Comparative Investigation of Different CO2 Capture Technologies for Coal to Ethylene Glycol Process
Abstract
:1. Introduction
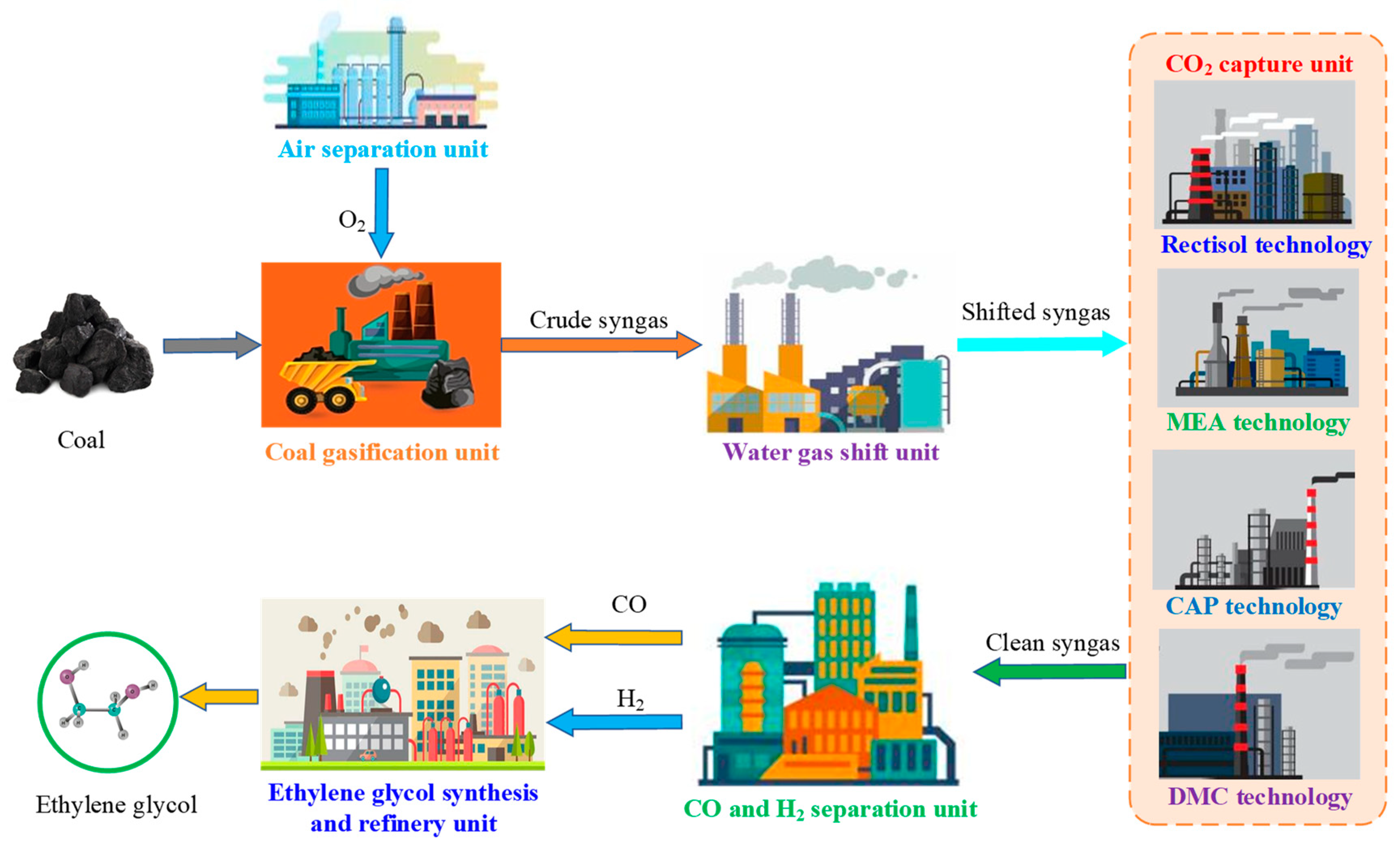
2. Process Description and Modeling
2.1. Air Separation Unit
2.2. Coal Gasification Unit
2.3. Water Gas Shift Unit
2.4. Acid Gas Removal/CO2 Capture Unit
2.4.1. Rectisol Technology
2.4.2. MEA Technology
2.4.3. CAP Technology
2.4.4. DMC Technology
2.5. CO and H2 Separation Unit
2.6. Ethylene Glycol Synthesis and Refinery Unit
2.7. Flowsheet Simulation and Validation
3. Comparison of the Four Different CO2 Capture Technologies
3.1. Comparison of Energy Consumption and Energy Efficiency
- In general, there is an ASU unit in a CTEG plant, which can simultaneously provide oxygen and cold nitrogen. The cold nitrogen is at very low temperature and has a relative high cooling ability. Therefore, the cold utility of the Rectisol and DMC processes can be greatly reduced by integrating with the cold nitrogen stream of the ASU unit, which will also reduce the amount of other coolants to cool the methanol. As a result, the energy consumption of CO2 capture of these two processes is lower than that of the MEA and CAP processes.
- The electricity consumption of the Rectisol process is significantly lower than that of the DMC process. Thus, although both the Rectisol and DMC are part of the physical absorption method and have a relative similar flowsheet, the CO2 capture and total energy consumptions of the Rectisol process are lower than those of the DMC process.
- As the MEA process is a chemical absorption method, it needs an amount of steam to heat the rich solution in the reboiler. In fact, the regeneration duty of the MEA process is about 4.08 GJ/tCO2, accounting for 78.46% of the total CO2 capture energy consumption. However, most of the CO2 of the Rectisol and DMC processes is discharged in the multistage flashes, resulting in low regeneration duty.
- In addition to the heat duty of CO2 regeneration column (1.26 GJ/tCO2), the CAP process requires additional steam to heat the ammonia regeneration column, which is about 1.42 GJ/tCO2. Thus, the energy consumption of the regeneration process of the CAP process is far less than that of the MEA process, resulting in a low CO2 capture energy consumption. Compared with the MEA and CAP processes, the Rectisol and DMC process requires less regeneration duty due to the high solubility of acid gas.
- As for the compression work, the MEA process consumes the highest electricity, 1.12 GJ/t CO2, because of the highest pressure of the CO2 stream from this process in comparison with other processes. It also leads to the high energy consumption of this process.
- As Figure 8 shows, the total energy consumption of the CTEG process with Rectisol, MEA, CAP and DMC technologies is 41.38 GJ/t, 48.44 GJ/t, 45.12 GJ/t, 41.85 GJ/t, respectively. Assuming that the ethylene glycol production of these four processes is the same up to 37.5 t/h, their total output energy is the same up to 19.10 GJ/T. Thus, the energy efficiency of the CTEG process with Rectisol, MEA, CAP and DMC technologies is 46.16%, 39.43%, 42.33% and 45.64%, respectively. Therefore, the CTEG process with Rectisol technology has the best thermodynamic performance.
3.2. Comparison of Total Capital Investment
3.3. Comparison of Capture and Production Costs
3.4. Comparison of Internal Rate of Return
3.5. Comparison of the Overall Performance
4. Conclusions
- The energy consumption of CO2 capture of the CTEG process with Rectisol technology is the lowest, 1.88 GJ/tCO2, and the CTEG process with MEA technology is the highest, 5.20 GJ/tCO2. That of the CTEG process with CAP and DMC technologies are 3.64 GJ/tCO2 and 2.10 GJ/tCO2.
- The investment cost of the Rectisol, CAP and DMC technologies is about 1.27, 1.21 and 1.18 times that of the MEA process, respectively. As a result, the total capital investment of the CTEG with Rectisol technology is the highest, 4.49 × 109 CNY, followed by the CAP technology, 4.45 × 109 CNY, DMC technology, 4.42 × 109 CNY, and MEA technology, 4.28 × 109 CNY.
- The CO2 capture cost of the Rectisol process is the lowest, CNY 169.5/tCO2, followed by the DMC process, CNY 193.2/tCO2, the CAP process CNY 232.6/tCO2, and the MEA process CNY 250.5/tCO2. Therefore, the total production cost of the CTEG process with the Rectisol process is the lowest, CNY 4631/tEG, and that of the MEA process is the highest, CNY 4750/tEG.
- The internal rate of return of the CTEG process with Rectisol technology is similar to the DMC technology, which is higher than that of the CTEG process with the MEA and CAP technologies.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| η | energy efficiency |
| salvage value of ith unit | |
| annual cost of the operational and maintenance of ith unit | |
| CRF | capital recovery factor |
| FOM | fixed cost |
| n | plant lifetime |
| N | number of stages |
| NCFj | net cash flow of jth year |
| P | pressure |
| ∆P | pressure drop |
| r | discount rate |
| RFi | ratio factor of ith capital investment |
| S | processing capacity |
| T | temperature |
| ∆T | temperature difference |
| total capital investment required for ith unit | |
| VOM | variable operation and maintenance cost |
| Wcol | work consumption of cooling equipment |
| Wcom | work consumption of compressor |
| Wpum | work consumption pump |
| ASU | air separation unit |
| AGR | acid gas removal |
| CG | coal gasification |
| CHS | CO and H2 separation |
| CTEG | coal to ethylene glycol |
| CCG | choren coal gasifier |
| CCR | CO coupling reactor |
| CAP | chilled ammonia process |
| CEPCI | Chemical Engineering Plant Cost Index |
| DM | dimethyl carbonate |
| EG | ethylene glycol |
| EGSR | ethylene glycol synthesis and refining |
| EI | equipment investment |
| H/C | hydrogen–carbon |
| IGCC | integrated gasification combined cycle |
| IRR | internal rate of return |
| MEA | mono-ethanol amine |
| NRTL | non-random-two-liquid |
| TCI | total capital investment |
| TPC | total production cost |
| WGS | water gas shift |
References
- Pang, J.; Zheng, M.; Sun, R.; Wang, A.; Wang, X.; Zhang, T. Synthesis of ethylene glycol and terephthalic acid from biomass for producing PET. Green Chem. 2016, 18, 342–359. [Google Scholar] [CrossRef]
- National Development and Reform Committee of China (NDRC). Long-Term Planning for Coal Chemical Industry Development. 2016. Available online: http://www.ndrc.gov.cn/ (accessed on 29 December 2016).
- Zhang, Y.; Yuan, Z.; Margni, M.; Bulle, C.; Hua, H.; Jiang, S.; Liu, X. Intensive carbon dioxide emission of coal chemical industry in China. Appl. Energy 2019, 236, 540–550. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.; He, S.; Gao, L. Coal to substitute natural gas based on combined coal-steam gasification and one-step methanation. Appl. Energy 2019, 240, 851–859. [Google Scholar] [CrossRef]
- Plaza, M.G.; Rubiera, F. Evaluation of a novel multibed heat-integrated vacuum and temperature swing adsorption post-combustion CO2 capture process. Appl. Energy 2019, 250, 916–925. [Google Scholar] [CrossRef]
- Diego, M.E.; Bellas, J.-M.; Pourkashanian, M. Techno-economic analysis of a hybrid CO2 capture system for natural gas combined cycles with selective exhaust gas recirculation. Appl. Energy 2019, 215, 778–791. [Google Scholar] [CrossRef]
- Wu, X.; Wang, M.; Liao, P.; Shen, J.; Li, Y. Solvent-based post-combustion CO2 capture for power plants: A critical review and perspective on dynamic modelling, system identification, process control and flexible operation. Appl. Energy 2020, 257, 113941. [Google Scholar] [CrossRef]
- Ye, R.; Lin, L.; Liu, C.; Chen, C.; Yao, Y. One-pot synthesis of cyclodextrin-doped Cu-SiO2 catalysts for efficient hydrogenation of dimethyl oxalate to ethylene glycol. ChemCatChem 2017, 9, 4587–4597. [Google Scholar] [CrossRef]
- Wei, R.; Yan, C.; Yang, A.; Shen, W.; Li, J. Improved process design and optimization of 200 kt/a ethylene glycol production using coal-based syngas. Chem. Eng. Res. Des. 2018, 132, 551–563. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, C.; Zhang, D.; Zhou, H. Development of a coke oven gas assisted coal to ethylene glycol process for high techno-economic performance and low emission. Ind. Eng. Chem. Res. 2018, 57, 7600–7612. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, X.; Zhu, S.; Huang, W.; Zhang, D. Efficient utilization of CO2 in a coal to ethylene glycol process integrated with dry/steam-mixed reforming: Conceptual design and techno-economic analysis. ACS Sustain. Chem. Eng. 2019, 7, 3496–3510. [Google Scholar] [CrossRef]
- Rezvani, S.; Huang, Y.; McIlveen-Wright, D.; Hewitt, N.J.; Mondol, J.D. Comparative assessment of coal fired IGCC systems with CO2 capture using physical absorption, membrane reactors and chemical looping. Fuel 2009, 88, 2463–2472. [Google Scholar] [CrossRef]
- Giuffrida, A.; Bonalumi, D.; Lozza, G. Amine-based post-combustion CO2 capture in air-blown IGCC systems with cold and hot gas clean-up. Appl. Energy 2013, 110, 44–54. [Google Scholar] [CrossRef]
- Shi, B.; Xu, W.; Wu, E.; Wu, W.; Kuo, P.-C. Novel design of integrated gasification combined cycle (IGCC) power plants with CO2 capture. J. Clean. Prod. 2018, 195, 176–186. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, B.; Chen, D.; Zhao, F.; Fei, W. Techno-economic assessment of China’s indirect coal liquefaction projects with different CO2 capture alternatives. Energy 2011, 36, 6559–6566. [Google Scholar] [CrossRef]
- Pettinau, A.; Ferrara, F.; Tola, V.; Cau, G. Techno-economic comparison between different technologies for CO2-free power generation from coal. Appl. Energy 2017, 193, 426–439. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, S.; Yu, P.; Yang, Q.; Zhang, D. Thermodynamic and techno-economic analysis of coal to ethylene glycol process (CtEG) with different coal gasifiers. Energy Convers. Manag. 2019, 191, 80–92. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, L.; Fan, J.; Jiang, P.; Li, L. MSW to synthetic natural gas: System modeling and thermodynamics assessment. Waste Manag. 2016, 48, 257–264. [Google Scholar] [CrossRef]
- Man, Y.; Yang, S.; Xiang, D.; Li, X.; Qian, Y. Environmental impact and techno-economic analysis of the coal gasification process with/without CO2 capture. J. Clean. Prod. 2014, 71, 59–66. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, D.; Zhou, H.; Zhang, C. Process simulation, analysis and optimization of a coal to ethylene glycol process. Energy 2018, 155, 521–534. [Google Scholar] [CrossRef]
- Qin, S.; Chang, S.; Yao, Q. Modeling, thermodynamic and techno-economic analysis of coal-to-liquids process with different entrained flow coal gasifiers. Appl. Energy 2018, 229, 413–432. [Google Scholar] [CrossRef]
- Mores, P.; Scenna, N.J.; Mussati, S.F. Post-combustion CO2 capture process: Equilibrium stage mathematical model of the chemical absorption of CO2 into monoethanolamine (MEA) aqueous solution. Chem. Eng. Res. Des. 2011, 89, 1587–1599. [Google Scholar] [CrossRef]
- Liu, X.; Yang, S.; Hu, Z.; Qian, Y. Simulation and assessment of an integrated acid gas removal process with higher CO2 capture rate. Comput. Chem. Eng. 2015, 83, 48–57. [Google Scholar] [CrossRef]
- Li, K.; Leigh, W.; Feron, P.; Yu, H.; Tade, M. Systematic study of aqueous monoethanolamine (MEA)-based CO2 capture process: Techno-economic assessment of the MEA process and its improvements. Appl. Energy 2016, 165, 648–659. [Google Scholar] [CrossRef]
- Lawal, A.; Wang, M.; Stephenson, P.; Yeung, H. Dynamic modelling of CO2 absorption for post combustion capture in coal-fired power plants. Fuel 2009, 88, 2455–2462. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Jiang, K.; Jones, T.; Feron, P.; Bennett, R.; Hollenkamp, A. CO2 regenerative battery for energy harvesting from ammonia-based post-combustion CO2 capture. Appl. Energy 2019, 247, 417–425. [Google Scholar] [CrossRef]
- Zhang, L.; Han, L.; Zhao, G.; Chai, R.; Zhang, Q.; Liu, Y.; Lu, Y. Structured Pd–Au/Cu-fiber catalyst for gas-phase hydrogenolysis of dimethyl oxalate to ethylene glycol. Chem. Commun. 2015, 51, 10547–10550. [Google Scholar] [CrossRef]
- He, C.; Feng, X. Process modeling and thermodynamic analysis of Lurgi fixed-bed coal gasifier in an SND plant. Appl. Energy 2013, 111, 742–757. [Google Scholar] [CrossRef]
- Yang, S.; Qian, Y.; Liu, Y.; Wang, Y.; Yang, S. Modeling, simulation, and techno-economic analysis of Lurgi gasification and BGL gasification for coal-to-SNG. Chem. Eng. Res. Des. 2017, 117, 355–368. [Google Scholar] [CrossRef]
- Vo, T.T.; Wall, D.M.; Ring, D.; Rajendran, K.; Murphy, J.D. Techno-economic analysis of biogas upgrading via amine scrubber, carbon capture and ex-situ methanation. Appl. Energy 2018, 212, 1191–1202. [Google Scholar] [CrossRef]
- Sproul, E.; Barlow, J.; Quinn, J. Time value of greenhouse gas emissions in life cycle assessment and tech-no-economic analysis. Environ. Sci. Technol. 2019, 53, 6073–6080. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Li, X.; Xu, G.; Xin, T.; Yang, Y.; Liu, W.; Wang, M. Energy, exergy and economic analyses of a novel solar-lignite hybrid power generation process using lignite pre-drying. Energy Convers. Manag. 2018, 170, 19–33. [Google Scholar] [CrossRef]
- Li, G.; Liu, Z.; Liu, T.; Shan, J.; Fang, Y.; Wang, Z. Techno-economic analysis of a coal to hydrogen process based on ash agglomerating fluidized bed gasification. Energy Convers. Manag. 2018, 164, 552–559. [Google Scholar] [CrossRef]
- Han, Z.; Xiao, K.; Zhao, Y.; Bai, Y. Comparison between MEA and chilled ammonia based carbon capture process. J. North China Electr. Power Univ. 2016, 43, 87–93. [Google Scholar]
- Wang, X.; Li, L.; Palazoglu, A.; El-Farra, N.H.; Shah, N. Optimization and control of offshore wind systems with energy storage. Energy Convers. Manag. 2018, 173, 426–437. [Google Scholar] [CrossRef]











| Technology | Equipment | Model | Operational Parameters | Equipment | Model | Operational Parameters |
|---|---|---|---|---|---|---|
| Rectisol a | Lean cooler | Heater | Tout = −58 °C, ∆P = 0 | CO2 Flash1 | Flash | P = 1.38 MPa, Q = 0 |
| Acid gas Absorber | Radfrac | N = 20, P = 5.5 MPa | H2S con. column | Radfrac | N = 20, P = 0.2 MPa | |
| SO2 Flash | Flash | P = 0.69 MPa, Q = 0 | H2S | Radfrac | N = 10, P = 0.3 MPa | |
| MEA b | Cooler | Heater | Tout = 40 °C, ∆P = 0 | Desorber | Radfrac | N = 11, P = 0.19 MPa |
| Absorber | Radfrac | N = 11, P = 0.12 MPa | Lean cooler | Heater | Tout = 40 °C, ∆P = 0 | |
| Rich-pump | Pump | P = 0.21 MPa, η = 0.8 | Lean pump | Pump | P = 0.21 MPa, η = 0.8 | |
| CAP c | Heat exchanger | HeatX | Tcold = 95 °C, ∆P = 0 | Separator | Flash2 | Tout = 40 °C, ∆P = 0 |
| Contact cooler | Radfrac | N = 5, P = 0.12 MPa | Rich-pump-2 | Pump | P = 0.14 MPa, η = 0.8 | |
| Absorber | Radfrac | N = 20, P = 0.12 MPa | Heat exchanger-2 | HeatX | ∆Tcold = 5 °C, ∆P = 0 | |
| Rich-pump-1 | Pump | P = 1.0 MPa, η = 0.8 | Lean pump | Pump | P = 0.14 MPa, η = 0.8 | |
| Heat exchanger-1 | HeatX | ∆Tcold = 5 °C, ∆P = 0 | NH3-desorber | Radfrac | N = 5, P = 0.12 MPa | |
| Desorber | Radfrac | P = 2.0 MPa | Cooler-2 | Heater | Tout = 40 °C, ∆P = 0 | |
| Lean cooler-1 | Heater | Tout = 25 °C, ∆P = 0 | Lean cooler-2 | Heater | Tout = 10 °C, ∆P = 0 | |
| Flash | Flash2 | Tout = 40 °C, ∆P = 0 | Mixer | Mixer | Adiabatic mixing | |
| DMC c | Absorber | Radfrac | N = 10, P = 2.84 MPa, ∆P = 0.02 MPa | Heat exchanger | HeatX | Tlean = 30 °C, ∆P = 0 |
| Flash | Flash2 | Tout = 30 °C, ∆P = 0 | Lean MEA | - | 35 mol% |
| Unit | Key Parameters | Value | Key Parameters | Value |
|---|---|---|---|---|
| ASU unit | O2 supplied mole purity | 99.8% | N2 supplied mole purity | 99.9% |
| CG unit | Gasification pressure | 4.0 MPa | Gasification temperature | 1500 °C |
| Oxygen to coal | 0.42 | Steam to coal | 0.25 | |
| WGS unit | Pressure | 3.65 MPa | Shift ratio | 49.25% |
| 1st shift reactor temperature | 350 °C | 2nd shift reactor temperature | 220 °C | |
| AGR unit | Shown in Table 1 | |||
| CHS unit | H2 recovery ratio | 95.17% | CO recovery ratio | 91.75% |
| EGSR unit | DMO reactor temperature | 130 °C | DMO reactor pressure | 0.5 MPa |
| Regeneration temperature | 40 °C | H2/DMO (mole ratio) | 42 | |
| EG reactor temperature | 235 °C | EG reactor pressure | 3.0 MPa | |
| Recovery column temperature | 90 °C | Pressure | 0.1 MPa | |
| Methanol recovery ratio | 99.5% | DMC removal ratio | 95.2% | |
| CO | H2 | CO2 | H2O | N2+Ar | H2S | Others | ||
|---|---|---|---|---|---|---|---|---|
| Crude syngas a | Simulation results | 59.58 | 27.37 | 12.09 | - | 0.68 | 0.25 | 0.03 |
| Industrial data b | 61.30 | 27.10 | 10.47 | - | 0.87 | 0.24 | 0.02 | |
| Shifted syngas | Simulation results | 23.17 | 43.83 | 32.04 | 0.22 | 0.53 | 0.18 | 0.03 |
| Industrial data c | 22.18 | 40.85 | 35.59 | 0.24 | 0.94 | 0.18 | 0.02 | |
| Clean syngas | Simulation results | 34.59 | 63.39 | - | - | 2.02 | - | - |
| Industrial data d | 34.60 | 63.96 | - | - | 1.43 | - | 0.01 | |
| H2 product | Simulation results | 0.01 | 99.91 | - | - | 0.04 | - | - |
| Industrial data d | - | 99.95 | - | - | 0.05 | - | - | |
| CO product | Simulation results | 99.06 | - | - | - | 0.94 | - | - |
| Industrial data d | 98.00 | - | - | - | 2.00 | - | - |
| Item | Value |
|---|---|
| Input coal | 118.98 t/h |
| Air consumption | 20,2600 Nm3/h |
| CO consumption | 1529 kmol/h |
| H2 consumption | 2846 kmol/h |
| Methanol consumption | 3.38 t/h |
| HNO3 consumption | 2.23 t/h |
| NaOH consumption | 0.54 t/h |
| CO2 captured (AGR unit) | 2.19 t/h |
| Captured CO2 purity | ≥95.0% |
| EG product | 37.50 t/h |
| Unit | Benchmark | sf | Sref | EIref (106 CNY) | CEPCIref | f |
|---|---|---|---|---|---|---|
| ASU a | Oxygen supply | 0.50 | 91.75 t/h | 105 | 584.6/2012 | 0.5 |
| CG a | Coal input | 0.67 | 114.21 t/h | 135.21 | 394.3/2001 | 1.0 |
| WGS b | Syngas input | 0.67 | 30,697 kmol/h | 11.56 | 525.4/2007 | 0.6 |
| Rectisol a | Syngas input | 0.67 | 200 kNm3/h | 52.3 | 584.6/2012 | 0.65 |
| MEA c | CO2 output | 0.67 | 66,509 t/d | 206.55 | 525.4/2007 | 0.65 |
| CAP d | CO2 output | 0.67 | 419.16 t/h | 117.15 | 584.6/2012 | 0.65 |
| DMC d | CO2 output | 0.67 | 66,509 t/d | 244.45 | 525.4/2007 | 0.8 |
| DMOS e | H2 input | 0.65 | 2845.50 kmol/h | 55.20 | 576.1/2014 | 0.6 |
| EGS e | DMO input | 0.65 | 74.21 t/h | 77.76 | 576.1/2014 | 0.6 |
| EGR e | EG output | 0.65 | 37.50 t/h | 84.32 | 576.1/2014 | 0.6 |
| HCS a | H2 output | 0.67 | 21.3 kg/s | 45.92 | 575.4/2008 | 1.0 |
| PSA f | H2 input | 0.70 | 944 Nm3/h | 0.83 | 576.1/2014 | 1.0 |
| Items | Price | Items | Price | |
|---|---|---|---|---|
| Raw material and | Raw coal | CNY 450/t | Fuel coal | CNY 200/t |
| utilities | Electricity | 0.65 kWh/t | Water | CNY 4.5/t |
| Steam | CNY 70/t | Ammonia | CNY 2200/t | |
| Methanol | CNY 2000/t | MEA | CNY 10, 600/t | |
| DMC | CNY 5500/t | NaOH | CNY 2000/t | |
| HNO3 | CNY 2500/t | Catalysts | CNY 300/t | |
| Product and | Ethylene glycol | CNY 7500/t | Light diol | CNY 1966/t |
| byproduct | Dimethyl carbonate | CNY 5500/t | Sulfur | CNY 1026/t |
| Mixed alcohol ester | CNY 7700/t | Heavy component | CNY 6154/t | |
| Sulfuric acid | CNY 250/t | Cinder | CNY 43/t |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Liao, Y.; Su, Y.; Wang, B.; Yang, Y.; Ji, D.; Li, H.; Zhou, H.; Wang, D. Comparative Investigation of Different CO2 Capture Technologies for Coal to Ethylene Glycol Process. Processes 2021, 9, 207. https://doi.org/10.3390/pr9020207
Ma Y, Liao Y, Su Y, Wang B, Yang Y, Ji D, Li H, Zhou H, Wang D. Comparative Investigation of Different CO2 Capture Technologies for Coal to Ethylene Glycol Process. Processes. 2021; 9(2):207. https://doi.org/10.3390/pr9020207
Chicago/Turabian StyleMa, Yanqing, Yitao Liao, Yi Su, Baojie Wang, Yong Yang, Dong Ji, Hongwei Li, Huairong Zhou, and Dongliang Wang. 2021. "Comparative Investigation of Different CO2 Capture Technologies for Coal to Ethylene Glycol Process" Processes 9, no. 2: 207. https://doi.org/10.3390/pr9020207
APA StyleMa, Y., Liao, Y., Su, Y., Wang, B., Yang, Y., Ji, D., Li, H., Zhou, H., & Wang, D. (2021). Comparative Investigation of Different CO2 Capture Technologies for Coal to Ethylene Glycol Process. Processes, 9(2), 207. https://doi.org/10.3390/pr9020207