31] |
| CdTe | 2.15–2.28 | [32] | CdTe-PPy | 1.45 | [33] |
| MoTe2 | 1.15 | [34] | CHI-PPY | 1.30–2.32 | [35] |
| OsTe2 | 4.5 | This work | OsTe2-PPy | 4.4 and 5.4 | This work |
| Electrode Materials | Detection Method | Linear Range (ng/mL) | Limit of Detection (ng/mL) | Ref. |
|---|---|---|---|---|
| Pd@Cu2O NPs | EIS | 10−5–1 × 102 | 2 × 10−6 | [56] |
| Au/Ag-rGO/Animated-GQD/Carboxyl-GQDs | ECL | 1 × 10−3–10 | 2.9 × 10−4 | [57] |
| AuNP-PANI/Au | DPV | 1 × 10−3–1 × 102 | 6 × 10−4 | [58] |
| Au-NS/POEGMA-co-GMA brush | EIS | 5 × 10−3–1 × 103 | 2.3 × 10−3 | [59] |
| GO/AuNPs | CV | - | 1.4 | [55] |
| TGA-OsTe2QD-PPy | CV | 0–15 | 0.36 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, R.; Feleni, U.; Iwuoha, E. Biocompatible Osmium Telluride-Polypyrrole Nanocomposite Material: Application in Prostate Specific Antigen Immunosensing. Processes 2021, 9, 2203. https://doi.org/10.3390/pr9122203
Gupta R, Feleni U, Iwuoha E. Biocompatible Osmium Telluride-Polypyrrole Nanocomposite Material: Application in Prostate Specific Antigen Immunosensing. Processes. 2021; 9(12):2203. https://doi.org/10.3390/pr9122203
Chicago/Turabian StyleGupta, Riya, Usisipho Feleni, and Emmanuel Iwuoha. 2021. "Biocompatible Osmium Telluride-Polypyrrole Nanocomposite Material: Application in Prostate Specific Antigen Immunosensing" Processes 9, no. 12: 2203. https://doi.org/10.3390/pr9122203






