Novel Anthracene HTM Containing TIPs for Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
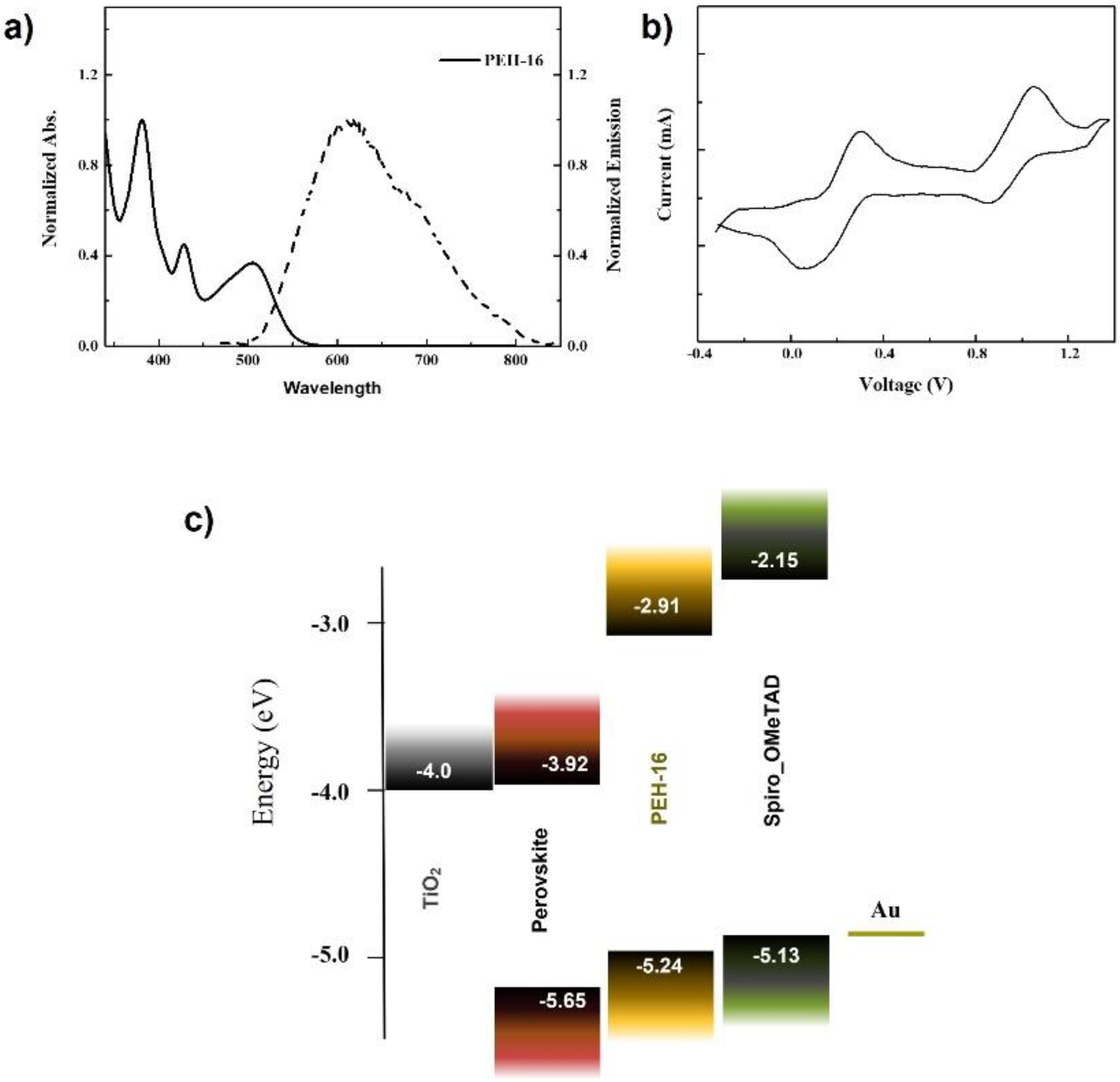
3. Results
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Graẗzel, M.; Mhaisalkar, S.; Sum, T.C. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- de Wolf, S.; Holovsky, J.; Moon, S.J.; Löper, P.; Niesen, B.; Ledinsky, M.; Haug, F.J.; Yum, J.H.; Ballif, C. Organometallic Halide Perovskites: Sharp Optical Absorption Edgeand Its Relation to Photovoltaic Performance. J. Phys. Chem. Lett. 2014, 5, 1035–1039. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [Green Version]
- NREL Research Cell Record Efficiency Chart. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies-rev210726.pdf (accessed on 30 October 2021).
- Chen, Y.; Xu, X.; Cai, N.; Qian, S.; Luo, R.; Huo, Y.; Tsang, S.-W. Rational Design of Dopant-Free Coplanar D-π-D Hole-Transporting Materials for High-Performance Perovskite Solar Cells with Fill Factor Exceeding 80%. Adv. Energy Mater. 2019, 9, 1901268. [Google Scholar] [CrossRef]
- Vegiraju, S.; Ke, W.; Priyanka, P.; Ni, J.-S.; Wu, Y.-C.; Spanopoulos, I.; Yau, S.L.; Marks, T.J.; Chen, M.-C.; Kanatzidis, M.G. Benzodithiophene Hole-Transporting Materials for Efficient Tin-Based Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1905393. [Google Scholar] [CrossRef]
- Zimmermann, I.; Aghazada, S.; Nazeeruddin, M.K. Lead and HTM Free Stable Two-Dimensional Tin Perovskites with Suitable Band Gap for Solar Cell Applications. Angew. Chem. Int. Ed. 2019, 58, 1072–1076. [Google Scholar] [CrossRef]
- Ke, W.; Priyanka, P.; Vegiraju, S.; Stoumpos, C.C.; Spanopoulos, I.; Soe, C.M.M.; Marks, T.J.; Chen, M.-C.; Kanatzidis, M.G. Dopant-Free Tetrakis-Triphenylamine Hole Transporting Material for Efficient Tin-Based Perovskite Solar Cells. J. Am. Chem. Soc. 2018, 140, 388–393. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, F.; Liu, Z.; Xiao, Y.; Wang, S.; Li, X. Dopant-free and low-cost molecular “bee” hole-transporting materials for efficient and stable perovskite solar cells. J. Mater. Chem. C 2017, 5, 11429–11435. [Google Scholar] [CrossRef]
- Li, H.; Fu, K.; Hagfeldt, A.; Grätzel, M.; Mhaisalkar, S.G.; Grimsdale, A.C. A Simple 3,4-Ethylenedioxythiophene Based Hole-Transporting Material for Perovskite Solar Cells. Angew. Chem. Int. Ed. 2014, 53, 4085–4088. [Google Scholar] [CrossRef]
- Abate, A.; Paek, S.; Giordano, F.; Correa-Baena, J.P.; Saliba, M.; Gao, P.; Matsui, T.; Ko, J.; Zakeeruddin, S.M.; Dahmen, K.H.; et al. Silolothiophene-linked triphenylamines as stable hole transporting materials for high efficiency perovskite solar cells. Energy Environ. Sci. 2015, 8, 2946–2953. [Google Scholar] [CrossRef]
- Rakstys, K.; Paek, S.; Drevilkauskaite, A.; Kanda, H.; Daskeviciute, S.; Shibayama, N.; Daskeviciene, M.; Gruodis, A.; Kamarauskas, E.; Jankauskas, V.; et al. Carbazole-Terminated Isomeric Hole-Transporting Materials for Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 19710–19717. [Google Scholar] [CrossRef] [PubMed]
- Luizys, P.; Xia, J.; Daskeviciene, M.; Kantminiene, K.; Kasparavicius, E.; Kanda, H.; Zhang, Y.; Jankauskas, V.; Rakstys, K.; Getautis, V.; et al. Branched Methoxydiphenylamine-Substituted Carbazole Derivatives for Efficient Perovskite Solar Cells: Bigger Is Not Always Better. Chem. Mater. 2021, 33, 7017–7027. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Orlandi, S.; Matsui, T.; Aghazada, S.; Cavazzini, M.; Correa-baena, J.P.; Gao, P.; Scopelliti, R.; Mosconi, E.; Dahmen, K.H.; et al. A molecularly engineered hole-transporting material for efficient perovskite solar cells. Nat. Energy 2016, 1, 15017. [Google Scholar] [CrossRef]
- Nawar, A.M.; Yahia, I.S. Fabrication and characterization of anthracene thin films for wide-scale organic optoelectronic applications based on linear/nonlinear analyzed optical dispersion parameters. Opt. Mater. 2017, 70, 1–10. [Google Scholar] [CrossRef]
- Yutaka, M.; Hiroshi, O.; Yasuhiro, K.; Jeon, H.W.; Yun, Y.; Takeshi, M.; Motoki, Y.; Toshiaki, I. Anthracene-Based Organic Small-Molecule Electron-InjectingMaterial for Inverted Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 11810–11817. [Google Scholar]
- Inoue, Y.; Tokito, S. Organic thin-film transistors based on anthracene oligomers. J. Appl. Phys. 2004, 95, 5795. [Google Scholar] [CrossRef]
- Choi, H.; Ko, H.M.; Cho, N.; Song, K.; Lee, J.K.; Ko, J. Electron-Rich Anthracene Semiconductors Containing Triarylamine for Solution-Processed Small-Molecule Organic Solar Cells. Chemsuschem 2012, 5, 2045–2052. [Google Scholar] [CrossRef]
- Kayani, K.Q.; Yaqoob, U.; Jabeen, S.; Iqbal, S.; Yaseen, M.; Khalid, M.; Akhter, M.S.; Iqbal, J. Tris-isopropyl-sily-ethynyl anthracene based small molecules for organic solar cells with efficient photovoltaic parameters. Comput. Theor. Chem. 2021, 1202, 113305. [Google Scholar] [CrossRef]
- Liu, X.; Kong, F.; Ghadari, R.; Jin, S.; Yu, T.; Chen, W.; Liu, G.; Tan, Z.; Chena, J.; Dai, S. Anthracene–arylamine hole transporting materials for perovskite solar cells. Chem. Commun. 2017, 53, 9558–9561. [Google Scholar] [CrossRef] [Green Version]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldtd, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.T.; Grancini, G.; Lee, Y.; Oveisi, E.; Ryu, J.; Almora, O.; Tschumi, M.; Schouwink, P.A.; Seo, G.; Heo, S.; et al. Selective growth of layered perovskites for stable and efficient photovoltaics. Energy Environ. Sci. 2018, 11, 952–959. [Google Scholar] [CrossRef]
- Park, J.-H.; Chung, D.S.; Park, J.-W.; Ahn, T.; Kong, H.; Jung, Y.K.; Lee, J.; Yi, M.H.; Park, C.E.; Kwon, S.-K.; et al. Soluble and Easily Crystallized Anthracene Derivatives: Precursors of Solution-Processable Semiconducting Molecules. Org. Lett. 2007, 9, 2573–2576. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
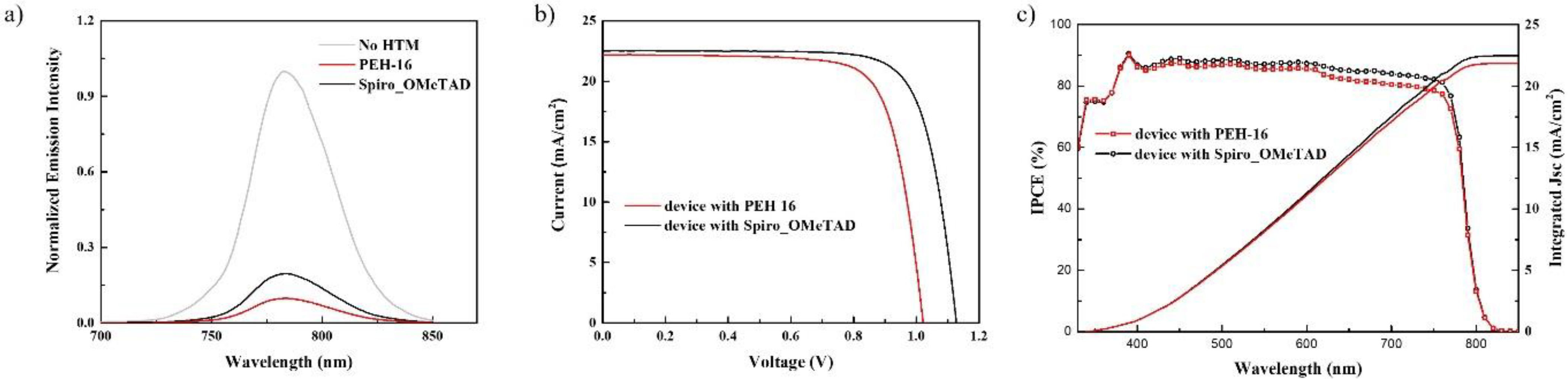
| Voc (V) | Jsc (mA cm−2) | ff | Efficiency (%) | ||
|---|---|---|---|---|---|
| PEH-16 | Best | 1.022 | 22.19 | 0.755 | 17.12 |
| Reverse scan | 1.036 | 22.46 | 0.710 | 16.51 | |
| Forward scan | 1.020 | 22.46 | 0.690 | 15.81 | |
| Spiro_OMeTAD | Best | 1.127 | 22.51 | 0.767 | 19.46 |
| Reverse scan | 1.117 | 22.58 | 0.750 | 18.90 | |
| Forward scan | 1.105 | 22.59 | 0.735 | 18.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paek, S. Novel Anthracene HTM Containing TIPs for Perovskite Solar Cells. Processes 2021, 9, 2249. https://doi.org/10.3390/pr9122249
Paek S. Novel Anthracene HTM Containing TIPs for Perovskite Solar Cells. Processes. 2021; 9(12):2249. https://doi.org/10.3390/pr9122249
Chicago/Turabian StylePaek, Sanghyun. 2021. "Novel Anthracene HTM Containing TIPs for Perovskite Solar Cells" Processes 9, no. 12: 2249. https://doi.org/10.3390/pr9122249
APA StylePaek, S. (2021). Novel Anthracene HTM Containing TIPs for Perovskite Solar Cells. Processes, 9(12), 2249. https://doi.org/10.3390/pr9122249





