Cyanidin-3-glucoside Lipophilic Conjugates for Topical Application: Tuning the Antimicrobial Activities with Fatty Acid Chain Length
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds’ Synthesis and Stability
2.1.1. Reagents and Extraction of Cy3glc
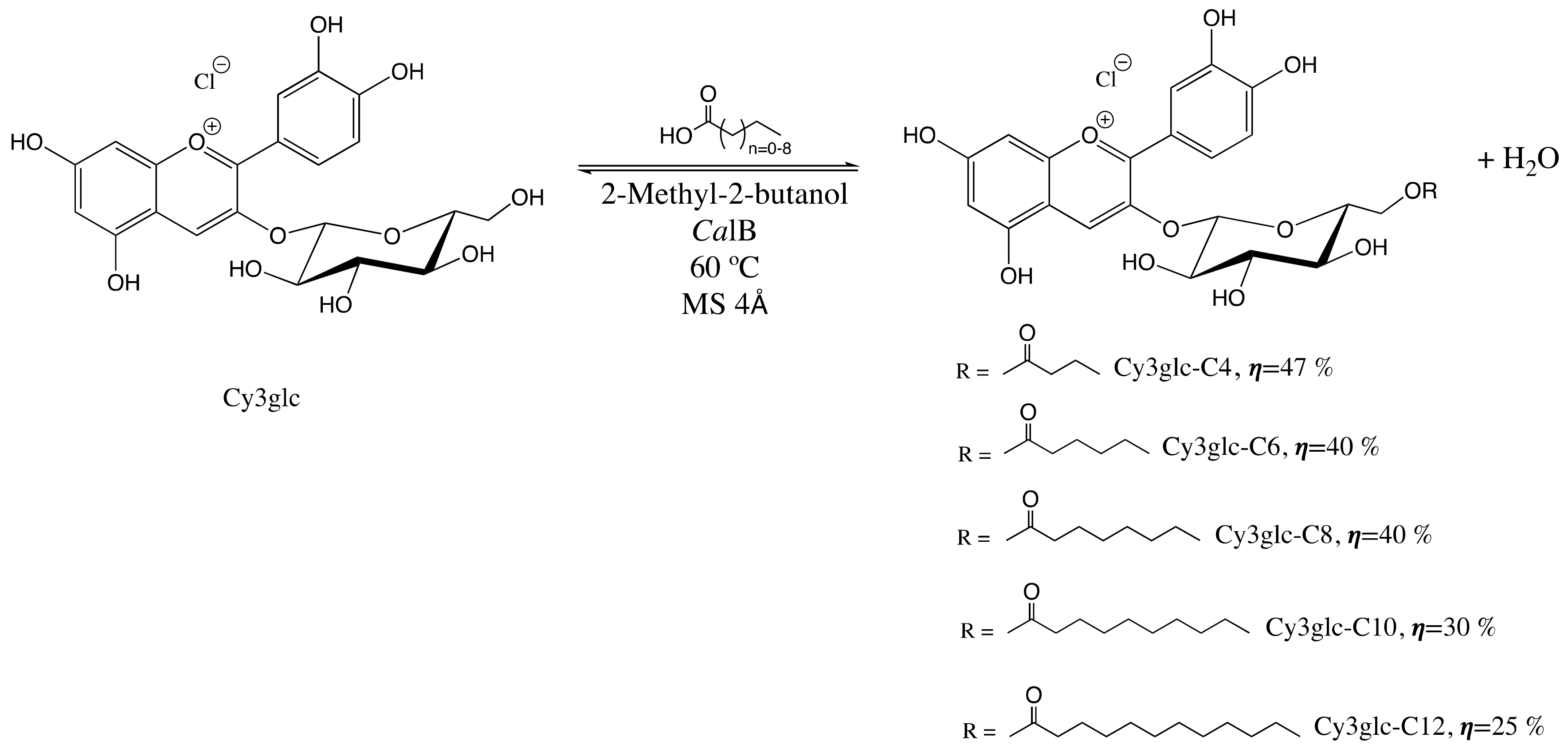
2.1.2. Enzymatic Synthesis and Purification of Cy3glc−Fatty Acid Derivatives
2.1.3. Stability
2.1.4. HPLC Analysis
2.2. Collagenase Activity
2.3. Elastase Activity
2.4. Tyrosinase Activity
2.5. Cell Culture Conditions
2.5.1. Compounds Stability and Enzymatic/Chemical Transformation
2.5.2. MTT Assay
2.5.3. Reactive Oxygen Species Experiments
2.6. Antibacterial Activity Assessment
2.7. Statistical Analysis
3. Results
3.1. Physiological Stability
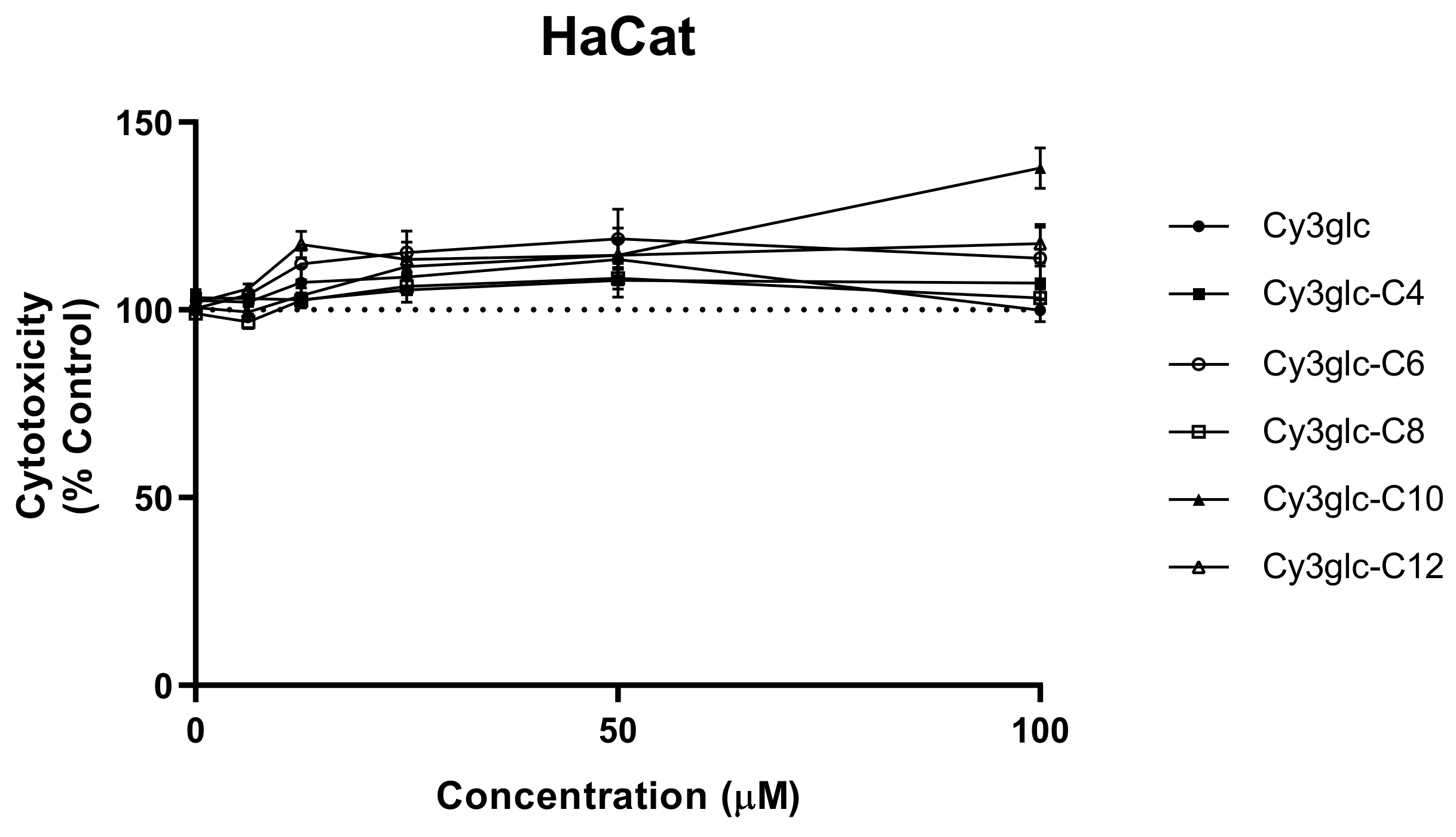
3.2. Cytotoxicity
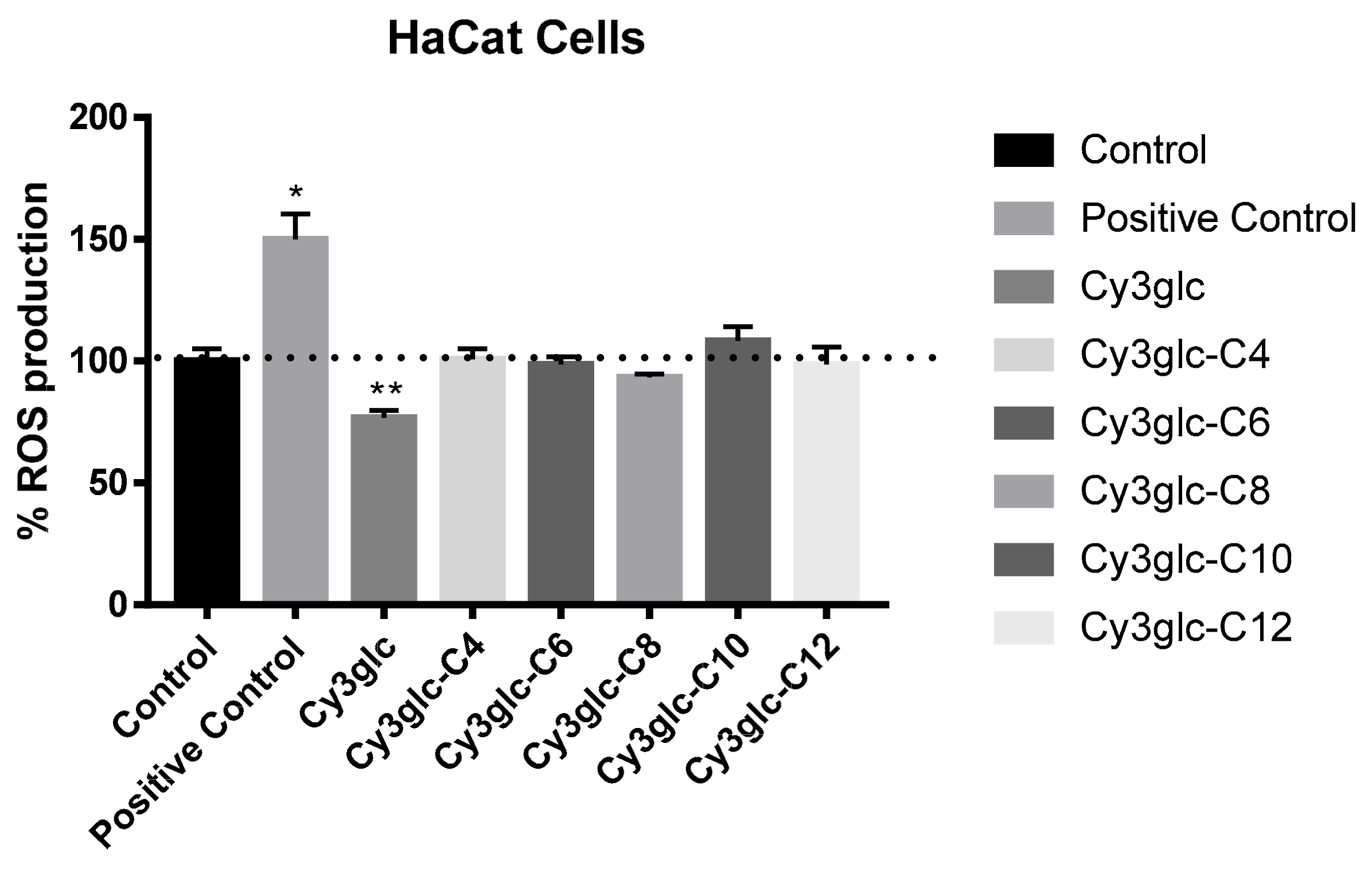
3.3. ROS Production
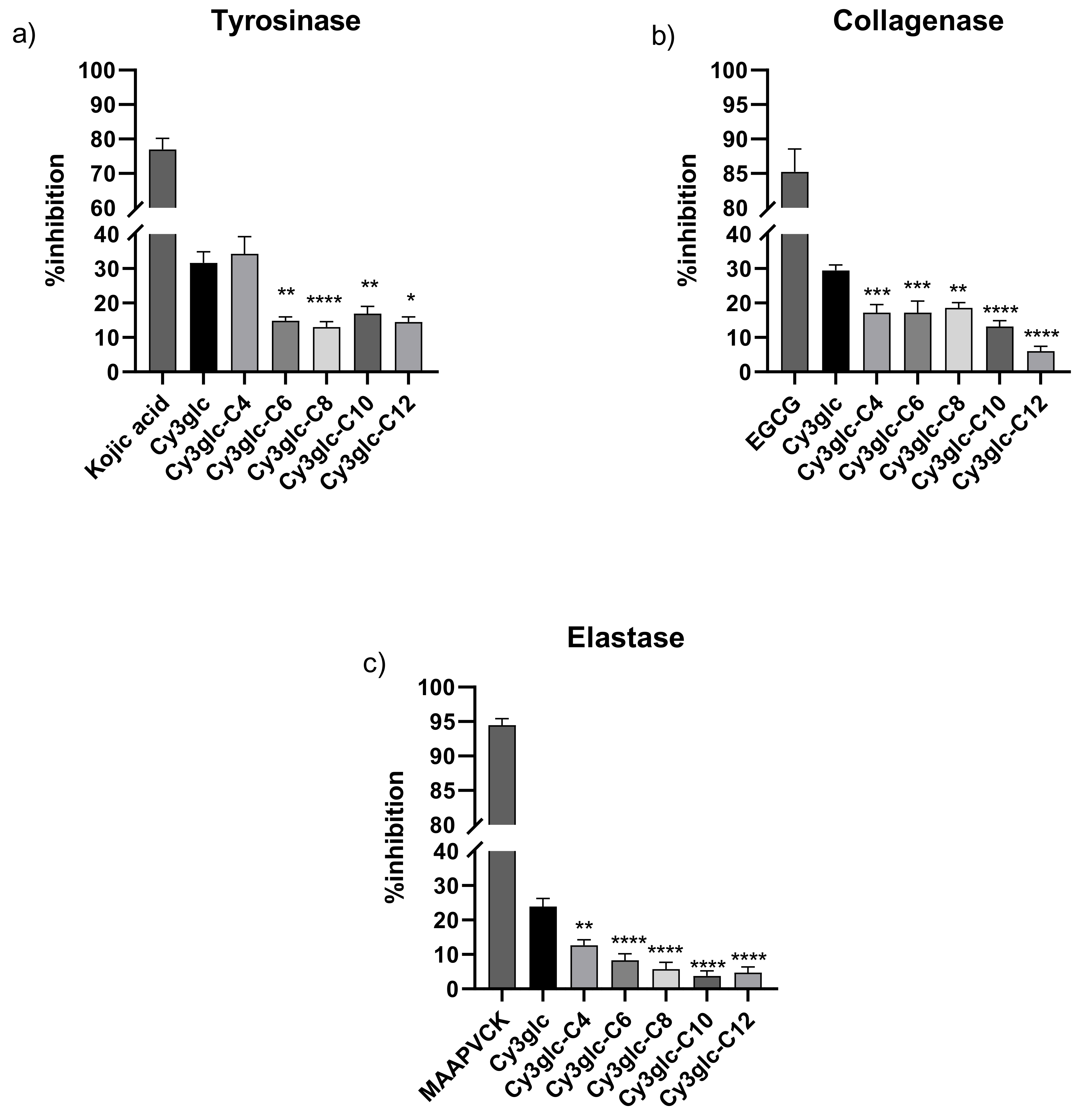
3.4. Enzymatic Activity
3.5. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Values
4. Discussion
5. Conclusions
Author Contributions
Funding

Acknowledgments
Conflicts of Interest
References
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wen, X.; Hao, D.; Zhang, N.; He, G.; Jiang, X. NF-κB signaling in skin aging. Mech. Ageing Dev. 2019, 184, 111–160. [Google Scholar] [CrossRef] [PubMed]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin From Solar Radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef]
- Evora, A.; de Freitas, V.; Mateus, N.; Fernandes, I. The effect of anthocyanins from red wine and blackberry on the integrity of a keratinocyte model using ECIS. Food Funct. 2017, 8, 3989–3998. [Google Scholar] [CrossRef]
- Nanashima, N.; Horie, K.; Maeda, H.; Tomisawa, T.; Kitajima, M.; Nakamura, T. Blackcurrant Anthocyanins Increase the Levels of Collagen, Elastin, and Hyaluronic Acid in Human Skin Fibroblasts and Ovariectomized Rats. Nutrients 2018, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Basílio, N.; Pina, F.; Fernandes, I.; De Freitas, V.; Mateus, N. Purple-Fleshed sweet potato acylated anthocyanins: Equilibrium network and photophysical properties. Food Chem. 2019, 288, 386–394. [Google Scholar] [CrossRef]
- Mattarei, A.; Biasutto, L.; Rastrelli, F.; Garbisa, S.; Marotta, E.; Zoratti, M.; Paradisi, C. Regioselective O-derivatization of quercetin via ester intermediates. An improved synthesis of rhamnetin and development of a new mitochondriotropic derivative. Molecules 2010, 15, 4722–4736. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Fernandes, V.C.; Araújo, P.; Mateus, N.; De Freitas, V. Synthesis, characterisation and antioxidant features of procyanidin B4 and malvidin-3-glucoside stearic acid derivatives. Food Chem. 2015, 174, 480–486. [Google Scholar] [CrossRef]
- Cruz, L.; Guimarães, M.; Araújo, P.; Évora, A.; de Freitas, V.; Mateus, N. Malvidin 3-Glucoside–Fatty Acid Conjugates: From Hydrophilic toward Novel Lipophilic Derivatives. J. Agric. Food. Chem. 2017, 65, 6513–6518. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Baréa, B.; Figueroa-Espinoza, M.C. Lipophilization and MS characterization of the main anthocyanins purified from hibiscus flowers. Food Chem. 2017, 230, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kortesniemi, M.; Yang, B.; Zheng, J. Enzymatic acylation of anthocyanins isolated from alpine bearberry (Arctostaphylos alpina) and lipophilic properties, thermostability, and antioxidant capacity of the derivatives. J. Agric. Food Chem. 2018, 66, 2909–2916. [Google Scholar] [CrossRef]
- Cruz, L.; Benohoud, M.; Rayner, C.M.; Mateus, N.; de Freitas, V.; Blackburn, R.S. Selective enzymatic lipophilization of anthocyanin glucosides from blackcurrant (Ribes nigrum L.) skin extract and characterization of esterified anthocyanins. Food Chem. 2018, 266, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.; Pérez-Gregorio, M.; Mateus, N.; de Freitas, V.; Galinha, C.F.; Crespo, J.G.; Portugal, C.A.M.; Cruz, L. An efficient method for anthocyanins lipophilization based on enzyme retention in membrane systems. Food Chem. 2019, 300, 125167. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Aulis, F.; Torres, A.; Sanchez-Mendoza, E.; Cruz, L.; Navarro-Ocana, A. New acylated cyanidin glycosides extracted from underutilized potential sources: Enzymatic synthesis, antioxidant activity and thermostability. Food Chem. 2020, 309, 125796. [Google Scholar] [CrossRef]
- Cruz, L.; Fernandes, I.; Guimaraes, M.; de Freitas, V.; Mateus, N. Enzymatic synthesis, structural characterization and antioxidant capacity assessment of a new lipophilic malvidin-3-glucoside-oleic acid conjugate. Food Funct. 2016, 7, 2754–2762. [Google Scholar] [CrossRef]
- Guimarães, M.; Mateus, N.; de Freitas, V.; Branco, L.C.; Cruz, L. Microwave-Assisted Synthesis and Ionic Liquids: Green and Sustainable Alternatives toward Enzymatic Lipophilization of Anthocyanin Monoglucosides. J. Agric. Food. Chem. 2020, 68, 7387–7392. [Google Scholar] [CrossRef]
- Mendoza, J.; Pina, F.; Basílio, N.; Guimarães, M.; De Freitas, V.; Cruz, L. Extending the stability of red and blue colors of malvidin-3-glucoside-lipophilic derivatives in the presence of SDS micelles. Dyes Pigment. 2018, 151, 321–326. [Google Scholar] [CrossRef]
- Guimarães, M.; Mateus, N.; de Freitas, V.; Cruz, L. Improvement of the Color Stability of Cyanidin-3-glucoside by Fatty Acid Enzymatic Acylation. J. Agric. Food. Chem. 2018, 66, 10003–10010. [Google Scholar] [CrossRef]
- Marquez-Rodriguez, A.S.; Guimarães, M.; Mateus, N.; de Freitas, V.; Ballinas-Casarrubias, M.L.; Fuentes-Montero, M.E.; Salas, E.; Cruz, L. Disaccharide anthocyanin delphinidin 3-O-sambubioside from Hibiscus sabdariffa L.: Candida antarctica lipase B-catalyzed fatty acid acylation and study of its color properties. Food Chem. 2021, 344, 128603. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. M07-A10:Methods for Dilution of Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—10th Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; Volume 35. [Google Scholar]
- Bessa, L.J.; Eaton, P.; Dematei, A.; Placido, A.; Vale, N.; Gomes, P.; Delerue-Matos, C.; Sa Leite, J.R.; Gameiro, P. Synergistic and antibiofilm properties of ocellatin peptides against multidrug-resistant Pseudomonas aeruginosa. Future Microbiol. 2018, 13, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.; Yoda, T.; Chahal, B.; Morita, M.; Tkagi, M.; Vestergaard, M. Structure-dependent interactions of polyphenols with a biomimetic membrane system. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 2670–2677. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.; Tran, K.; Reddy, S.; Malik, A. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Vercauteren, J. Compositions Comprising Stilbene Polyphenol Derivatives and Use Thereof for Combating the Ageing of Living Organisms and Diseases Affecting Same. U.S. Patent 2,0100,310,615 12 September 2007. [Google Scholar]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Santos, J.; La, V.D.; Bergeron, C.; Grenier, D. Inhibition of host- and bacteria-derived proteinases by natural anthocyanins. J. Periodontal Res. 2011, 46, 550–557. [Google Scholar] [CrossRef]
- Afaq, F.; Zaid, M.A.; Khan, N.; Dreher, M.; Mukhtar, H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009, 18, 553–561. [Google Scholar] [CrossRef]
- Xue, Y.-L.; Miyakawa, T.; Hayashi, Y.; Okamoto, K.; Hu, F.; Mitani, N.; Furihata, K.; Sawano, Y.; Tanokura, M. Isolation and tyrosinase inhibitory effects of polyphenols from the leaves of persimmon, Diospyros kaki. J. Agric. Food. Chem. 2011, 59, 6011–6017. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Biswas, R.; Sharma, A.; Banerjee, S.; Biswas, S.; Katiyar, C.K. Validation of medicinal herbs for anti-tyrosinase potential. J. Herbal Med. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- Tsuda, T.; Osawa, T. Inhibition of Tyrosinase Activity by the Anthocyanin Pigments Isolated from Phaseolus vulgaris L. Food Sci. Technol. Int. 1997, 3, 82–83. [Google Scholar] [CrossRef]
- Melzig, M.F.; Loser, B.; Ciesielski, S. Inhibition of neutrophil elastase activity by phenolic compounds from plants. Die Pharm. 2001, 56, 967–970. [Google Scholar]
- Lim, W.-C.; Kim, H.; Kim, Y.-J.; Jeon, B.-N.; Kang, H.-B.; Ko, H. Catechol inhibits epidermal growth factor-induced epithelial-to-mesenchymal transition and stem cell-like properties in hepatocellular carcinoma cells. Sci. Rep. 2020, 10, 7620. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, C.G.; Garbois, G.D.; Amaral, L.M.; Diniz, C.C.; Le Hyaric, M. Relationship between structure and antibacterial activity of lipophilic N-acyldiamines. Biomed. Pharmacother. 2010, 64, 287–290. [Google Scholar] [CrossRef]
- Richter, M.F.; Hergenrother, P.J. The challenge of converting Gram-positive-only compounds into broad-spectrum antibiotics. Ann. N. Y. Acad. Sci. 2019, 1435, 18–38. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of Specialized Biomolecules against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol 2018, 4, 482–501. [Google Scholar] [CrossRef]


| Compound | P. aeruginosa ATCC 27853 | E. coli ATCC 25922 | S. aureus ATCC 29213 | E. faecalis ATCC 29212 |
|---|---|---|---|---|
| MIC (MBC) | ||||
| Cy3glc | >512 (-) | >512 (-) | >512 (-) | >512 (-) |
| Cy3glc-C4 | >512 (-) | >512 (-) | >512 (-) | >512 (-) |
| Cy3glc-C6 | >512 (-) | 128 (128) | 8 (16) | 4 (>32) |
| Cy3glc-C8 | 256 (256) | 32 (32) | 2 (4) | 2 (16) |
| Cy3glc-C10 | 128 (128) | 16 (32) | 2 (2) | 1 (>8) |
| Cy3glc-C12 | >512 (-) | >512 (-) | >512 (-) | >512 (-) |
| Compound | P. aeruginosa PA004 | P. aeruginosa PA002 | MRSA Sa1 | MRSA S007 |
|---|---|---|---|---|
| MIC (MBC) | ||||
| Cy3glc | >512 (-) | >512 (-) | >512 (-) | >512 (-) |
| Cy3glc-C4 | >512 (-) | >512 (-) | >512 (-) | >512 (-) |
| Cy3glc-C6 | >512 (-) | >512 (-) | 16 (64) | 16 (64) |
| Cy3glc-C8 | 128 (128) | >512 (-) | 8 (32) | 8 (8) |
| Cy3glc-C10 | 64 (64) | 512 (512) | 8 (16) | 4 (8) |
| Cy3glc-C12 | >512 (-) | >512 (-) | >512 (-) | >512 (-) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, H.; Correia, P.; Bessa, L.J.; Guimarães, M.; Gameiro, P.; Freitas, V.d.; Mateus, N.; Cruz, L.; Fernandes, I. Cyanidin-3-glucoside Lipophilic Conjugates for Topical Application: Tuning the Antimicrobial Activities with Fatty Acid Chain Length. Processes 2021, 9, 340. https://doi.org/10.3390/pr9020340
Oliveira H, Correia P, Bessa LJ, Guimarães M, Gameiro P, Freitas Vd, Mateus N, Cruz L, Fernandes I. Cyanidin-3-glucoside Lipophilic Conjugates for Topical Application: Tuning the Antimicrobial Activities with Fatty Acid Chain Length. Processes. 2021; 9(2):340. https://doi.org/10.3390/pr9020340
Chicago/Turabian StyleOliveira, Hélder, Patrícia Correia, Lucinda J. Bessa, Marta Guimarães, Paula Gameiro, Victor de Freitas, Nuno Mateus, Luís Cruz, and Iva Fernandes. 2021. "Cyanidin-3-glucoside Lipophilic Conjugates for Topical Application: Tuning the Antimicrobial Activities with Fatty Acid Chain Length" Processes 9, no. 2: 340. https://doi.org/10.3390/pr9020340
APA StyleOliveira, H., Correia, P., Bessa, L. J., Guimarães, M., Gameiro, P., Freitas, V. d., Mateus, N., Cruz, L., & Fernandes, I. (2021). Cyanidin-3-glucoside Lipophilic Conjugates for Topical Application: Tuning the Antimicrobial Activities with Fatty Acid Chain Length. Processes, 9(2), 340. https://doi.org/10.3390/pr9020340













