Optimisation of a Side Inlet for H2 Entry into an Ultrasonic Spray Pyrolysis Device
Abstract
:1. Introduction
2. Materials and Methods
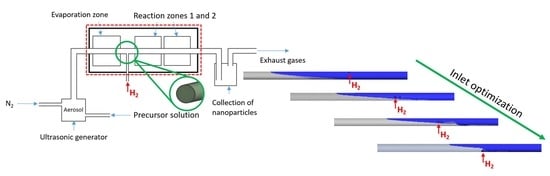
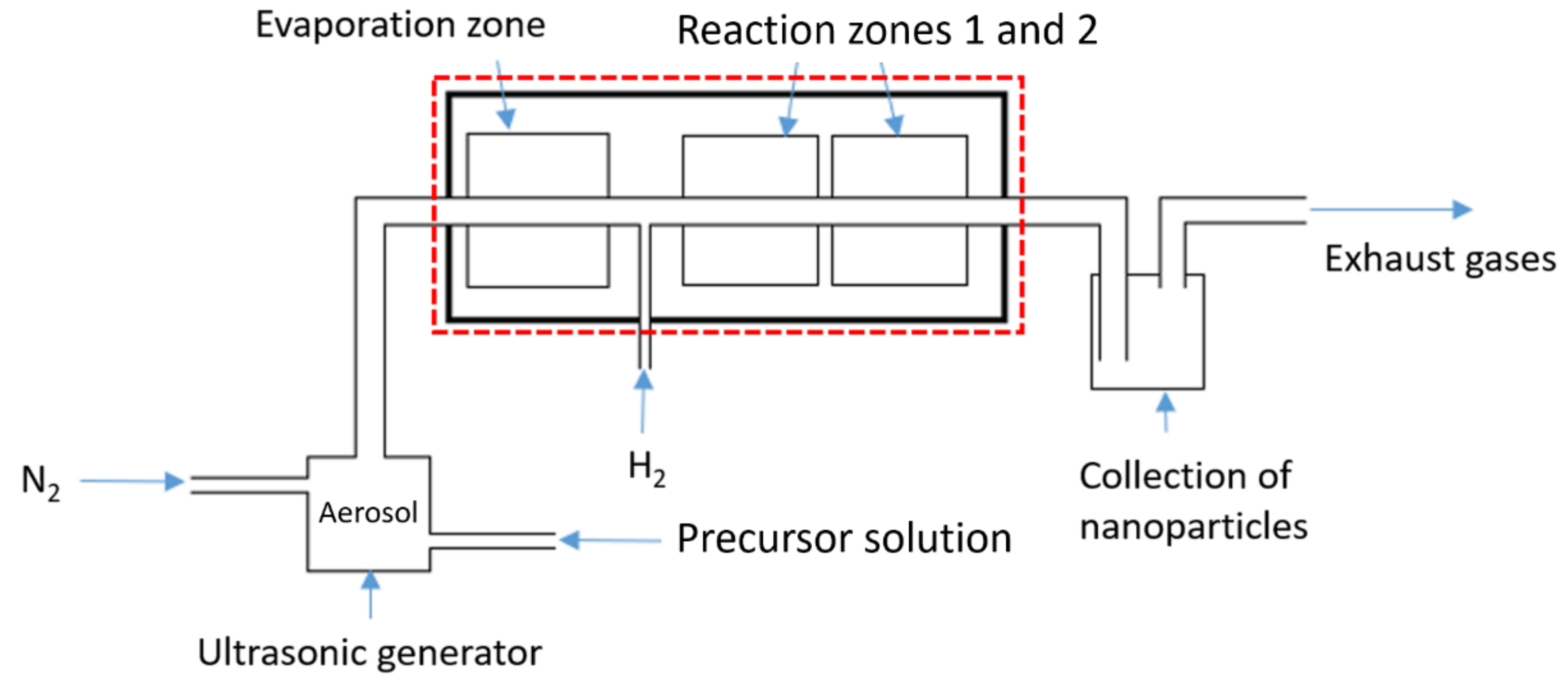
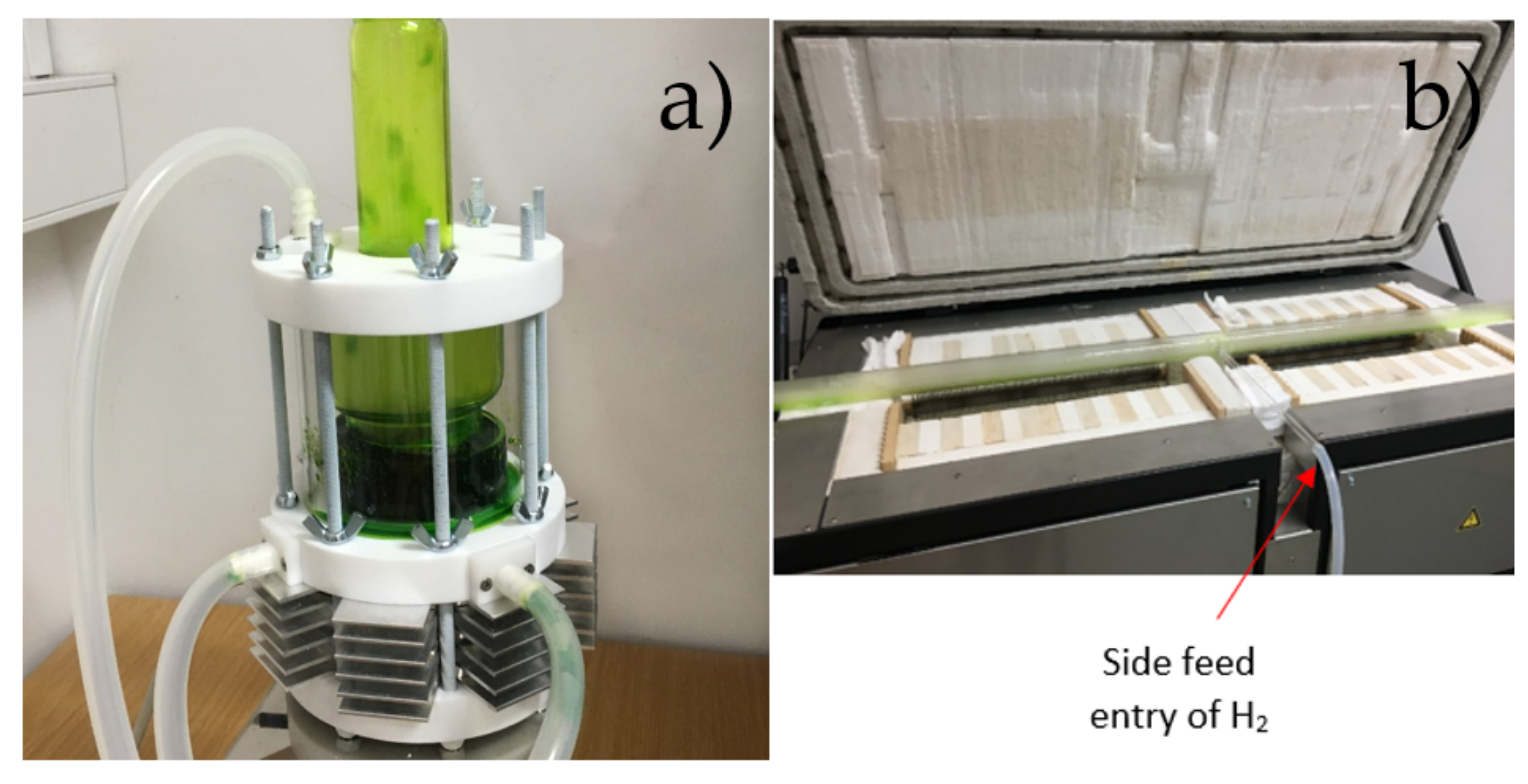
2.1. USP Device
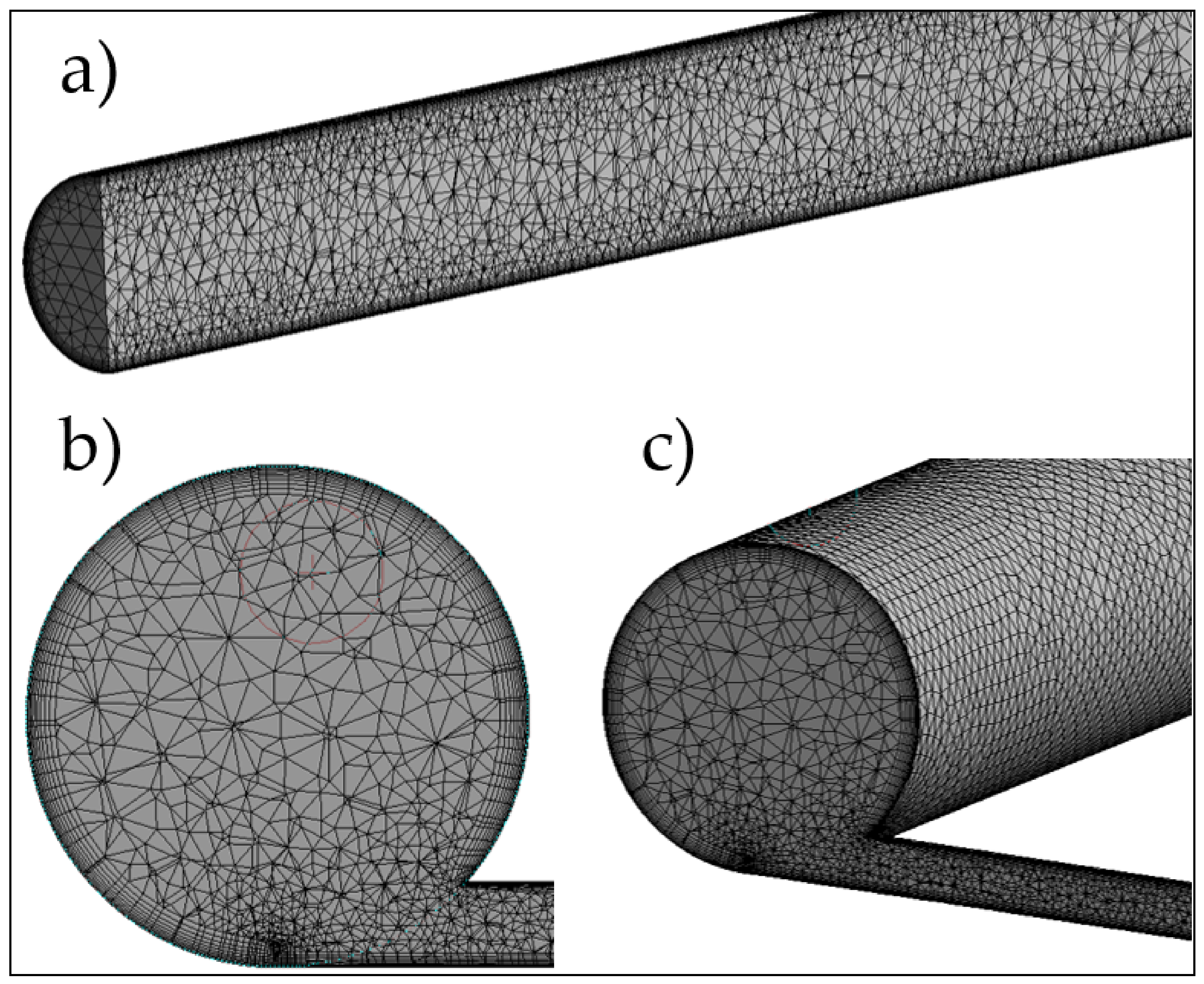
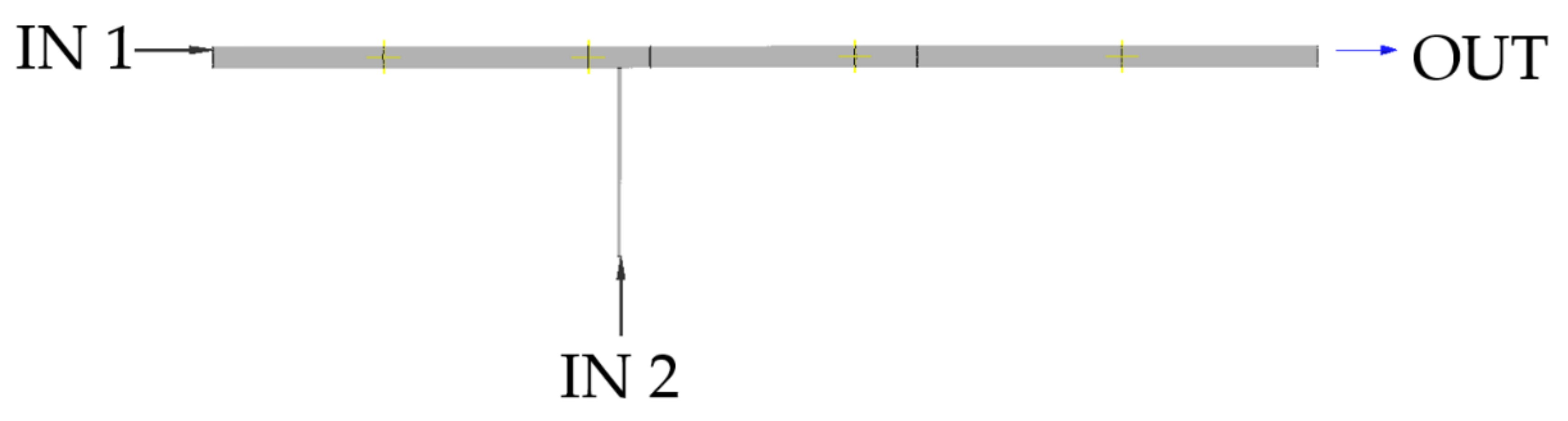
2.2. Numerical Model—Description of the Geometry
2.2.1. Description of the Geometry
2.2.2. New Geometries—A Case Study
2.3. Conditions
2.3.1. Conditions for Basic Geometry Disregarding Temperature
2.3.2. Conditions for Basic Geometry Taking into Account Temperature
2.3.3. New Geometries
2.4. Experimental Setup
3. Results
3.1. Results of the Numerical Simulations
3.1.1. Results of Simulations on the Basic Geometry
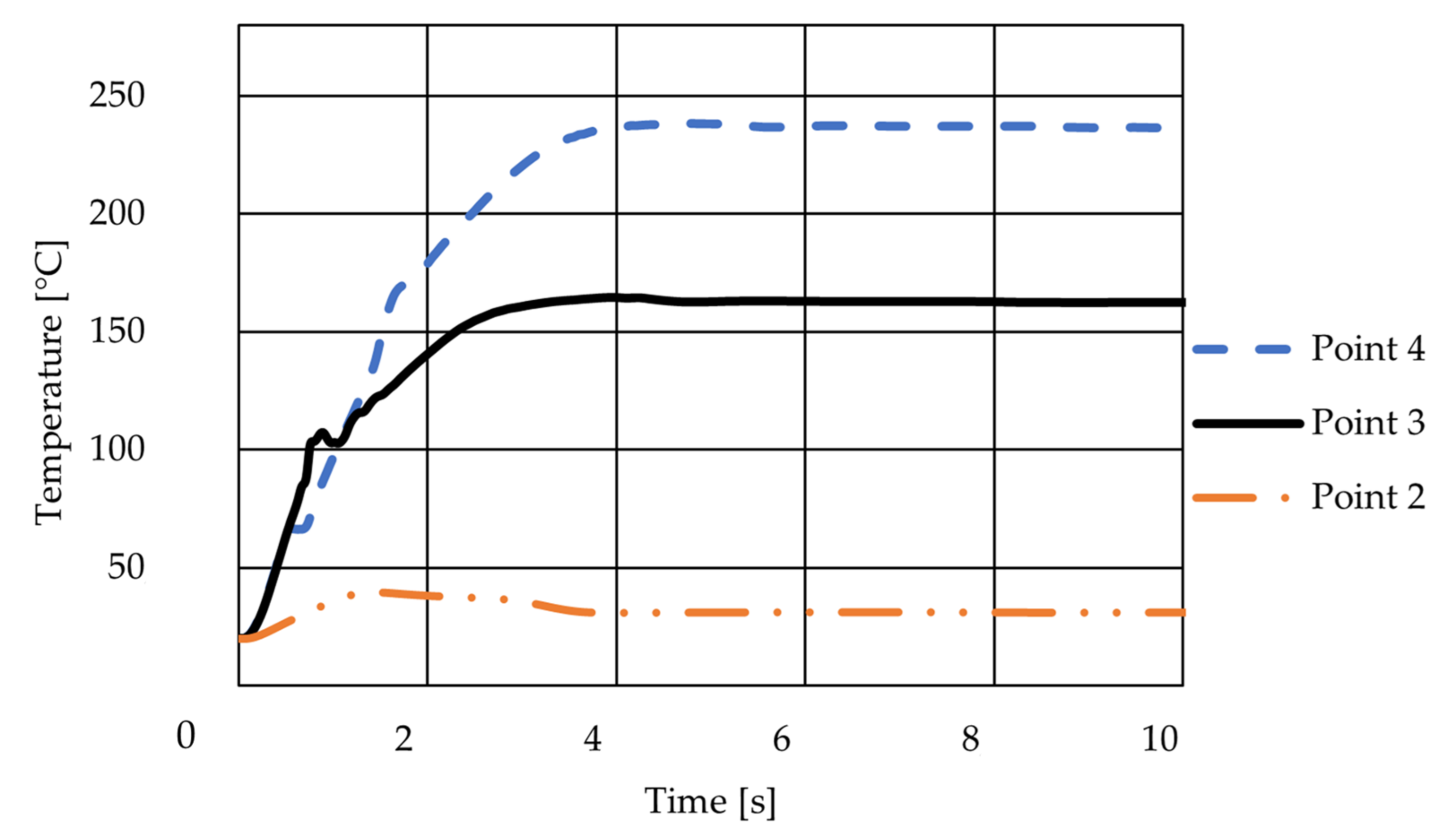
3.1.2. The Results of the Simulation of the Basic Geometry with Regard to Temperature
3.1.3. Results of Simulations on the New Geometries
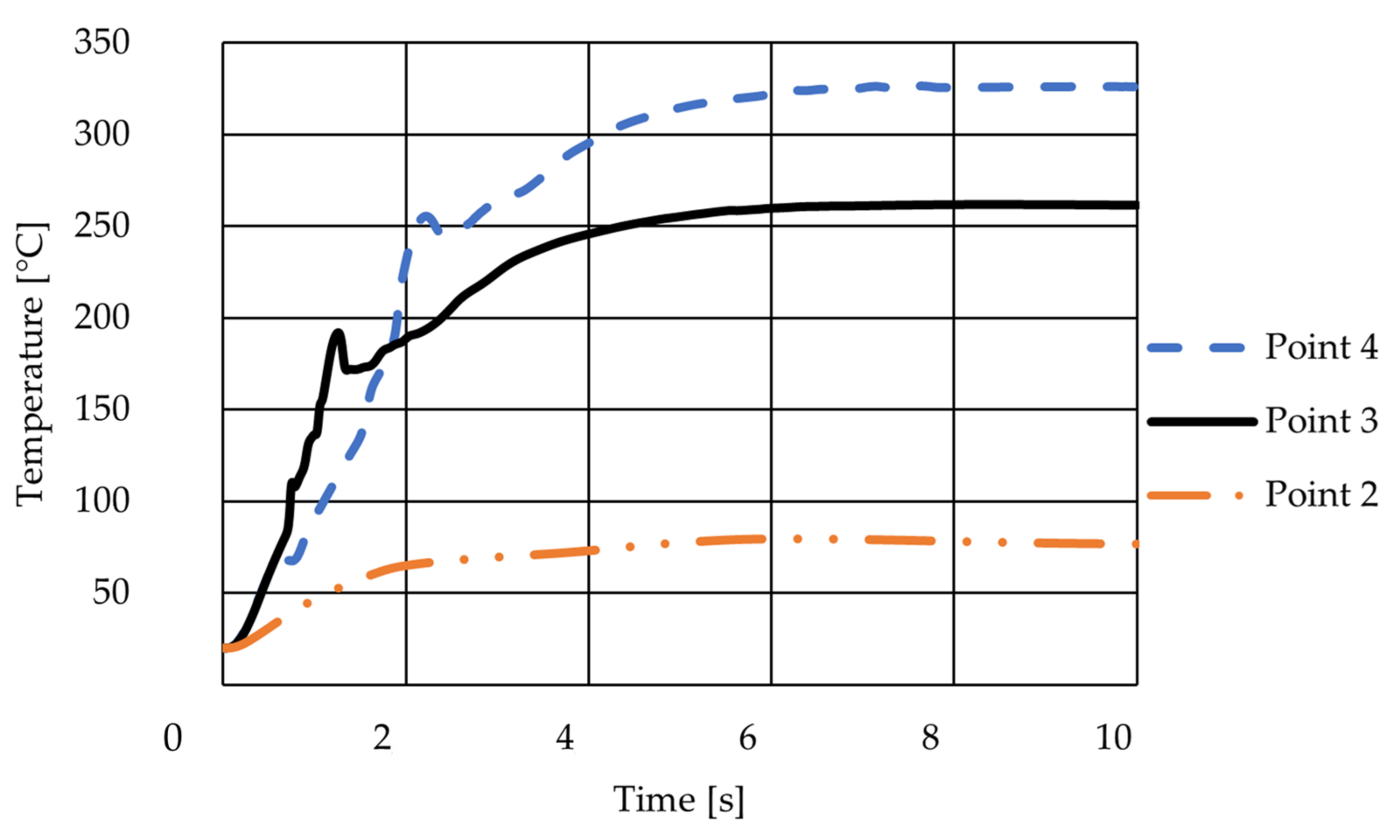
3.1.4. Temperature-Based Simulation on Geometry No. 2 by Entering the Side Inlet at the Bottom of the Reaction Tube
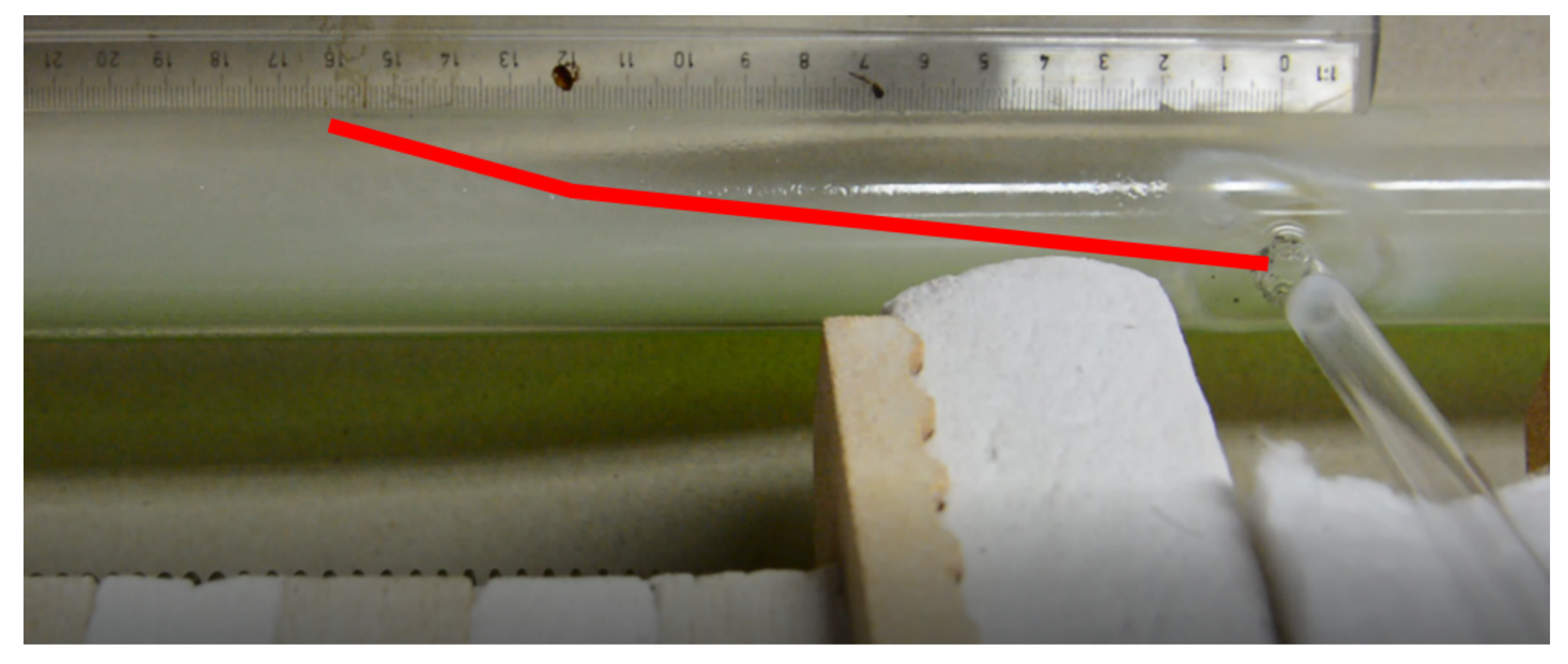
3.2. Results of Experimental Tests
4. Discussion
5. Conclusions
- The results of numerical simulations, in which the H2 inlets above and below were compared, show that placing the inlet on the bottom is the best proposed design solution for the USP device.
- H2, which entered from the side inlet into the reaction tube of the USP device, escaped back into the evaporation zone because N2 displaced it at lower flows (5 L/min). The leakage profile H2 was wedge-shaped.
- To prevent H2 leakage back into the evaporation zone, it was necessary to increase the N2 carrier gas flow to at least 10 L/min.
- The experiment showed that the turbulence zone occurred approximately 15 mm from the side inlet in the reaction tube after H2 entered at a flow rate of 5 L/min and a N2 flow rate of 10 L/min.
- Due to the high flow of N2, cooling occurred in the reaction tube of the USP device, so it was necessary to ensure higher temperatures in all three zones of the USP device with temperature control, or to extend the lengths of the zones in the USP device.
- The results of the validation with the experiment showed that the numerical simulations give results with reasonable accuracy and are able to predict the flow in the glass tube of the USP device qualitatively. The numerical approach provides results with sufficient accuracy and can provide trends for the future optimisation of USP devices.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CFD | Computer fluid dynamics |
| RANS | Reynolds-averaged Navier–Stokes |
| USP | Ultrasonic spray pyrolysis |
References
- Shariq, M.; Maric, N.; Gorse, G.K.; Kargl, R.; Rudolf, R. Synthesis of Gold Nanoparticles with the Ultrasonic Spray Pyrolysis and Estimation of their Usage in 3D Printing. Micro Nanosyst. 2018, 10, 102–109. [Google Scholar] [CrossRef]
- Rahman, M.T.; Rebrov, E.V. Microreactors for gold nanoparticles synthesis: From faraday to flow. Processes 2014, 2, 466–493. [Google Scholar] [CrossRef] [Green Version]
- Majerič, P.; Feizpour, D.; Friedrich, B.; Jelen, Ž.; Anžel, I.; Rudolf, R. Morphology of composite Fe@Au submicron particles, produced with ultrasonic spray pyrolysis and potential for synthesis of Fe@Au core-shell particles. Materials 2019, 12, 3326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasfi, A.S.; Humud, H.R.; Fadhil, N.K. Synthesis of core-shell Fe3O4-Au nanoparticles by electrical exploding wire technique combined with laser pulse shooting. Opt. Laser Technol. 2019, 111, 720–726. [Google Scholar] [CrossRef]
- Ma, C.; Shao, H.; Zhan, S.; Hou, P.; Zhang, X.; Chai, Y.; Liu, H. Bi-phase dispersible Fe3O4@Au core–shell multifunctional nanoparticles: Synthesis, characterization and properties. Compos. Interfaces 2019, 26, 537–549. [Google Scholar] [CrossRef]
- Majerič, P.; Rudolf, R. Advances in ultrasonic spray pyrolysis processing of noble metal nanoparticles-Review. Materials 2020, 13, 3485. [Google Scholar] [CrossRef] [PubMed]
- Tiyyagura, H.R.; Majerič, P.; Bračič, M.; Anžel, I.; Rudolf, R. Gold inks for inkjet printing on photo paper: Complementary characterisation. Nanomaterials 2021, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, J.; Yuan, S.; Yan, W.C. CFD simulation of ultrasonic atomization pyrolysis reactor: The influence of droplet behaviors and solvent evaporation. Int. J. Chem. React. Eng. 2021, 19, 167–178. [Google Scholar] [CrossRef]
- Ternik, P.; Rudolf, R.; Primož, T. Evaporation of water droplets in the 1st stage of the ultrasonic spray pyrolysis device. Anal. Pazu 2016, 6, 14–19. [Google Scholar]
- ANSYS CFX Brochure. Available online: https://pdf.directindustry.com/pdf/ansys/ansys-cfx-brochure/9123-233568.html (accessed on 25 October 2021).
- Škerget, L. Mehanika Tekočin; Faculty of Mechanical Engineering, University of Maribor: Maribor, Slovenia, 1994. [Google Scholar]
- Anderson, J.D. Computational Fluid Dynamics: The Basic with Application; McGraw-Hill Education: New York, NY, USA, 1995; Volume 1, ISBN 0070016852. [Google Scholar]
- Denton, J.D. Some Limitations of Turbomachinery CFD. Turbo Expo Power Land Sea Air 2010, 44021, 735–745. [Google Scholar] [CrossRef]
- Matula, G.; Bogovic, J.; Stoppic, S.; Friedrich, B. Scale up of Ultrasonic spray pyrolysis process for nano-power production—Part I. Heat Treat. Rep. 2013, 1, 2–5. [Google Scholar]
- Rudolf, R.; Majerič, P.; Štager, V.; Albreht, B. Process for the Production of Gold Nanoparticles by Modified Ultrasonic Spray Pyrolysis. Patent Application No. P-202000079, 5 May 2020. [Google Scholar]
- Alkan, G.; Diaz, F.; Matula, G.; Stopic, S.; Friedrich, B. Scaling up of nanopowder collection in the process of ultrasonic spray pyrolysis. World Metall—ERZMETALL 2017, 70, 97–101. [Google Scholar]
- Heraeus Properties of Fused Silica. Available online: https://www.heraeus.com/en/hca/fused_silica_quartz_knowledge_base_1/properties_1/properties_hca.html (accessed on 25 October 2021).
- Kitamura, R.; Pilon, L.; Jonasz, M. Optical constants of silica glass from extreme ultraviolet to far infrared at near room Temperature. Appl. Opt. 2007, 46, 8118–8133. [Google Scholar] [CrossRef] [PubMed]
- Kraut, B. Krautov strojniški priročnik, 15th ed.; Puhar, J., Stropnik, J., Eds.; Littera Picta: Ljubljana, Slovenia, 2011. [Google Scholar]
- Universal Industrial Gasses, I. Hydrogen (H2) Properties, Uses, Applications Hydrogen Gas and Liquid Hydrogen. Available online: http://www.uigi.com/hydrogen.html (accessed on 25 October 2021).
- Greenwood, N.N.; Earnshaw, A. Chemistry of the elements, 2nd ed.; Elsevier: Oxford, UK, 1997. [Google Scholar]
- Omoniyi, O.; Bacquart, T.; Moore, N.; Bartlett, S.; Williams, K.; Goddard, S.; Lipscombe, B.; Murugan, A.; Jones, D. Hydrogen gas quality for gas network injection: State of the art of three hydrogen production methods. Processes 2021, 9, 1056. [Google Scholar] [CrossRef]
- Nice, K. How Fuel Processors Work. Available online: https://auto.howstuffworks.com/fuel-efficiency/fuel-consumption/fuel-processor.htm (accessed on 25 October 2021).
- Turner, J.A. Sustainable Hydrogen Production. Science 2004, 305, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, P. A Sustainable Approach to the Supply of Nitrogen; Parker Hannifin Corporation: Lancaster, NY, USA, 2013. [Google Scholar]
- Hoskins, D. What Is the Fractional Distillation of Air? 2018. Available online: https://sciencing.com/fractional-distillation-air-7148479.html (accessed on 25 October 2021).
- Li, X.; Wang, S. Flow field and pressure loss analysis of junction and its structure optimization of aircraft hydraulic pipe system. Chin. J. Aeronaut. 2013, 26, 1080–1092. [Google Scholar] [CrossRef] [Green Version]
- Qian, S.; Frith, J.; Kasahara, N. Classification of Flow Patterns in Angled T-Junctions for the Evaluation of High Cycle Thermal Fatigue. J. Press. Vessel. Technol. Trans. ASME 2015, 137, 325–334. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.; Lee, J.C.; Ko, K.; Seo, Y. Development of a hydrocyclone for ultra-low flow rates. Chem. Eng. Res. Des. 2020, 156, 100–107. [Google Scholar] [CrossRef]
- Guo, H.F.; Chen, Z.Y.; Yu, C.W. Simulation of the effect of geometric parameters on tangentially injected swirling pipe airflow. Comput. Fluids 2009, 38, 1917–1924. [Google Scholar] [CrossRef]
- Koksal, M.; Hamdullahpur, F. Gas mixing in circulating fluidized beds with secondary air injection. Chem. Eng. Res. Des. 2004, 82, 979–992. [Google Scholar] [CrossRef]
- Raghavan, A.; Ghoniem, A.F. Simulation of supercritical water-hydrocarbon mixing in a cylindrical tee at intermediate Reynolds number: Formulation, numerical method and laminar mixing. J. Supercrit. Fluids 2014, 92, 31–46. [Google Scholar] [CrossRef]
- Frank, T.; Lifante, C.; Prasser, H.M.; Menter, F. Simulation of turbulent and thermal mixing in T-junctions using URANS and scale-resolving turbulence models in ANSYS CFX. Nucl. Eng. Des. 2010, 240, 2313–2328. [Google Scholar] [CrossRef] [Green Version]
- Selvam, P.K.; Kulenovic, R.; Laurien, E. Large eddy simulation on thermal mixing of fluids in a T-junction with conjugate heat transfer. Nucl. Eng. Des. 2015, 284, 238–246. [Google Scholar] [CrossRef]
- Sakowitz, A.; Mihaescu, M.; Fuchs, L. Effects of velocity ratio and inflow pulsations on the flow in a T-junction by Large Eddy Simulation. Comput. Fluids 2013, 88, 374–385. [Google Scholar] [CrossRef]
- Ayhan, H.; Sökmen, C.N. CFD modeling of thermal mixing in a T-junction geometry using les model. Nucl. Eng. Des. 2012, 253, 183–191. [Google Scholar] [CrossRef]
- Lin, C.H.; Ferng, Y.M. Investigating thermal mixing and reverse flow characteristics in a T-junction using CFD methodology. Appl. Therm. Eng. 2016, 102, 733–741. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, Y.; Xu, K.; Chen, Y.; Zou, J. Investigation on mixing behavior and heat transfer in a horizontally arranged tee pipe under turbulent mixing of hot and cold fluid. Ann. Nucl. Energy 2019, 127, 139–155. [Google Scholar] [CrossRef]
- Selvam, P.K.; Kulenovic, R.; Laurien, E.; Kickhofel, J.; Prasser, H.M. Thermal mixing of flows in horizontal T-junctions with low branch velocities. Nucl. Eng. Des. 2017, 322, 32–54. [Google Scholar] [CrossRef]
- Qian, S.; Kanamaru, S.; Kasahara, N. High-accuracy CFD prediction methods for fluid and structure temperature fluctuations at T-junction for thermal fatigue evaluation. Nucl. Eng. Des. 2015, 288, 98–109. [Google Scholar] [CrossRef]
- Smith, B.L.; Mahaffy, J.H.; Angele, K. A CFD benchmarking exercise based on flow mixing in a T-junction. Nucl. Eng. Des. 2013, 264, 80–88. [Google Scholar] [CrossRef]
- Kuczaj, A.K.; Komen, E.M.J.; Loginov, M.S. Large-Eddy Simulation study of turbulent mixing in a T-junction. Nucl. Eng. Des. 2010, 240, 2116–2122. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.C.; Hong, W.T.; Chen, N. Ultrasonically dispersed dyed water mists as a substitute for colored powders. Case Stud. Fire Saf. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]



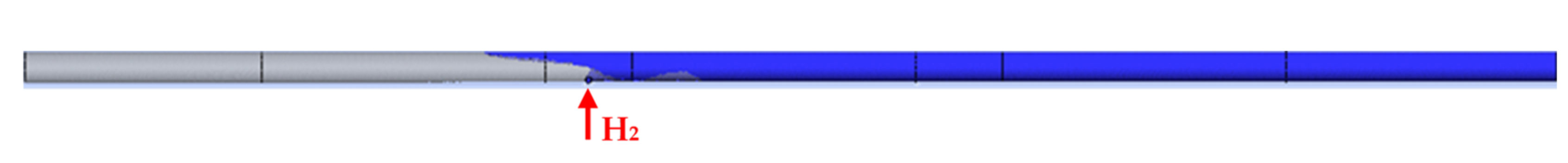
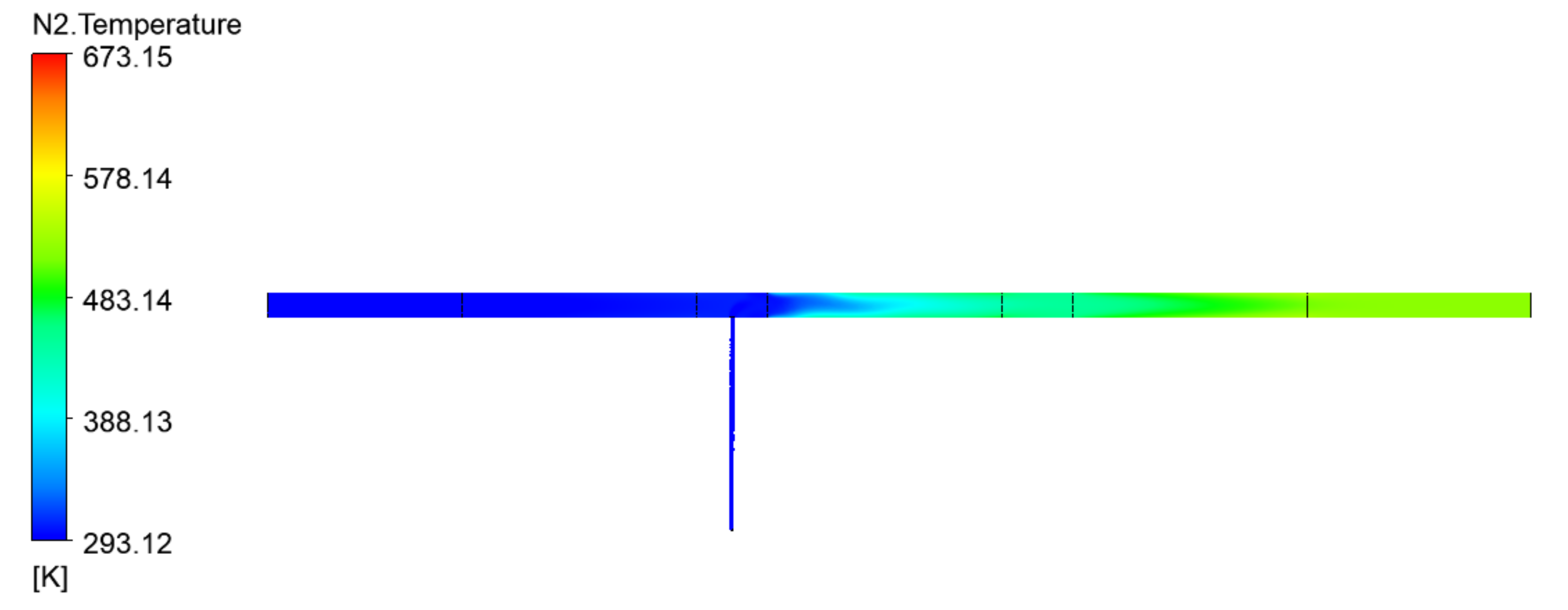


| The Shape of the Geometry | Image of the Model |
|---|---|
| 1. Side inlet at the top of the reaction tube | 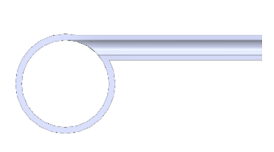 |
| 2. Side inlet at the bottom of the reaction tube |  |
| 3. Side inlet enters the reaction tube at an angle of 22.5° | 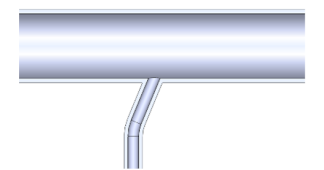 |
| 4. Side inlet enters the reaction tube at an angle of 45° |  |
| 5. Side inlet enters the reaction tube with a radius of 25 mm | 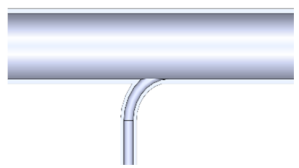 |
| 6. Side inlet enters the reaction tube with a radius of 35 mm | 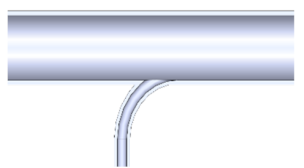 |
| 7. Side inlet enters the reaction tube in the centre of the reaction tube with a radius of 20 mm | 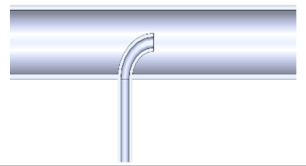 |
| 8. The shape of basic geometry, but the side inlet has a diameter of 10 mm |  |
| Volume Flow (L/min) | Mass Flow (kg/s) | |
|---|---|---|
| Simulation 1 | ||
| H2 | 10 | 0.00001500 |
| N2 | 5 | 0.00010417 |
| Simulation 2 | ||
| H2 | 5 | 0.00000750 |
| N2 | 5 | 0.00010417 |
| Simulation 3 | ||
| H2 | 5 | 0.00000750 |
| N2 | 10 | 0.00020833 |
| Simulation 4 | ||
| H2 | 10 | 0.00001500 |
| N2 | 10 | 0.00020833 |
| Simulation 6 | ||
| H2 | 5 | 0.0000075 |
| N2 | 15 | 0.0003125 |
| Flow N2 (L/min) | Flow H2 (L/min) | |
|---|---|---|
| 1. | 5 | 5 |
| 2. | 10 | 5 |
| 3. | 15 | 5 |
| Selected Flow | Image of the Simulation |
|---|---|
|
1. Flow H2 = 5 L/min Flow N2 = 5 L/min | 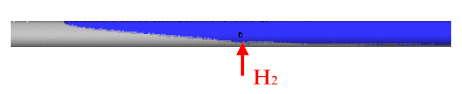 |
|
2. Flow H2 = 10 L/min Flow N2 = 5 L/min |  |
|
3. Flow H2 = 5 L/min Flow N2 = 10 L/min | 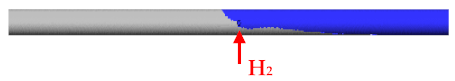 |
|
4. Flow H2 = 10 L/min Flow N2 = 10 L/min |  |
|
5. Flow H2 = 5 L/min Flow N2 = 15 L/min | 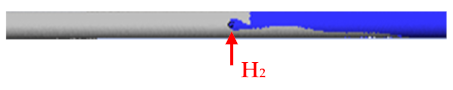 |
| The Shape of the Geometry | Image of the Model |
|---|---|
| 1. Side inlet at the top of the reaction tube |  |
| 2. Side inlet at the bottom of the reaction tube |  |
| 3. The side inlet enters the reaction tube at an angle of 22.5° |  |
| 4. The side inlet enters the reaction tube at a 45° angle |  |
| 5. The side inlet enters the reaction tube with a radius of 25 mm |  |
| 6. The side inlet enters the reaction tube with a radius of 35 mm |  |
| 7. The side inlet enters the reaction tube in the centre of the reaction tube with a radius of 20 mm |  |
| 8. The shape is of basic geometry but has a side inlet diameter of 10 mm |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelen, Ž.; Kandare, D.; Lešnik, L.; Rudolf, R. Optimisation of a Side Inlet for H2 Entry into an Ultrasonic Spray Pyrolysis Device. Processes 2021, 9, 2256. https://doi.org/10.3390/pr9122256
Jelen Ž, Kandare D, Lešnik L, Rudolf R. Optimisation of a Side Inlet for H2 Entry into an Ultrasonic Spray Pyrolysis Device. Processes. 2021; 9(12):2256. https://doi.org/10.3390/pr9122256
Chicago/Turabian StyleJelen, Žiga, Domen Kandare, Luka Lešnik, and Rebeka Rudolf. 2021. "Optimisation of a Side Inlet for H2 Entry into an Ultrasonic Spray Pyrolysis Device" Processes 9, no. 12: 2256. https://doi.org/10.3390/pr9122256
APA StyleJelen, Ž., Kandare, D., Lešnik, L., & Rudolf, R. (2021). Optimisation of a Side Inlet for H2 Entry into an Ultrasonic Spray Pyrolysis Device. Processes, 9(12), 2256. https://doi.org/10.3390/pr9122256