A Review on the Synthesis, Characterization, and Modeling of Polymer Grafting
Abstract
:1. Introduction
2. Chemistry of Polymer Grafting
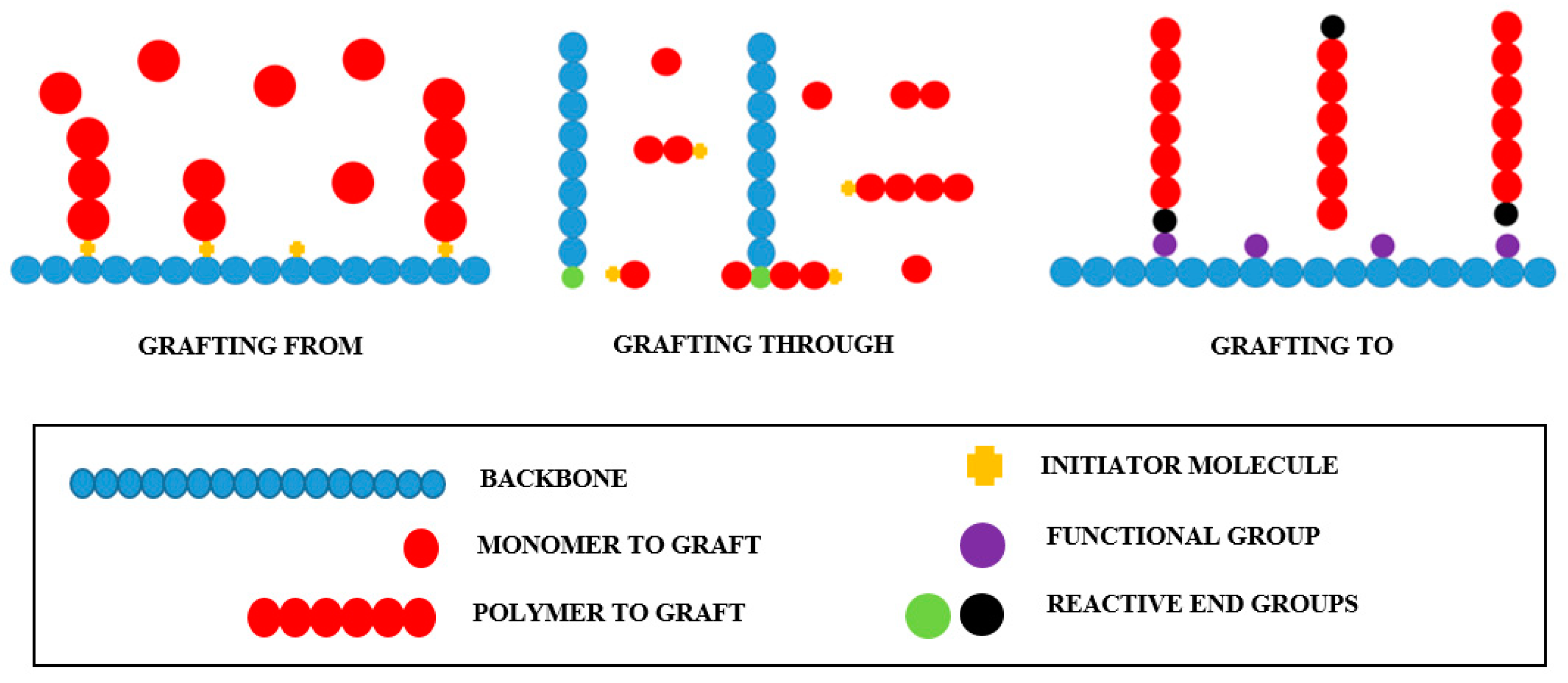
2.1. Types of Polymer Grafting
2.2. Main Backbones Used in Polymer Grafting
2.3. Backbone Functionalization Methods
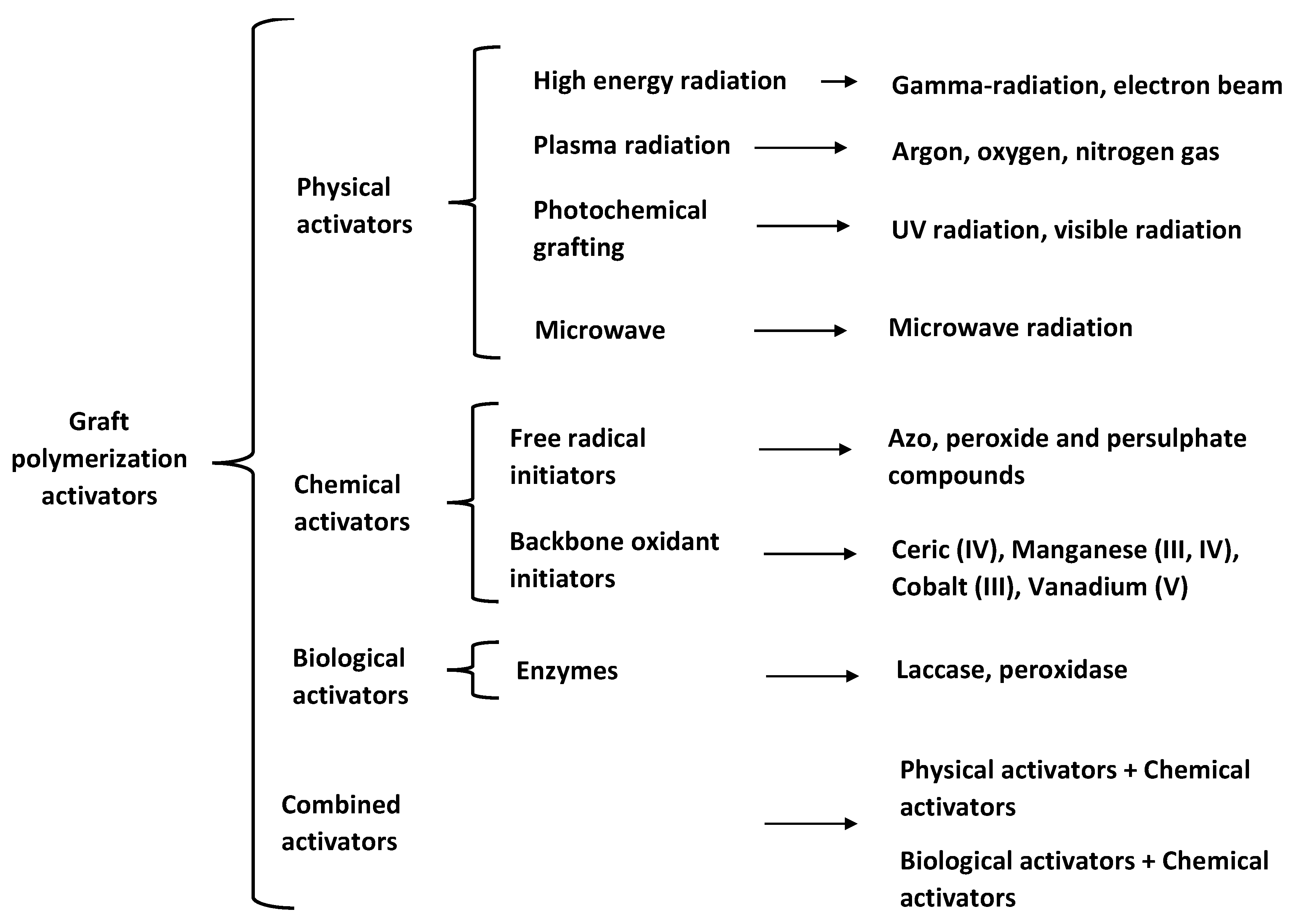
2.4. Backbone Activation Methods
2.4.1. Physical Activators
2.4.2. Chemical Activators
2.4.3. Biological Activators
2.4.4. Combined Activators
2.5. Polymer Grafting by Free-Radical Polymerization
3. Backbones and Supports Used in Polymer Grafting
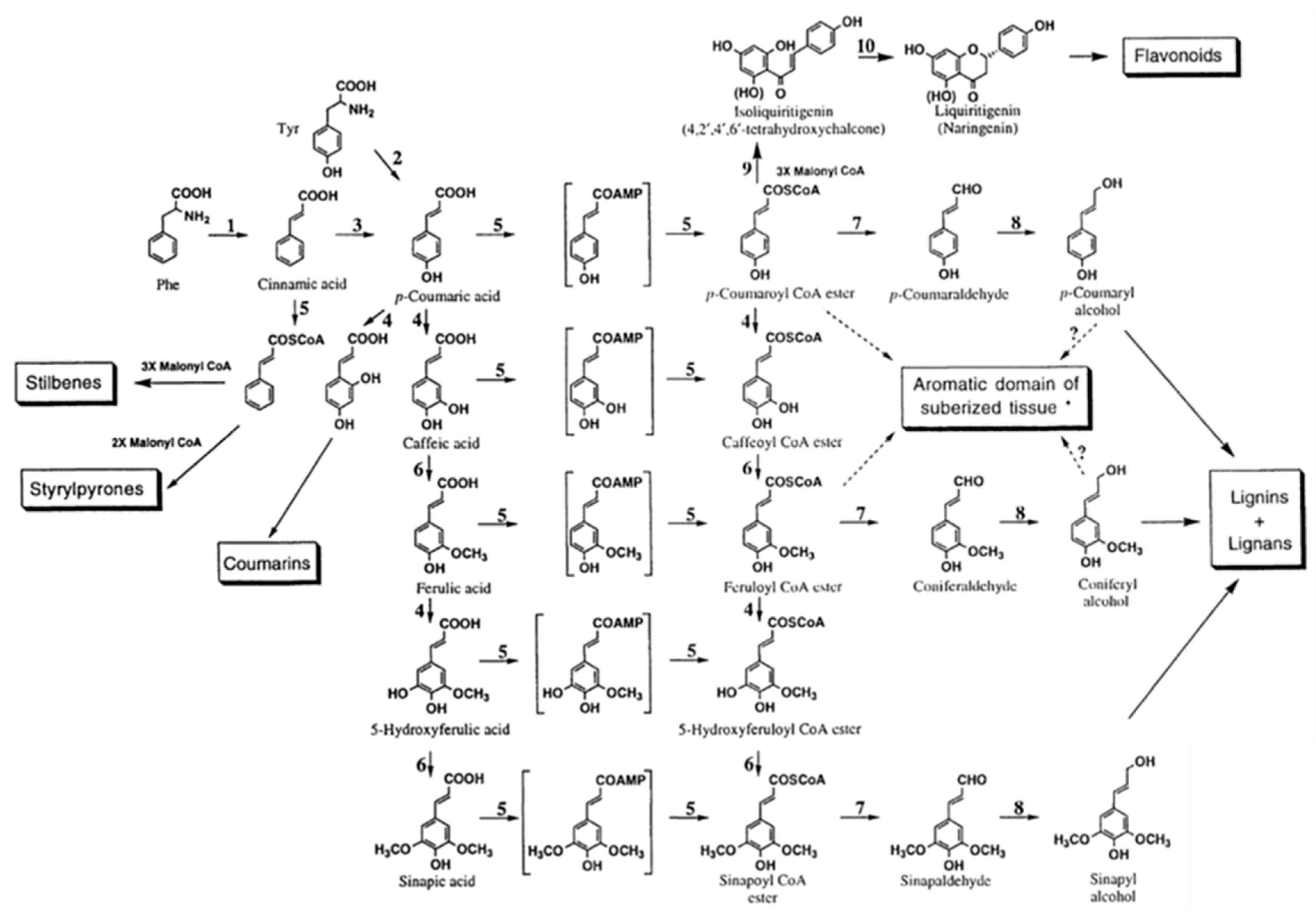
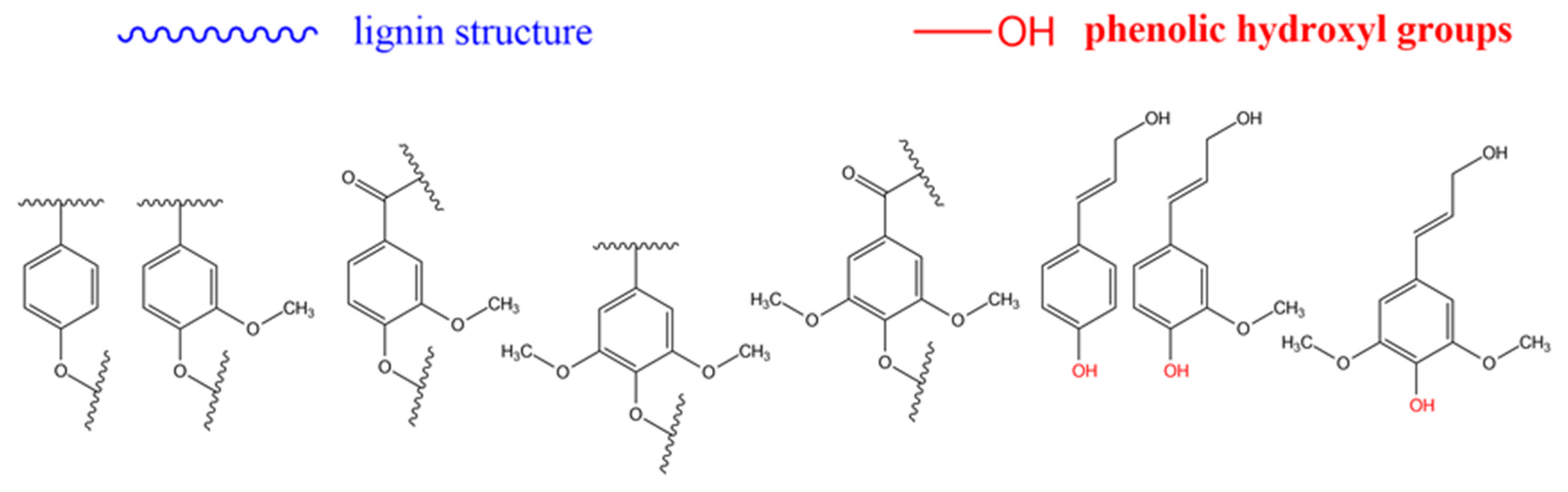
3.1. Cellulose, Lignin, and Lignocellulosic Biomasses as Backbones
3.2. Polymer Backbones
4. Characterization Techniques Used for Polymer Grafted Materials
5. Modeling of Polymer Grafting
5.1. Literature review on Modeling of Polymer Grafting
5.2. Modeling of Polymer Branching and Crosslinking
5.3. Main Modeling Equations for Polymer Grafting
6. Nomenclature, Symbols, Abbreviations, and Chemical Structures
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hadjichristidis, N.; Pitsikalis, M.; Iatrou, H.; Driva, P.; Chatzichris, M.; Sakellariou, G.; Lohse, D. Graft copolymers. In Encyclopedia of Polymer Science and Technology, 2nd ed.; Matyjaszewski, K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 1–38. ISBN 978-047-144-026-0. [Google Scholar]
- Slagman, S.; Zuilhof, H.; Franssen, M.C.R. Laccase-Mediated Grafting on Biopolymers and Synthetic Polymers: A Critical Review. ChemBioChem 2017, 19, 288–311. [Google Scholar] [CrossRef] [Green Version]
- Stannett, V.T. Block and graft copolymerization. In Journal of Polymer Science: Polymer Letters, 1st ed.; Ceresa, R.J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1973; Volume 1, pp. 669–670. [Google Scholar] [CrossRef]
- Meier, D.J. Theory of block copolymers. I. Domain formation in A-B block copolymers. J. Polym. Sci. C Polym. Symp. 1969, 26, 81–98. [Google Scholar] [CrossRef]
- Helfand, E.; Block Copolymer Theory. III. Statistical Mechanics of the Microdomain Structure. Macromolecules 1975, 8, 552–556. [Google Scholar] [CrossRef]
- Helfand, E.; Wasserman, Z.R. Block Copolymer Theory. 4. Narrow Interphase Approximation. Macromolecules 1976, 9, 879–888. [Google Scholar] [CrossRef]
- Helfand, E. Block copolymers, polymer-polymer interfaces, and the theory of inhomogeneous polymers. Acc. Chem. Res. 1975, 8, 295–299. [Google Scholar] [CrossRef]
- Blanchette, J.A.; Nielsen, L.E. Characterization of graft polymers. J. Polym. Sci. 1956, 20, 317–326. [Google Scholar] [CrossRef]
- Merret, F.M. Graft polymers with preset molecular configurations. J. Polym. Sci. 1957, 24, 467–477. [Google Scholar] [CrossRef]
- Gluckman, M.S.; Kampf, M.J.; O’brien, L.J.; Fox, T.G.; Graham, R.K. Graft copolymers from polymers having pendant mercaptan groups. II. Synthesis and characterization. J. Polym. Sci. 1959, 37, 411–423. [Google Scholar] [CrossRef]
- Miller, M.L. Block and graft polymers I. Graft polymers from acrylamide and acrylonitrile. Can. J. Chem. 1957, 36, 303–308. [Google Scholar] [CrossRef]
- Beevers, R.B.; White, E.F.T.; Brown, L. Physical properties of vinyl polymers. Part 3.—X-ray scattering in block, random and graft methyl methacrylate + acrylonitrile copolymers. Trans. Faraday Soc. 1960, 56, 1535–1541. [Google Scholar] [CrossRef]
- Oster, G.; Oster, G.K.; Moroson, H. Ultraviolet induced crosslinking and grafting of solid high polymers. J. Polym. Sci. 1959, XXXIV, 671–684. [Google Scholar] [CrossRef]
- Kobayashi, Y. Gamma-ray–induced graft copolymerization of styrene onto cellulose and some chemical properties of the grafted polymer. J. Polym. Sci. 1961, 51, 359–372. [Google Scholar] [CrossRef]
- Bridgeford, D.J. Catalytic Deposition and Grafting of Olefin Polymers into Cellulosic Materials. Ind. Eng. Chem. Prod. Res. Dev. 1962, 1, 45–52. [Google Scholar] [CrossRef]
- Huang, R.Y.-M.; Immergut, B.; Immergut, E.H.; Rapson, W.H. Grafting vinyl polymers onto cellulose by high energy radiation. I. High energy radiation-induced graft copolymerization of styrene onto cellulose. J. Polym. Sci. A Gen. Pap. 1963, 1, 1257–1270. [Google Scholar] [CrossRef]
- McManus, N.; Zhu, S.-H.; Tzoganakis, C.; Penlidis, A. Grafting of ethylene-ethyl acrylate-maleic anhydride terpolymer with amino-terminated polydimethylsiloxane during reactive processing. J. Appl. Polym. Sci. 2006, 101, 4230–4237. [Google Scholar] [CrossRef]
- Cadena, L.-E.; Gauthier, M. Phase-segregated dendrigraft copolymer architectures. Polymers 2010, 2, 596–622. [Google Scholar] [CrossRef]
- Aridi, T.; Gauthier, M. Chapter 6. Arborescent polymers with a mesoscopic scale. In Complex Macromolecular Architectures: Synthesis, Characterization, and Self-Assembly, 1st ed.; Hadjichristidis, N., Hirao, A., Tezuka, Y., Du Prez, F., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 169–194. ISBN 978-047-082-514-3. [Google Scholar]
- Moingeon, F.; Wu, Y.; Cadena-Sánchez, L.; Gauthier, M. Synthesis of arborescent styrene homopolymers and copolymers from epoxidized substrates. J. Polym. Sci. A Polym. Chem. 2012, 50, 1819–1826. [Google Scholar] [CrossRef]
- Whitton, G.; Gauthier, M. Arborescent polypeptides from γ-benzyl l -glutamic acid. J. Polym. Sci. A Polym. Chem. 2013, 51, 5270–5279. [Google Scholar] [CrossRef]
- Aridi, T.; Gauthier, M. Synthesis of arborescent polymers by click grafting. Mater. Res. Soc. Symp. Proc. 2014, 1613, 23–31. [Google Scholar] [CrossRef]
- Dockendorff, J.; Gauthier, M. Synthesis of arborescent polystyrene-g-[poly(2-vinylpyridine)-b- polystyrene] core-shell-corona copolymers. J. Polym. Sci. A Polym. Chem. 2014, 52, 1075–1085. [Google Scholar] [CrossRef]
- Whitton, G.; Gauthier, M. Arborescent micelles: Dendritic poly(γ-benzyl l -glutamate) cores grafted with hydrophilic chain segments. J. Polym. Sci. A Polym. Chem. 2016, 54, 1197–1209. [Google Scholar] [CrossRef]
- Gauthier, M.; Whitton, G. Arborescent unimolecular micelles: Poly(γ-benzyl L-glutamate) core grafted with a hydrophilic shell by copper(I)-catalyzed azide-alkyne cycloaddition coupling. Polymers 2017, 9, 540. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, M.; Aridi, T. Synthesis of arborescent polystyrene by “click” grafting. J. Polym. Sci. A Polym. Chem. 2019, 57, 1730–1740. [Google Scholar] [CrossRef]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef] [PubMed]
- Wohlhauser, S.; Delepierre, G.; Labet, M.; Morandi, G.; Thielemans, W.; Weder, C.; Zoppe, J.O. Grafting Polymers from Cellulose Nanocrystals: Synthesis, Properties, and Applications. Macromolecules 2018, 51, 6157–6189. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.W.; Hudson, S.M. Review of vinyl graft copolymerization featuring recent advances toward controlled radical-based reactions and illustrated with chitin/chitosan trunk polymers. Chem. Rev. 2001, 101, 3245–3274. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K. Recent Advances in Graft Copolymerization and Applications of Chitosan: A Review. ACS Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Kaur, L.; Gupta, G.D. A review on microwave assisted grafting of polymers. Int. J. Pharm. Sci. Res. 2017, 8, 422–426. [Google Scholar] [CrossRef]
- Brodin, M.; Vallejos, M.; Opedal, M.T.; Area, M.C.; Chinga-Carrasco, G. Lignocellulosics as sustainable resources for production of bioplastics—A review. J. Clean. Prod. 2017, 162, 646–664. [Google Scholar] [CrossRef]
- Niphadkar, S.; Bagade, P.; Ahmed, S. Bioethanol production: Insight into past, present and future perspectives. Biofuels 2018, 9, 229–238. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef]
- Neuling, U.; Kaltschmitt, M. Review of Biofuel Production—Feedstock, Processes and Markets. J. Oil Palm Res. 2019, 29, 137–167. [Google Scholar] [CrossRef] [Green Version]
- Vega-Hernández, M.Á.; Rosas-Aburto, A.; Vivaldo-Lima, E.; Vázquez-Torres, H.; Cano-Díaz, G.S.; Pérez-Salinas, P.; Hernández-Luna, M.G.; Alcaraz-Cienfuegos, J.; Zolotukhin, M.G. Development of polystyrene composites based on blue agave bagasse by in situ RAFT polymerization. J. Appl. Polym. Sci. 2019, 136, 47089. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.; Ayoub, A.; Prochazka, F.; Fernández-de-Alba, C.; Mignard, N.; Taha, M.; Becquart, F. Towards thermoplastic hemicellulose: Chemistry and characteristics of poly-(ε-caprolactone) grafting onto hemicellulose backbones. Mater. Des. 2018, 153, 298–307. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, Z.; Xu, X.; Liu, X.; Liu, L.; Huang, G.; Liu, L.; Wang, H.; Song, P. Grafting Lignin with Bioderived Polyacrylates for Low-Cost, Ductile, and Fully Biobased Poly(lactic acid) Composites. ACS Sustain. Chem. Eng. 2020, 8, 2267–2276. [Google Scholar] [CrossRef]
- Fox, T.G.; Gluckman, M.S.; Gornick, F.; Graham, R.K.; Gratch, S. Graft copolymers from polymers having pendant mercaptan groups. I. Kinetic considerations. J. Polym. Sci. 1959, XXXVII, 397–409. [Google Scholar] [CrossRef]
- Zimmerman, J. Molecular weight distributions of vinyl polymers grafted to a solid polymeric substrate by irradiation (theoretical). J. Polym. Sci. 1960, XLIV, 107–116. [Google Scholar] [CrossRef]
- Hernández-Ortiz, J.C.; Van Steenberge, P.H.M.; Duchateau, J.N.E.; Toloza, C.; Schreurs, F.; Reyniers, M.-F.; Marin, G.B.; D’hooge, D.R. A two-phase stochastic model to describe mass transport and kinetics during reactive processing of polyolefins. Chem. Eng. J. 2019, 377, 119980. [Google Scholar] [CrossRef]
- Gandhi, A.; Verma, S.; Imam, S.S.; Vyas, M. A review on techniques for grafting of natural polymers and their applications. Plant Arch 2019, 19, 972–978. [Google Scholar]
- Wei, L.; McDonald, A.G. A Review on Grafting of Biofibers for Biocomposites. Materials 2016, 9, 303. [Google Scholar] [CrossRef]
- De Jesús Muñoz Prieto, E.; Rivas, B.; Sánchez, J. Natural polymer grafted with syntethic monomer by microwave for water treatment—A review. Cienc. Desarro. 2012, 4, 219–240. [Google Scholar] [CrossRef] [Green Version]
- Barsbay, M.; Güven, O. A short review of radiation-induced raft-mediated graft copolymerization: A powerful combination for modifying the surface properties of polymers in a controlled manner. Radiat. Phys. Chem. 2009, 78, 1054–1059. [Google Scholar] [CrossRef]
- Francis, R.; Joy, N.; Aparna, E.P.; Vijayan, R. Polymer Grafted Inorganic Nanoparticles, Preparation, Properties, and Applications: A Review. Polym. Rev. 2014, 54, 268–347. [Google Scholar] [CrossRef]
- Garcia-Valdez, O.; Champagne, P.; Cunningham, M.F. Graft modification of natural polysaccharides via reversible deactivation radical polymerization. Prog. Polym. Sci. 2018, 76, 151–173. [Google Scholar] [CrossRef]
- Sun, H.; Yang, L.; Thompson, M.P.; Schara, S.; Cao, W.; Choi, W.C.; Hu, Z.; Zang, N.; Tan, W.; Gianneschi, N.C. Recent Advances in Amphiphilic Polymer–Oligonucleotide Nanomaterials via Living/Controlled Polymerization Technologies. Bioconjugate Chem. 2019, 30, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.C. Synthesis of functional polyolefin copolymers with graft and block structures. Prog. Polym. Sci. 2002, 27, 39–85. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, Y.; Li, X.; Liu, X.; Yeung, K.W.K.; Wu, S.; Wang, X.; Cui, Z.; Yang, X.; Chu, P.K. Surface functionalization of biomaterials by radical polymerization. Prog. Mater. Sci. 2016, 83, 191–235. [Google Scholar] [CrossRef]
- Ayyavoo, J.; Nguyen, T.P.N.; Jun, B.-M.; Kim, I.-C.; Kwon, Y.N. Protection of polymeric membranes with antifouling surfacing via surface modifications. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 190–201. [Google Scholar] [CrossRef]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Sun, H.; Choi, W.; Zang, N.; Battistella, C.; Thompson, M.P.; Cao, W.; Zhou, X.; Forman, C.; Gianneschi, N.C. Bioactive Peptide Brush Polymers via Photoinduced Reversible-Deactivation Radical Polymerization. Angew. Chem. Int. Ed. 2019, 58, 17359–17364. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Grafted cellulose: A bio-based polymer for durable applications. Polym. Bull. 2018, 75, 2213–2242. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, A. Lignin. Biosynthesis and Transformation for Industrial Applications; Springer Series on Polymer and Composite Materials; Springer Nature: Cham, Switzerland, 2020; pp. 1–252. ISBN 978-303-040-663-9. [Google Scholar]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Argüelles-Monal, W.M.; Lizardi-Mendoza, J.; Fernández-Quiroz, D.; Recillas-Mota, M.T.; Montiel-Herrera, M. Chitosan Derivatives: Inducing new functionalities with a controlled molecular architecture for innovative materials. Polymers 2018, 10, 342. [Google Scholar] [CrossRef] [Green Version]
- Kurita, K. Controlled functionalization of the polysaccharide chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Lele, V.V.; Kumari, S.; Niju, H. Syntheses, characterization and applications of graft copolymers of sago starch. Starch 2018, 70, 1700133. [Google Scholar] [CrossRef]
- Radhakrishnan, B.; Ranjan, R.; Brittain, W.J. Surface initiated polymerization from silica nanoparticles. Soft Matter 2006, 2, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.C.; Radzinsky, S.C.; Matson, J.B. Graft polymer synthesis by RAFT transfer-to. J. Polym. Sci. A Polym. Chem. 2017, 55, 2865–2876. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A. Radiation and industrial polymers. Prog. Polym. Sci. 2000, 25, 371–401. [Google Scholar] [CrossRef]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef] [Green Version]
- Ngo, T.H.A.; Tran, D.T.; Dinh, C.H. Surface photochemical graft polymerization of acrylic acid onto polyamide thin film composite membranes. J. Appl. Polym. Sci. 2017, 134, 44418. [Google Scholar] [CrossRef]
- Singh, V.; Tiwari, A.; Tripathi, D.N.; Sanghi, R. Microwave assisted synthesis of guar-g-polyacrylamide. Carbohydr. Polym. 2004, 58, 1–6. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Misra, B.N. Grafting: A versatile means to modify polymers technics, factors and applications. Prog. Polym. Sci. 2004, 29, 767–814. [Google Scholar] [CrossRef]
- Sosnik, A.; Gotelli, G.; Abraham, G.A. Microwave-assisted polymer synthesis (MAPS) as a tool in biomaterials science: How new and how powerful. Prog. Polym. Sci. 2011, 36, 1050–1078. [Google Scholar] [CrossRef]
- Kumar, D.; Pandey, J.; Raj, V.; Kumar, P. A review on the modification of polysaccharide through graft copolymerization for various potential applications. Open Med. Chem. J. 2017, 11, 109–126. [Google Scholar] [CrossRef]
- Fan, G.; Zhao, J.; Zhang, Y.; Guo, Z. Grafting modification of kevlar fiber using horseradish peroxidase. Polym. Bull. 2006, 56, 507–515. [Google Scholar] [CrossRef]
- Cannatelli, M.D.; Ragauskas, A.J. Conversion of lignin into value-added materials and chemicals via laccase-assisted copolymerization. Appl. Microbiol. Biotechnol. 2016, 100, 8685–8691. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Wu, L.; Zhang, Z.; Xu, T. Atom transfer radical polymerization (ATRP): A versatile and forceful tool for functional membranes. Prog. Polym. Sci. 2014, 39, 124–144. [Google Scholar] [CrossRef]
- Crawford, D.E. Extrusion-back to the future: Using an established technique to reform automated chemical synthesis. Beilstein J. Org. Chem. 2017, 13, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Moad, G. The synthesis of polyolefin graft copolymers by reactive extrusion. Prog. Polym. Sci. 1999, 24, 81–142. [Google Scholar] [CrossRef]
- Moad, G. Chemical modification of starch by reactive extrusion. Prog. Polym. Sci. 2011, 36, 218–237. [Google Scholar] [CrossRef]
- Russell, K.E. Free radical graft polymerization and copolimerization at higher temperatures. Prog. Polym. Sci. 2002, 27, 1007–1038. [Google Scholar] [CrossRef]
- Monties, B. Les Polymères Végétaux: Polymères Pariétaux et Alimentaires non Azotés, 1st ed.; Gauthier-Villars: Paris, France, 1980; ISBN 978-204-010-480-1. [Google Scholar]
- Casarrubias-Cervantes, R.A. Análisis Fisicoquímico de Procesos de Pretratamiento de Materiales Lignocelulósicos para su Uso en Polímeros Conductores. Bachelor Degree, Facultad de Química—Universidad Nacional Autónoma de México, Ciudad Universitaria, CDMX, 2019, Biblioteca Digital UNAM. Available online: http://132.248.9.195/ptd2019/abril/0788572/Index.html (accessed on 25 May 2020).
- Koshijima, T.; Muraki, E. Radiation Grafting of Methyl Methacrylate onto Lignin. J. Jpn. Wood Res. Soc. 1964, 10, 110–115. [Google Scholar]
- Koshijima, T.; Muraki, E. Degradation of Lignin-Methyl Metacrylate Graft Copolymer by γ-Ray Irradiation. J. Jpn. Wood Res. Soc. 1964, 10, 116–119. [Google Scholar]
- Koshijima, T.; Timell, T.E. Factors Affecting Number Average Molecular Weights Determination of Hardwood Xylan. J. Jpn. Wood Res. Soc. 1966, 12, 166–172. [Google Scholar]
- Koshijima, T.; Muraki, E. Solvent Effects upon Radiation-Induced Graft-copolymerization of Styrene onto Lignin. J. Jpn. Wood Res. Soc. 1966, 12, 139. [Google Scholar]
- Koshijima, T. Oxidation of Lignin-Styrene Graft polymer. J. Jpn. Wood Res. Soc. 1966, 12, 114. [Google Scholar]
- Koshijima, T.; Muraki, E. Radial Grafting on Lignin (II). Grafting of styrene into Lignin by Initiators. J. Jpn. Wood Res. Soc. 1967, 13, 355–358. [Google Scholar]
- Koshijima, T.; Muraki, E.; Naito, K.; Adachi, K. Radical Grafting on Lignin. IV. Semi-Conductive Properties of Lignin-Styrene Graftpolymer. J. Jpn. Wood Res. Soc. 1968, 14, 52–54. [Google Scholar]
- Meister, J.J. Modification of Lignin. J. Macromol. Sci. Polymer Rev. 2002, 42, 235–289. [Google Scholar] [CrossRef]
- Hon, D.N.S. Chemical Modification of Lignocellulosic Materials, 1st ed.; Marcel Dekker, Inc.: New York, NY, USA, 1996; ISBN 978-082-479-472-9. [Google Scholar]
- McDowall, D.J.; Gupta, B.S.; Stannett, V.T. Grafting of vinyl monomers to cellulose by ceric ion initiation. Prog. Polym. Sci. 1984, 10, 1–50. [Google Scholar] [CrossRef]
- Bhattacharyya, S.N.; Maldas, D. Graft copolymerization onto cellulosics. Prog. Polym. Sci. 1984, 10, 171–270. [Google Scholar] [CrossRef]
- Hon, D.N.S. Graft Copolymerization of Lignocellulosic Fibers; ACS Symposium Series 187; American Chemical Society: Washington, DC, USA, 1982; ISBN 978-084-120-721-9. [Google Scholar]
- Mansour, O.Y.; Nagaty, A. Grafting of synthetic polymers to natural polymers by chemical processes. Prog. Polym. Sci. 1985, 11, 91–165. [Google Scholar] [CrossRef]
- Feldman, D.; Lacasse, M.; Bernaczuk, L.M. Lignin-polymer sytems and some applications. Prog. Polym. Sci. 1986, 12, 271–299. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Möller, M. Celluloses and polyoses/hemicelluloses. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-008-087-862-1. [Google Scholar]
- Pantelakis, S.; Tserpes, K. Revolutionizing Aircraft Materials and Processes; Springer Nature AG: Cham, Switzerland, 2020; ISBN 978-303-035-346-9. [Google Scholar]
- Rol, F.; Belgacem, M.N.; Gandinia, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- Zahran, M.K.; Morsy, M.; Mahmoud, R. Grafting of acrylic monomers onto cotton fabric using an activated cellulose thiocarbonate–azobisisobutyronitrile redox system. J. Appl. Polym. Sci. 2003, 91, 1261–1274. [Google Scholar] [CrossRef]
- Chauhan, G.S.; Lal, H.; Sharma, R.; Sarwade, B.D. Grafting of a styrene–acrylonitrile binary monomer mixture onto cellulose extracted from pine needles. J. Appl. Polym. Sci. 2001, 83, 2000–2007. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mokhtar, S.M. Chemically induced graft copolymerization of itaconic acid onto cellulose fibers. Polym. Test. 2002, 21, 337–343. [Google Scholar] [CrossRef]
- Gupta, K.C.; Sahoo, S. Grafting of N,N ′-methylenebisacrylamide onto cellulose using Co(III)-acetylacetonate complex in aqueous medium. J. Appl. Polym. Sci. 2000, 76, 906–912. [Google Scholar] [CrossRef]
- Gupta, K.C.; Sahoo, S. Co(III) acetylacetonate-complex-initiated grafting of N-vinyl pyrrolidone on cellulose in aqueous media. J. Appl. Polym. Sci. 2001, 81, 2286–2296. [Google Scholar] [CrossRef]
- Gupta, K.C.; Khandekar, K. Temperature-Responsive Cellulose by Ceric(IV) Ion-Initiated Graft Copolymerization of N-Isopropylacrylamide. Biomacromolecules 2003, 4, 758–765. [Google Scholar] [CrossRef]
- Gupta, K.C.; Khandekar, K. Graft copolymerization of acrylamide–methylacrylate comonomers onto cellulose using ceric ammonium nitrate. J. Appl. Polym. Sci. 2002, 86, 2631–2642. [Google Scholar] [CrossRef]
- Gupta, K.C.; Khandekar, K. Graft copolymerization of acrylamide onto cellulose in presence of comonomer using ceric ammonium nitrate as initiator. J. Appl. Polym. Sci. 2006, 101, 2546–2558. [Google Scholar] [CrossRef]
- Gupta, K.C.; Sahoo, S. Graft Copolymerization of Acrylonitrile and Ethyl Methacrylate Comonomers on Cellulose Using Ceric Ions. Biomacromolecules 2001, 2, 239–247. [Google Scholar] [CrossRef]
- Gupta, K.C.; Sahoo, S.; Khandekar, K. Graft Copolymerization of Ethyl Acrylate onto Cellulose Using Ceric Ammonium Nitrate as Initiator in Aqueous Medium. Biomacromolecules 2002, 3, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.C.; Khandekar, K. Ceric(IV) ion-induced graft copolymerization of acrylamide and ethyl acrylate onto cellulose. Polym. Int. 2005, 55, 139–150. [Google Scholar] [CrossRef]
- Toledano-Thompson, T.; Loría-Bastarrachea, M.I.; Aguilar-Vega, M.J. Characterization of henequen cellulose microfibers treated with an epoxide and grafted with poly(acrylic acid). Carbohydr. Polym. 2005, 62, 67–73. [Google Scholar] [CrossRef]
- Mansour, O.Y.; Nagieb, Z.A.; Basta, A.H. Graft polymerization of some vinyl monomers onto alkali-treated cellulose. J. Appl. Polym. Sci. 1991, 43, 1147–1158. [Google Scholar] [CrossRef]
- Semsarilar, M.; Ladmiral, V.; Perrier, S. Synthesis of a cellulose supported chain transfer agent and its application to RAFT polymerization. J. Polym. Sci. A Polym. Chem. 2010, 48, 4361–4365. [Google Scholar] [CrossRef]
- Cankaya, N.; Temüz, M. Characterization and monomer reactivity ratios of grafted cellulose with n-(4-nitrophenyl)acrylamide and methyl methacrylate by atom transfer radical polymerization. Cell. Chem. Technol. 2012, 46, 551–558. [Google Scholar]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Rapid synthesis of graft copolymers from natural cellulose fibers. Carbohydr. Polym. 2013, 98, 820–828. [Google Scholar] [CrossRef]
- Routray, C.; Tosh, B. Graft copolymerization of methyl methacrylate (mma) onto cellulose acetate in homogeneous medium: Effect of solvent, initiator and homopolymer inhibitor. Cell. Chem. Technol. 2013, 47, 171–190. [Google Scholar]
- Cankaya, N.; Temüz, M.M. Monomer reactivity ratios of cellulose grafted with N-cyclohexylacrylamide and methyl methacrylate by atom transfer radical polymerization. Cell. Chem. Technol. 2014, 48, 209–215. [Google Scholar]
- Popescu, O.; Dunca, S.; Grigoriu, A. Antibacterial action of silver applied on cellulose fibers grafted with monochlorotriazinyl-β-cyclodextrin. Cell. Chem. Technol. 2013, 47, 247–255. [Google Scholar]
- Popescu, O.; Grigoriu, A.; Diaconescu, R.M.; Vasluianu, E. Optimization of the cellulosic materials functionalization with monochlorotriazinyl-β-cyclodextrin in basic medium. Ind. Textilá 2012, 63, 68–75. [Google Scholar]
- Sun, Z.; Chen, F. Homogeneous grafting copolymerization of methylmethacrylate onto cellulose using ammonium persulfate. Cell. Chem. Technol. 2014, 48, 217–223. [Google Scholar]
- Dai, L.; Shen, Y.; Li, D.; Xiao, S.; He, J. Cellulose-graft-poly(l-lactide) as a degradable drugdelivery system: Synthesis, degradation and drug release. Cell. Chem. Technol. 2014, 48, 237–245. [Google Scholar]
- Xiaoming, S.; Songlin, W.; Shanshan, G.; Fushan, C.; Fusheng, L. Study on grafting copolymerization of methyl methacrylate onto cellulose under heterogeneous conditions. Cell. Chem. Technol. 2016, 50, 65–70. [Google Scholar]
- Yin, Y.; Jiang, T.X.; Wang, H.; Gao, W. Modification of cellulose nanocrystal via SI-ATRP of styrene and themechanism of its reinforcement of polymethylmethacrylate. Carbohydr. Polym. 2016, 142, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Paula, E.L.; Roig, F.; Mas, A.; Habas, J.P.; Mano, V.; Vargas Pereira, F.; Robin, J.J. Effect of surface-grafted cellulose nanocrystals on the thermal and mechanical properties of PLLA based nanocomposites. Eur. Polym. J. 2016, 84, 173–187. [Google Scholar] [CrossRef]
- Zhao, C.; Li, J.; He, B.; Zhao, L. Fabrication of hydrophobic biocomposite by combining cellulosic fibers with polyhydroxyalkanoate. Cellulose 2017, 24, 2265–2274. [Google Scholar] [CrossRef]
- Badawy, S.M. Functional cellulosic filter papers prepared by radiation-induced graft copolymerization for chelation of rare earth elements. Cell. Chem. Technol. 2017, 51, 551–558. [Google Scholar]
- Çankaya, N.; Temüz, M.M.; Yakuphanoglu, F. Grafting of some monomers onto cellulose by atom transfer radical polymerization. Electrical conductivity and thermal properties of resulting copolymers. Cell. Chem. Technol. 2018, 52, 19–26. [Google Scholar]
- Müssig, J.; Kelch, M.; Gebert, B.; Hohe, J.; Luke, M.; Bahners, T. Improvement of the fatigue behaviour of cellulose/polyolefin composites using photo-chemical fibre surface modification bio-inspired by natural role models. Cellulose 2020, 27, 5815–5827. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Z.; Han, Y.; Yang, S.; Fan, D.; Li, G.; Wang, S. Combination of water soluble chemical grafting and gradient freezing to fabricate elasticity enhanced and anisotropic nanocellulose aerogels. Appl. Nanosci. 2020, 10, 411–419. [Google Scholar] [CrossRef]
- Eraghi Kazzaz, A.; Hosseinpour Feizi, Z.; Fatehi, P. Grafting strategies for hydroxy groups of lignin for producing materials. Green Chem. 2019, 21, 5714–5752. [Google Scholar] [CrossRef] [Green Version]
- Abe, A.; Dusek, K.; Kobayashi, S. Biopolymers. Lignin, Proteins, Bioactive Nanocomposites, 1st ed.; Springer: Heidelberg/Berlin, Germany, 2010; Volume 232, ISBN 978-364-213-630-6. [Google Scholar]
- Huang, J.; Fu, S.; Gan, L. Lignin Chemistry and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-012-813-963-9. [Google Scholar]
- Marton, J. Lignin. Structure and Reactions, 1st ed.; Advances in Chemistry Series 59; American Chemical Society: Washington, DC, USA, 1966; ISBN 978-084-122-239-7. [Google Scholar]
- Glasser, W.G.; Sarkanen, S. Lignin. Properties and Materials, 1st ed.; ACS Symposium Series 397; American Chemical Society: Washington, DC, USA, 1989; ISBN 978-084-121-248-0. [Google Scholar]
- Lewis, N.G.; Sarkanen, S. Lignin and Lignan Biosynthesis; ACS Symposium Series 697; American Chemical Society: Washington, DC, USA, 1998; ISBN 084-123-566-X. [Google Scholar]
- Katahira, R.; Elder, T.J.; Beckham, G.T. Chapter 1 A brief introduction to lignin structure. In Lignin Valorization. Emerging Approaches, 1st ed.; Beckham, G.T., Ed.; The Royal Society of Chemistry: Croydon, London, UK, 2018; pp. 1–20. ISBN 978-178-801-035-1. [Google Scholar]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, L.; Wang, Z.; Wilbon, P.A.; Wang, C.; Chu, F.; Tang, C. Lignin and soy oil-derived polymeric biocomposites by “grafting from” RAFT polymerization. Green Chem. 2016, 18, 4974–4981. [Google Scholar] [CrossRef]
- Yu, Y.; Fu, S.; Song, P.; Lou, X.; Jin, Y.; Lu, F.; Wu, Q.; Ye, J. Functionalized lignin by grafting phosphorus-nitrogen improves the thermal stability and flame retardancy of polypropylene. Polym. Degrad. Stabil. 2012, 97, 541–546. [Google Scholar] [CrossRef]
- Prieur, B.; Meub, M.; Wittermann, M.; Klein, R.; Bellayer, S.; Fontaine, G.; Bourbigot, S. Phosphorylation of lignin: Characterization and investigation of the thermal decomposition. RSC Adv. 2017, 7, 16866–16877. [Google Scholar] [CrossRef] [Green Version]
- Prieur, B.; Meub, M.; Wittemann, M.; Klein, R.; Bellayer, S.; Fontaine, G.; Bourbigot, S. Phosphorylation of lignin to flame retard acrylonitrile butadiene styrene (ABS). Polym. Degrad. Stabil. 2016, 127, 32–43. [Google Scholar] [CrossRef]
- Liu, H.; Chung, H. Lignin-Based Polymers via Graft Copolymerization. J. Polym. Sci. A Polym. Chem. 2017, 55, 3515–3528. [Google Scholar] [CrossRef]
- Gupta, C.; Washburn, N.R. Polymer-grafted lignin surfactants prepared via Reversible Addition−Fragmentation Chain-Transfer polymerization. Langmuir 2014, 30, 9303–9312. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Lokupitiya, H.N.; Tang, C. Lignin biopolymers in the age of controlled polymerization. Polymers 2019, 11, 1176. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Kadla, J.F. Preparation of a thermoresponsive lignin-based biomaterial through atom transfer radical polymerization. Biomacromolecules 2010, 11, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, J.; Korich, K.; Li, S.; Ma, S.; Ploehn, H.J.; Iovine, P.M.; Wang, C.; Chu, F.; Tang, C. Combining renewable gum rosin and lignin: Towards hydrophobic polymer composites by controlled polymerization. J. Polym. Sci. A Polym. Chem. 2011, 49, 3728–3738. [Google Scholar] [CrossRef]
- Diao, B.; Zhang, Z.; Zhu, J.; Li, J. Biomass-based thermogelling copolymers consisting of lignin and grafted poly (N-isopropylacrylamide), poly (ethylene glycol), and poly (propylene glycol). RSC Adv. 2014, 4, 42996–43003. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Wang, C.; Liu, Y.; Xu, Y.; Tang, C.; Chu, F. UV-Absorbent Lignin-Based Multi-Arm Star Thermoplastic Elastomers. Macromol. Rapid Commun. 2015, 36, 398–404. [Google Scholar] [CrossRef]
- Gao, G.; Dallmeyer, J.I.; Kadla, J.F. Synthesis of lignin nanofibers with ionic-responsive shells: Water-expandable lignin-based nanofibrous mats. Biomacromolecules 2012, 13, 3602–3610. [Google Scholar] [CrossRef] [PubMed]
- Hilburg, S.L.; Elder, A.N.; Chung, H.; Ferebee, R.L.; Bockstaller, M.R.; Washburn, N.R. A universal route towards thermoplastic lignin composites with improved mechanical properties. Polymer 2014, 55, 995–1003. [Google Scholar] [CrossRef]
- Shah, T.; Gupta, C.; Ferebee, R.L.; Bockstaller, M.R.; Washburn, N.R. Extraordinary toughening and strengthening effect in polymer nanocomposites using lignin-based fillers synthesized by ATRP. Polymer 2015, 72, 406–412. [Google Scholar] [CrossRef] [Green Version]
- Kai, D.; Low, Z.W.; Liow, S.S.; Abdul Karim, A.; Ye, H.; Jin, G.; Li, K.; Loh, X.J. Development of lignin supramolecular hydrogels with mechanically responsive and self-healing properties. ACS Sustain. Chem. Eng. 2015, 3, 2160–2169. [Google Scholar] [CrossRef]
- Li, H.; Pang, Z.; Gao, P.; Wang, L. Fe (III)-catalyzed grafting copolymerization of lignin with styrene and methyl methacrylate through AGET ATRP using triphenyl phosphine as a ligand. RSC Adv. 2015, 5, 54387–54394. [Google Scholar] [CrossRef]
- Liu, X.; Yin, H.; Zhang, Z.; Diao, B.; Li, G. Functionalization of lignin through ATRP grafting of poly(2-dimethylaminoethyl methacrylate) for gene delivery. Colloids Surf. B Biointerfaces 2015, 125, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Silmore, K.S.; Gupta, C.; Washburn, N.R. Tunable Pickering emulsions with polymer-grafted lignin nanoparticles (PGLNs). J. Colloid Interface Sci. 2016, 466, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, C.; Nadelman, E.; Washburn, N.R.; Kurtis, K.E. Lignopolymer Superplasticizers for Low-CO2 Cements. ACS Sustain. Chem. Eng. 2017, 5, 4041–4049. [Google Scholar] [CrossRef]
- Gupta, C.; Sverdlove, M.J.; Washburn, N.R. Molecular architecture requirements for polymer-grafted lignin superplasticizers. Soft Matter 2015, 11, 2691–2699. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Lu, X.; Xie, J.; Feng, B.; Han, Q. Synthesis of a novel tunable lignin-based star copolymer and its flocculation performance in the treatment of kaolin suspension. Sep. Purif. Technol. 2019, 210, 355–363. [Google Scholar] [CrossRef]
- Nemoto, T.; Konishi, G.-I.; Tojo, Y.; An, Y.C.; Funaoka, M. Functionalization of lignin: Synthesis of lignophenol–graft–poly (2-ethyl-2-oxazoline) and its application to polymer blends with commodity polymer. J. Appl. Polym. Sci. 2012, 123, 2636–2642. [Google Scholar] [CrossRef]
- Mahata, D.; Jana, M.; Jana, A.; Mukherjee, A.; Mondal, N.; Saha, T.; Sen, S.; Nando, G.B.; Mukhopadhyay, C.K.; Chakraborty, R.; et al. Lignin-graft-polyoxazoline conjugated triazole a novel anti-infective ointment to control persistent inflammation. Sci. Rep. 2017, 7, 46412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, Y.L.; Olsson, J.V.; Li, R.J.; Frank, C.W.; Waymouth, R.M.; Billington, S.L.; Sattely, E.S. A renewable lignin-lactide copolymer and application in biobased composite. ACS Sustain. Chem. Eng. 2013, 1, 1231–1238. [Google Scholar] [CrossRef]
- Liu, X.; Zong, E.; Jiang, J.; Fu, S.; Wang, J.; Xu, B.; Li, W.; Lin, X.; Xu, Y.; Wang, C.; et al. Preparation and characterization of Lignin–graft–poly (ε-caprolactone) copolymers based on lignocellulosic butanol residue. Int. J. Biol. Macromol. 2015, 81, 521–529. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, L.; Lu, X.; He, C. Biodegradable and renewable poly (lactide)-lignin composites: Synthesis, interface and toughening mechanism. J. Mater. Chem. A 2015, 3, 3699–3709. [Google Scholar] [CrossRef]
- Kai, D.; Zhang, K.; Liow, S.S.; Loh, X.J. New dual functional phb-grafted lignin copolymer: Synthesis, mechanical properties, and biocompatibility studies. ACS Appl. Bio Mater. 2018, 2, 127–134. [Google Scholar] [CrossRef]
- Pérez–Camargo, R.A.; Saenz, G.; Laurichesse, S.; Casas, M.T.; Puiggalí, J.; Avérous, L.; Müller, A.J. Nucleation crystallization, and thermal fractionation of poly (ε-caprolactone)-grafted-lignin: Effect of grafted chains length and lignin content. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1736–1750. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Synthesis, thermal properties, rheological and mechanical behaviors of lignins-grafted-poly(ε-caprolactone). Polymer 2013, 54, 3882–3890. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Molinari, V.; Esposito, D.; Tauer, K.; Antonietti, M. Lignin-based polymeric surfactants for emulsion polymerization. Polymer 2017, 112, 418–426. [Google Scholar] [CrossRef]
- Tapdiqov, S.Z. A drug-loaded gel based on graft radical co-polymerization of n-vinylpyrrolidone and 4-vinylpyridine with chitosan. Cell. Chem. Technol. 2020, 54, 429–438. [Google Scholar] [CrossRef]
- Cheaburu-Yilmaz, C.N. On the development of chitosan-graft-poly(n-isopropylacrylamide) by raft polymerization technique. Cell. Chem. Technol. 2020, 54, 1–10. [Google Scholar] [CrossRef]
- Kadokawa, J.-I. Preparation and Grafting Functionalization of Self-Assembled Chitin Nanofiber Film. Coatings 2016, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, G.A.; Sayed, A.; Thabit, M.; Safwat, G. Chitosan biopolymer based nanocomposite hydrogels for removal of methylene blue dye. SN Appl. Sci. 2020, 2, 968. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Liu, B.; Zou, L.; Sun, C.; Li, W. Preparation and characterization of PLLA/chitosan-graft-poly(ε-caprolactone) (CS-g-PCL) composite fibrous mats: The microstructure, performance and proliferation assessment. Int. J. Biol. Macromol. 2020, 162, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.-I. Fabrication of nanostructured a nd microstructured chitin materials through gelation with suitable dispersion media. RSC Adv. 2015, 5, 12736–12746. [Google Scholar] [CrossRef]
- Stefan, J.; Lorkowska-Zawicka, B.; Kaminski, K.; Szczubialka, K.; Nowakowska, M.; Korbut, R. The current view on biological potency of cationically modified chitosan. J. Physiol. Pharmacol. 2014, 65, 341–347. [Google Scholar] [PubMed]
- Jiang, T.; Deng, M.; James, R.; Nair, L.S.; Laurencin, C.T. Micro- and nanofabrication of chitosan structures for regenerative engineering. Acta Biomater. 2014, 10, 1632–1645. [Google Scholar] [CrossRef]
- Lai, G.-J.; Shalumon, K.T.; Chen, S.-H.; Chen, J.P. Composite chitosan/silk fibroin nanofibers for modulation of osteogenic differentiation and proliferation of human mesenchymal stem cells. Carbohydr. Polym. 2014, 111, 288–297. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, T.; Chen, X.; Liu, Y. Applications of chitosan based biomaterials: A focus on dependent antimicrobial properties. Mar. Life Sci. Technol. 2020, 2, 398–413. [Google Scholar] [CrossRef]
- Wen, J.; Li, Y.; Wang, L.; Chen, X.; Cao, Q.; He, N. Carbon Dioxide Smart Materials Based on Chitosan. Prog. Chem. 2020, 32, 417–422. (In Chinese) [Google Scholar]
- Sashiwa, H.; Aiba, S.-I. Chemically modified chitin and chitosan as biomaterials. Prog. Polym. Sci. 2004, 29, 887–908. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.-M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Mittal, H.; Ray, S.S.; Kaith, B.S.; Bhatia, J.K.; Sharma, S.J.; Alhassan, S.M. Recent progress in the structural modification of chitosan for applications in diversified biomedical fields. Eur. Polym. J. 2018, 109, 402–434. [Google Scholar] [CrossRef]
- Iyer, B.V.S.; Yashin, V.V.; Hamer, M.J.; Kowalewski, T.; Matyjaszewski, K.; Balazsa, A.C. Ductility, toughness and strain recovery in self-healing dualcross-linked nanoparticle networks studied by computer simulations. Prog. Polym. Sci. 2015, 40, 121–137. [Google Scholar] [CrossRef] [Green Version]
- Derry, M.J.; Fielding, L.A.; Armes, S.P. Polymerization-induced self-assembly of block copolymer nanoparticles via RAFT non-aqueous dispersion polymerization. Prog. Polym. Sci. 2016, 52, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Bednarek, M. Branched aliphatic polyesters by ring-opening (co)polymerization. Prog. Polym. Sci. 2016, 58, 27–58. [Google Scholar] [CrossRef]
- Yildirim, I.; Weber, C.; Schubert, U.S. Old meets new: Combination of PLA and RDRP to obtain sophisticated macromolecular architectures. Prog. Polym. Sci. 2018, 76, 111–150. [Google Scholar] [CrossRef]
- Wang, W.; Lu, W.; Goodwin, A.; Wang, H.; Yin, P.; Kang, N.-G.; Hong, K.; Mays, J.W. Recent advances in thermoplastic elastomers from living polymerizations: Macromolecular architectures and supramolecular chemistry. Prog. Polym. Sci. 2019, 95, 1–31. [Google Scholar] [CrossRef]
- Mocny, P.; Klok, H.-A. Complex polymer topologies and polymer—nanoparticle hybrid films prepared via surface-initiated controlled radical polymerization. Prog. Polym. Sci. 2020, 100, 101185. [Google Scholar] [CrossRef]
- Vivaldo-Lima, E.; Jaramillo-Soto, G.; Penlidis, A. Nitroxide-mediated polymerization (NMP). In Encyclopedia of Polymer Science and Technology, 1st ed.; John Wiley & Sons: New York, NY, USA, 2016; pp. 1–48. ISBN 978-047-144-026-0. [Google Scholar]
- Olivier, A.; Meyer, F.; Raquez, J.-M.; Damman, P.; Dubois, P. Surface-initiated controlled polymerization as a convenient method for designing functional polymer brushes: From self-assembled monolayers to patterned surfaces. Prog. Polym. Sci. 2012, 37, 157–181. [Google Scholar] [CrossRef]
- Radzevicius, P.; Krivorotova, T.; Makuska, R. Synthesis by one-pot RAFT polymerization and properties of amphiphilic pentablock copolymers with repeating blocks of poly(2-hydroxyethyl methacrylate) and poly(butyl methacrylate). Eur. Polym. J. 2017, 87, 69–83. [Google Scholar] [CrossRef]
- Chmielarz, P.; Fantin, M.; Park, S.; Isse, A.A.; Gennaro, A.; Magenau, A.J.; Sobkowiak, A.; Matyjaszewski, K. Electrochemically mediated atom transfer radical polymerization (eATRP). Prog. Polym. Sci. 2017, 69, 47–78. [Google Scholar] [CrossRef]
- Maharana, T.; Pattanaik, S.; Routaray, A.; Nath, N.; Sutar, A.K. Synthesis and characterization of poly(lactic acid) based graft copolymers. React. Funct. Polym. 2015, 93, 47–67. [Google Scholar] [CrossRef]
- Mehta, A.; Pandey, J.P.; Sen, G. Synthesis of Diallyl dimethyl ammonium chloride grafted polyvinyl pyrrolidone (PVP-g-DADMAC) and its applications. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2021, 263, 114750. [Google Scholar] [CrossRef]
- El-Sayed, N.; Awad, H.; El-Sayed, G.M.; Nagieb, Z.A.; Kamel, S. Synthesis and characterization of biocompatible hydrogel based on hydroxyethyl cellulose-g-poly(hydroxyethyl methacrylate). Polym. Bull. 2020, 77, 6333–6347. [Google Scholar] [CrossRef]
- Mahdavi, H.; Mazinani, N.; Heidari, A.A. Poly(vinylidene fluoride) (PVDF)/PVDF-g-polyvinylpyrrolidone (PVP)/TiO2 mixed matrix nanofiltration membranes: Preparation and characterization. Polym. Int. 2020, 69, 1187–1195. [Google Scholar] [CrossRef]
- Oliveira, T.S.; Brazil, T.R.; Guerrini, L.M.; Rezende, M.C.; Oliveira, M.P. Synthesis and characterization of poly (acrylonitrile-g-lignin) by semi-batch solution polymerization and evaluation of their potential application as carbon materials. J. Polym. Res. 2020, 27, 340. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, P.; Lan, G.; Liu, Y.; Cai, Q.; Xi, J. High crosslinked sodium carboxyl methylstarch-g-poly (acrylic acid-co-acrylamide) resin for heavy metal adsorption: Its characteristics and mechanisms. Environ. Sci. Pollut. Res. 2020, 27, 38617–38630. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Xu, J.; Wang, Q.; Fan, X. Synthesis of partly debranched starch-g-poly(2-acryloyloxyethyl trimethyl ammonium chloride) catalyzed by horseradish peroxidase and the effect on adhesion to polyester/cotton yarn. Process Biochem. 2020, 97, 176–182. [Google Scholar] [CrossRef]
- Xu, R.-M.; Yang, T.T.; Vidovic, E.; Jia, R.-N.; Zhang, J.-M.; Mi, Q.-Y.; Zhang, J. Cellulose Acetate Thermoplastics with High Modulus, Dimensional Stability and Anti-migration Properties by Using CA-g-PLA as Macromolecular Plasticizer. Chin. J. Polym. Sci. 2020, 38, 1141–1148. [Google Scholar] [CrossRef]
- Wu, Q.; Tiraferri, A.; Li, T.; Xie, W.; Chang, H.; Bai, Y.; Liu, B. Superwettable PVDF/PVDF-g-PEGMA Ultrafiltration Membranes. ACS Omega 2020, 5, 23450–23459. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, S.J.; Aghamohammadi-Bavil, O.; Foroutan, R.; Arsalani, N. Removal of malachite green using carboxymethyl cellulose-g-polyacrylamide/montmorillonite nanocomposite hydrogel. Int. J. Biol. Macromol. 2020, 159, 1122–1131. [Google Scholar] [CrossRef]
- Gürsel, U.; Taran, S.; Gökçen, M.; Ari, Y.; Alli, A. Ultraviolet illumination responsivity of the Au/n-Si diodes with and without poly (linolenic acid)-g-poly (caprolactone)-g-poly (t-butyl acrylate) interfacial layer. Surf. Rev. Lett. 2020, 27, 1950207. [Google Scholar] [CrossRef]
- Song, P.; Guo, R.; Ma, W.; Wang, L.; Ma, F.; Wang, R. Synthesis of CO2-based polycarbonate-g-polystyrene copolymers via NMRP. Chem. Commun. 2020, 56, 9493–9496. [Google Scholar] [CrossRef] [PubMed]
- Klimovica, K.; Pan, S.; Lin, T.-W.; Peng, X.; Ellison, C.J.; LaPointe, A.M.; Bates, F.S.; Coates, G.W. Compatibilization of iPP/HDPE Blends with PE-g-iPP Graft Copolymers. ACS Macro. Lett. 2020, 9, 1161–1166. [Google Scholar] [CrossRef]
- Li, W.; Yu, Z.; Wu, Y.; Liu, Q. Preparation, characterization of feather protein-g-poly(sodium allyl sulfonate) and its application as a low-temperature adhesive to cotton and viscose fibers for warp sizing. Eur. Polym. J. 2020, 136, 109945. [Google Scholar] [CrossRef]
- Czarnecka, E.; Nowaczyk, J. Semi-Natural superabsorbents based on Starch-g-poly(acrylic acid): Modification, synthesis and application. Polymers 2020, 12, 1794. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Gangadharappa, H.V.; Osmani, R.A.M.; Gowda, D.V. Design and development of polymethylmethacrylate-grafted gellan gum (PMMA-g-GG)-based pH-sensitive novel drug delivery system for antidiabetic therapy. Drug Deliv. and Transl. Res. 2020, 10, 1002–1018. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Patel, M.; Sung, J.-S.; Kim, J.H. Preparation and characterization of bioinert amphiphilic P(VDF-co-CTFE)-g-POEM graft copolymer. Polym. Plast. Technol. Mater. 2020, 59, 1077–1087. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Wu, J.; Ni, Q. Preparation, characterization of poly(acrylic acid)-g-feather protein-g-poly(methyl acrylate) and application in improving adhesion of protein to PLA fibers for sizing. React. Funct. Polym. 2020, 152, 104607. [Google Scholar] [CrossRef]
- Deng, J.-R.; Zhao, C.-L.; Wu, Y.-X. Antibacterial and pH-responsive Quaternized Hydroxypropyl Cellulose-g-Poly(THF-co-epichlorohydrin) Graft Copolymer: Synthesis, Characterization and Properties. Chin. J. Polym. Sci. 2020, 38, 704–714. [Google Scholar] [CrossRef]
- Cuggino, J.C.; Ambrosioni, F.E.; Picchio, M.L.; Nicola, M.; Jiménez Kairuz, A.F.; Gatti, G.; Minari, R.J.; Calderon, M.; Alvarez Igarzabal, C.I.; Gugliotta, L.M. Thermally self-assembled biodegradable poly(casein-g-N-isopropylacrylamide) unimers and their application in drug delivery for cancer therapy. Int. J. Biol. Macromol. 2020, 154, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.R.; Seggiani, M.; Cinelli, P.; Elnaby, H.M.H.; Azaam, M.M. Swelling capacity of sugarcane bagasse-g-poly(acrylamide)/attapulgite superabsorbent composites and their application as slow release fertilizer. Eur. Polym. J. 2020, 133, 109769. [Google Scholar] [CrossRef]
- Jiang, P.; Ji, H.; Li, G.; Chen, S.; Lv, L. Structure formation in pH-sensitive micro porous membrane from well-defined ethyl cellulose-g-PDEAEMA via non-solvent-induced phase separation process. J. Macromol. Sci. Pure Appl. Chem. 2020, 57, 461–471. [Google Scholar] [CrossRef]
- Huang, Q.; Xu, Z.; Cai, C.; Lin, J. Micelles with a Loose Core Self-Assembled from Coil-g-Rod Graft Copolymers Displaying High Drug Loading Capacity. Macromol. Chem. Phys. 2020, 221, 2000121. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Zhou, D.; Ji, P.; Zhou, X.; Zhang, Y.; He, P. Synthesis and Water Absorbing Properties of KGM-g-P(AA-AM-(DMAEA-EB)) via Grafting Polymerization Method. Polym. Sci. Ser. B 2020, 62, 238–244. [Google Scholar] [CrossRef]
- Erdoğan, M.K.; Akdemir, Ö.; Hamitbeyli, A.; Karakışla, M. Preparation of hydrophilic woven fabrics: Surface modification of poly(ethylene terephthalate) by grafting of poly(vinyl alcohol) and poly(vinyl alcohol)-g-(N-vinyl-2-pyrrolidone). J. Appl. Polym. Sci. 2020, 137, 48584. [Google Scholar] [CrossRef]
- Worzakowska, M. The preparation, physicochemical and thermal properties of the high moisture, solvent and chemical resistant starch-g-poly(geranyl methacrylate) copolymers. J. Thermal. Anal. Calorim. 2020, 140, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Delorme, V.; Lichon, L.; Mahindad, H.; Hunger, S.; Laroui, N.; Daurat, M.; Godefroy, A.; Coudane, J.; Gary-Bobo, M.; Van Den Berghe, H. Reverse poly(ε-caprolactone)-g-dextran graft copolymers. Nano-carriers for intracellular uptake of anticancer drugs. Carbohydr. Polym. 2020, 232, 115764. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.A.; Kim, K.; Karade, S.S.; Kim, H.; Kim, J.H. High-performance solid-state bendable supercapacitors based on PEGBEM-g-PAEMA graft copolymer electrolyte. Chem. Eng. J. 2020, 384, 123308. [Google Scholar] [CrossRef]
- Tian, B.; Cai, Y.; Zhang, X.; Fan, H.; Li, B.-G. Design of Well-Defined Polyethylene-g-poly-methyltrifluorosiloxane Graft Copolymers via Direct Copolymerization of Ethylene with Polyfluorosiloxane Macromonomers. Ind. Eng. Chem. Res. 2020, 59, 4557–4567. [Google Scholar] [CrossRef]
- Öztürk, T.; Meyvacı, E.; Arslan, T. Synthesis and characterization of poly(vinyl chloride-g-ε-caprolactone) brush type graft copolymers by ring-opening polymerization and “click” chemistry. J. Macromol. Sci. Pure Appl. Chem. 2020, 57, 171–180. [Google Scholar] [CrossRef]
- Zha, X.; Sadi, M.S.; Yang, Y.; Luo, T.; Huang, N. Adhesion of cornstarch-g-poly (2-hydroxyethyl acrylate) to cotton fibers in sizing. J. Adhes. Sci. Technol. 2020, 34, 461–479. [Google Scholar] [CrossRef]
- Nicolas, C.; Zhang, W.; Choppé, E.; Fontaine, L.; Montembault, V. Polynorbornene-g-poly(ethylene oxide) Through the Combination of ROMP and Nitroxide Radical Coupling Reactions. J. Polym. Sci. 2020, 58, 645–653. [Google Scholar] [CrossRef]
- Ilhan, E.; Karahaliloglu, Z.; Kilicay, E.; Hazer, B.; Denkbas, E.B. Potent bioactive bone cements impregnated with polystyrene-g-soybean oil-AgNPs for advanced bone tissue applications. Mater. Technol. 2020, 35, 179–194. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Li, Y.; Zhang, Q.; Huang, J.; Wu, Q.; Wang, S. Fabrication of cellulose nanocrystal-g-poly(acrylic acid-co-acrylamide) aerogels for efficient Pb(II) removal. Polymers 2020, 12, 333. [Google Scholar] [CrossRef] [Green Version]
- Mo, X.-Z.; Wei, F.-X.; Tan, D.-F.; Pang, J.-Y.; Lan, C.-B. The compatibilization of PLA-g-TPU graft copolymer on polylactide/thermoplastic polyurethane blends. J. Polym. Res. 2020, 27, 33. [Google Scholar] [CrossRef]
- Guleria, A.; Kumari, G.; Lima, E.C. Cellulose-g-poly-(acrylamide-co-acrylic acid) polymeric bioadsorbent for the removal of toxic inorganic pollutants from wastewaters. Carbohydr. Polym. 2020, 228, 115396. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Li, T.; Hu, M.; Chen, C.; Liu, B.; Crittenden, J.; Chu, L.-Y.; Ng, H.Y. Performance improvement for thin-film composite nanofiltration membranes prepared on PSf/PSf-g-PEG blended substrates. Sep. Purif. Technol. 2020, 230, 115855. [Google Scholar] [CrossRef]
- Savaş, B.; Öztürk, T. Synthesis and characterization of poly(vinyl chloride-g-methyl methacrylate) graft copolymer by redox polymerization and Cu catalyzed azide-alkyne cycloaddition reaction. J. Macromol. Sci. Pure Appl. Chem. 2020, 1–7. [Google Scholar] [CrossRef]
- Ahuja, D.; Rainu, A.S.; Singh, M.; Kaushik, A. Encapsulation of NPK fertilizer for slow release using sodium carboxymethyl cellulose-g-poly (AA-C0-AM-C0-AMPS)/ Montmorillonite clay-based nanocomposite hydrogels for sustainable agricultural applications. Trends Carbohydr. Res. 2020, 12, 15–23. [Google Scholar]
- Lu, Y.; Wu, F.; Duan, W.; Zhou, X.; Kong, W. Engineering a “PEG-g-PEI/DNA nanoparticle-in- PLGA microsphere” hybrid controlled release system to enhance immunogenicity of DNA vaccine. Mater. Sci. Eng. C 2020, 106, 110394. [Google Scholar] [CrossRef]
- Grebenik, E.A.; Surin, A.M.; Bardakova, K.N.; Dermina, T.S.; Minaev, N.V.; Veryasova, N.N.; Artyukhova, M.A.; Krasilnikova, I.A.; Bakaeva, Z.V.; Sorokina, E.G.; et al. Chitosan-g-oligo(L,L-lactide) copolymer hydrogel for nervous tissue regeneration in glutamate excitotoxicity: In vitro feasibility evaluation. Biomed. Mater. 2020, 15, 015011. [Google Scholar] [CrossRef] [PubMed]
- Barth, H.G.; Jackson, C.; Boyes, B.E. Size Exclusion Chromatography. Anal. Chem. 1994, 66, 595–620. [Google Scholar] [CrossRef] [PubMed]
- Hamielec, A.; Gloor, P.; Zhu, S. Kinetics of, free radical modification of polyolefins in extruders—Chain scission, crosslinking and grafting. Can. J. Chem. Eng. 1991, 69, 611–618. [Google Scholar] [CrossRef]
- Chaimberg, M.; Cohen, Y. Kinetic Modeling of Free-Radical Graft Polymerization. AIChE J. 1994, 40, 294–311. [Google Scholar] [CrossRef]
- Guillot, J.; Leroux, D. Modelling of size-exclusion chromatograms from molecular weight distribution calculations. Application to the grafting of polymers onto functionalized silica. Macromol. Chem. Phys. 1994, 195, 1463–1470. [Google Scholar] [CrossRef]
- Hojabr, S.; Baker, W.; Russell, K.; McLellan, P.; Huneault, M. Melt grafting of glycidyl methacrylate onto polyethylene: An experimental and mathematical modeling study. Int. Polym. Proc. 1998, 13, 118–128. [Google Scholar] [CrossRef]
- Machado, A.; Gaspar-Cunha, A.; Covas, J. Modelling of the grafting of maleic anhydride onto polyethylene in an extruder. Mater. Sci. Forum 2004, 455–456, 763–766. [Google Scholar] [CrossRef]
- Giudici, R. Mathematical modeling of the crafting of maleic anhydride onto polypropylene. Macromol. Symp. 2007, 259, 354–364. [Google Scholar] [CrossRef]
- Diaconescu, R.; Grigoriu, A.-M.; Luca, C. Neural network modeling of monochlorotriazinyl-β-cyclodextrin grafting on cellulosic supports. Cell. Chem. Technol. 2007, 41, 385–390. [Google Scholar]
- Luca, C.; Grigoriu, A.-M.; Diaconescu, R.; Secula, M. Modeling and simulation of monochlorotriazinyl-β-cyclodextrin paper grafting by artificial neural network. Rev. Chim. 2011, 62, 1033–1038. [Google Scholar]
- Grigoriu, A.; Racu, C.; Diaconescu, R.; Grigoriu, A.-M. Modeling of the simultaneous process of wet spinning-grafting of bast fibers using artificial neural networks. Textile Res. J. 2012, 82, 324–335. [Google Scholar] [CrossRef]
- Tong, G.-S.; Liu, T.; Hu, G.-H.; Hoppe, S.; Zhao, L.; Yuan, W.-K. Modelling of the kinetics of the supercritical CO2 assisted grafting of maleic anhydride onto isotactic polypropylene in the solid state. Chem. Eng. Sci. 2007, 62, 5290–5294. [Google Scholar] [CrossRef]
- Wang, J.; Ran, Y.; Ding, L.; Wang, D. Advances in supercritical CO2 assisted grafting of polypropylene in solid state. Chem. React. Eng. Technol. 2008, 24, 173–177. [Google Scholar]
- Casis, N.; Estenoz, D.; Vega, J.; Meira, G. Bulk prepolymerization of styrene in the presence of polybutadiene: Determination of grafting efficiency by size exclusion chromatography combined with a new extended model. J. Appl. Polym. Sci. 2009, 111, 1508–1522. [Google Scholar] [CrossRef]
- Badel, T.; Beyou, E.; Bounor-Legaré, V.; Chaumont, P.; Flat, J.; Michel, A. Free radical graft copolymerization of methyl methacrylate onto polyolefin backbone: Kinetics modeling through model compounds approach. Macromol. Chem. Phys. 2009, 210, 1087–1095. [Google Scholar] [CrossRef]
- Gianoglio Pantano, I.; Asteasuain, M.; Sarmoria, C.; Brandolin, A. Graft copolymers for blend compatibilization. Mathematical modeling of the grafting process. In Proceedings of the 2010 AIChE Annual Meeting Conference Proceedings, Salt Lake City, UT, USA, 7–12 November 2010; pp. 1–14. [Google Scholar]
- Gianoglio Pantano, I.; Asteasuain, M.; Sarmoria, C.; Brandolin, A. Graft Copolymers for Blend Compatibilization: Mathematical Modeling of the Grafting Process. Macromol. React. Eng. 2012, 6, 406–418. [Google Scholar] [CrossRef]
- Gianoglio Pantano, I.A.; Brandolin, A.; Sarmoria, C. Mathematical modeling of the graft reaction between polystyrene and polyethylene. Polym. Degrad. Stabil. 2011, 96, 416–425. [Google Scholar] [CrossRef]
- Aguiar, L.; Pessôa-Filho, P.; Giudici, R. Mathematical modeling of the grafting of maleic anhydride onto poly(propylene): Model considering a heterogeneous medium. Macromol. Theory Simul. 2011, 20, 837–849. [Google Scholar] [CrossRef]
- Damodaran, V.; Fee, C.; Popat, K. Modeling of PEG grafting and prediction of interfacial force profile using x-ray photoelectron spectroscopy. Surf. Interface Anal. 2012, 44, 144–149. [Google Scholar] [CrossRef]
- Zhou, D.; Gao, X.; Wang, W.-J.; Zhu, S. Termination of surface radicals and kinetic modeling of ATRP grafting from flat surfaces by addition of deactivator. Macromolecules 2012, 45, 1198–1208. [Google Scholar] [CrossRef]
- Nasef, M.; Shamsaei, E.; Ghassemi, P.; Aly, A.; Yahaya, A. Modeling, prediction, and multifactorial optimization of radiation-induced grafting of 4-vinylpyridine onto poly(vinylidene fluoride) films using statistical simulator. J. Appl. Polym. Sci. 2013, 127, 1659–1666. [Google Scholar] [CrossRef]
- Nasef, M.M.; Ali, A.; Saidi, H.; Ahmad, A. Modeling and optimization aspects of radiation induced grafting of 4-vinylpyridene onto partially fluorinated films. Radiat. Phys. Chem. 2014, 94, 123–128. [Google Scholar] [CrossRef]
- Wu, L.L.; Bu, Z.; Gong, C.; Li, B.-G.; Hungenberg, K.-D. Graft Copolymerization of Styrene and Acrylonitrile in the Presence of Poly(propylene glycol): Modeling and Simulation of Semi-Batch and Continuous Processes. Macromol. React. Eng. 2012, 6, 384–394. [Google Scholar] [CrossRef]
- Xie, X.-L.; Tong, Z.-F.; Huang, Z.-Q.; Zhang, Y.-Q. Kinetics model of graft co-polymerization of acrylamide onto mechanically-activated starch in inverse emulsion. J. Chem. Eng. Chin. Univ. 2014, 28, 567–573. [Google Scholar]
- Liu, X.; Nomura, M. Kinetic modeling and simulation of emulsion grafting copolymerization of styrene and acrylonitrile in the presence of polybutadiene seed latex particles. Ind. Eng. Chem. Res. 2014, 53, 17580–17588. [Google Scholar] [CrossRef]
- Zhang, M.; Jia, Y. Kinetic study on free radical grafting of polyethylene with acrylic acid by reactive extrusion. J. Appl. Polym. Sci. 2014, 131, 40990. [Google Scholar] [CrossRef]
- Sirirat, T.; Vatanatham, T.; Hansupalak, N.; Rempel, G.; Arayapranee, W. Kinetics and modeling of methyl methacrylate graft copolymerization in the presence of natural rubber latex. Korean J. Chem. Eng. 2015, 32, 980–992. [Google Scholar] [CrossRef]
- Oliveira, D.; Dias, R.; Costa, M. Modeling RAFT Gelation and Grafting of Polymer Brushes for the Production of Molecularly Imprinted Functional Particles. Macromol. Symp. 2016, 370, 52–65. [Google Scholar] [CrossRef]
- Saeb, M.; Rezaee, B.; Shadman, A.; Formela, K.; Ahmadi, Z.; Hemmati, F.; Kermaniyan, T.; Mohammadi, Y. Controlled grafting of vinylic monomers on polyolefins: A robust mathematical modeling approach. Des. Monomers Polym. 2017, 20, 250–268. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Ortiz, J.; Van Steenberge, P.; Reyniers, M.-F.; Marin, G.; D’hooge, D.; Duchateau, J.; Remerie, K.; Toloza, C.; Vaz, A.; Schreurs, F. Modeling the reaction event history and microstructure of individual macrospecies in postpolymerization modification. AIChE J. 2017, 63, 4944–4961. [Google Scholar] [CrossRef]
- Hernández-Ortiz, J.; Van Steenberge, P.; Duchateau, J.; Toloza, C.; Schreurs, F.; Reyniers, M.-F.; Marin, G.; D’hooge, D. Sensitivity Analysis of Single-Phase Isothermal Free Radical–Induced Grafting of Polyethylene. Macromol. Theory Simul. 2018, 27, 1800036. [Google Scholar] [CrossRef]
- Hernández-Ortiz, J.; Van Steenberge, P.; Duchateau, J.; Toloza, C.; Schreurs, F.; Reyniers, M.-F.; Marin, G.; D’hooge, D. The Relevance of Multi-Injection and Temperature Profiles to Design Multi-Phase Reactive Processing of Polyolefins. Macromol. Theory Simul. 2019, 28, 1900035. [Google Scholar] [CrossRef]
- Penlidis, A.; MacGregor, J.F.; Hamielec, A.E. Dynamic modeling of emulsion polymerization reactors. AIChE J. 1985, 31, 881–889. [Google Scholar] [CrossRef]
- Iedema, P.D.; Grcev, S.; Hoefsloot, H.C.J. Molecular weight distribution modeling of radical polymerization in a CSTR with long chain branching through transfer to polymer and terminal double bond. Macromolecules 2003, 36, 458–476. [Google Scholar] [CrossRef]
- Dias, R.; Costa, M. A new look at kinetic modeling of nonlinear free radical polymerizations with terminal branching and chain transfer to polymer. Macromolecules 2003, 36, 8853–8863. [Google Scholar] [CrossRef] [Green Version]
- Krallis, A.; Kiparissides, C. Mathematical modeling of the bivariate molecular weight-Long chain branching distribution of highly branched polymers: A population balance approach. Chem. Eng. Sci. 2007, 62, 5304–5311. [Google Scholar] [CrossRef]
- Kryven, I.; Iedema, P.D. A novel approach to population balance modeling of reactive polymer modification leading to branching. Macromol. Theory Simul. 2013, 22, 89–106. [Google Scholar] [CrossRef]
- Wang, R.; Luo, Y.; Li, B.-G.; Zhu, S. Modeling of Branching and Gelation in RAFT Copolymerization of Vinyl/Divinyl Systems. Macromolecules 2009, 42, 85–94. [Google Scholar] [CrossRef]
- Yaghini, N.; Iedema, P.D. Molecular weight and branching distribution modeling in radical polymerization with transfer to polymer and scission under gel conditions and allowing for multiradicals. Macromolecules 2014, 47, 4851–4863. [Google Scholar] [CrossRef]
- Penlidis, A.; Vivaldo-Lima, E.; Hernández-Ortiz, J.; Saldívar-Guerra, E. Chapter 12: Polymer Reaction Engineering. In Handbook of Polymer Synthesis, Characterization, and Processing, 1st ed.; John Wiley & Sons: New York, NY, USA, 2013; pp. 251–271. ISBN 978-047-063-032-7. [Google Scholar]
- Zhu, S.; Hamielec, A. Polymerization kinetic modeling and macromolecular. In Polymer Science: A Comprehensive Reference, 1st ed.; Matyjaszewski, K., Möller, M., Eds.; Elsevier B.V: London, UK, 2012; Chapter 4.32; Volume 4, pp. 779–831. ISBN 978-008-087-862-1. [Google Scholar]
- Quintero-Ortega, I.; Vivaldo-Lima, E.; Luna-Bárcenas, G.; Alvarado, J.; Louvier-Hernández, J.; Sanchez, I. Modeling of the Free-Radical Copolymerization Kinetics with Cross Linking of Vinyl/Divinyl Monomers in Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2005, 44, 2823–2844. [Google Scholar] [CrossRef]
- Dong, P.; Sun, H.; Quan, D. Synthesis of poly(L-lactide-co-5-amino-5-methyl-1,3-dioxan-2-ones) [P(L-LA-co-TAc)] containing amino groups via organocatalysis and post-polymerization functionalization. Polymer 2016, 97, 614–622. [Google Scholar] [CrossRef]

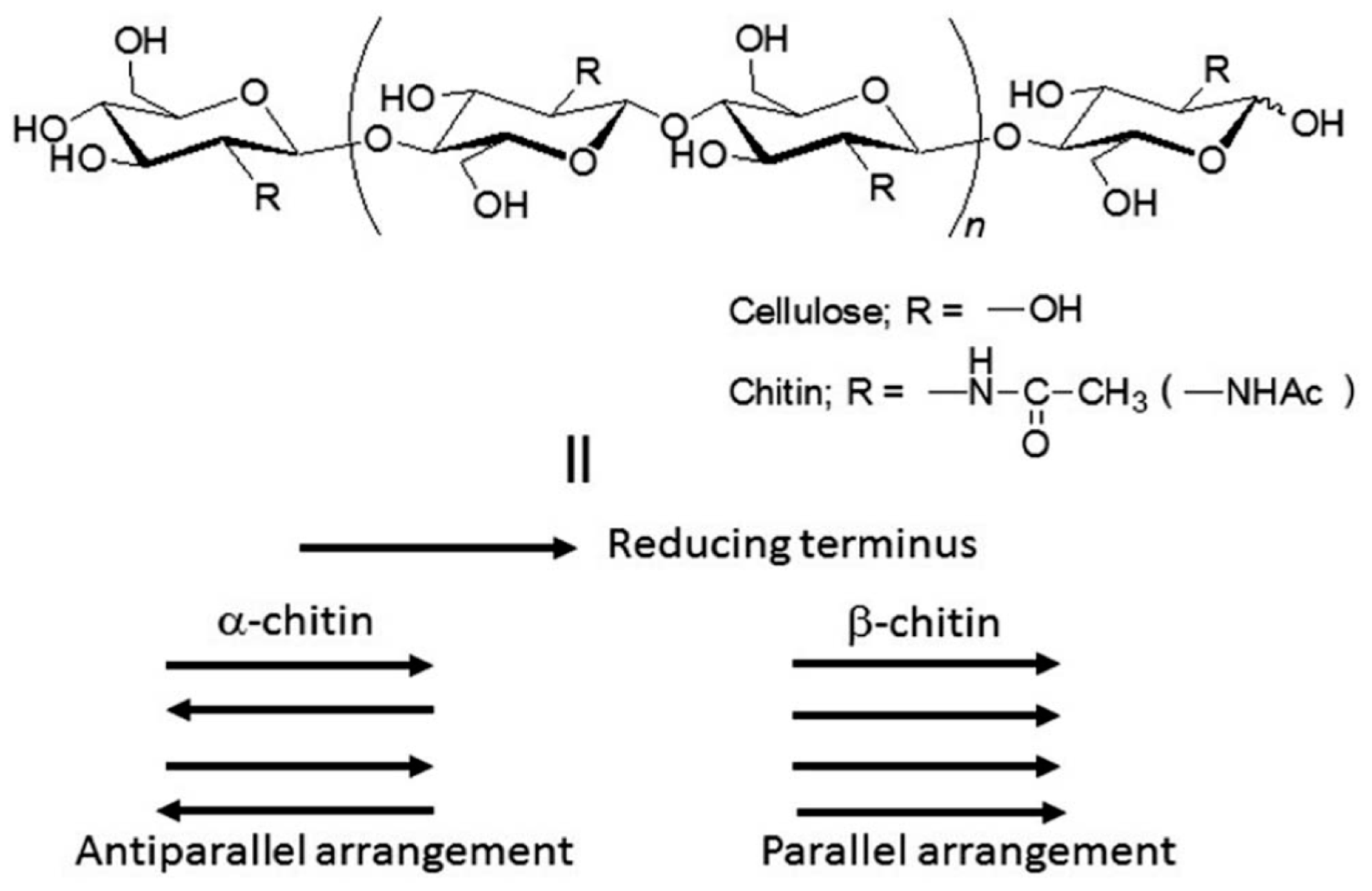
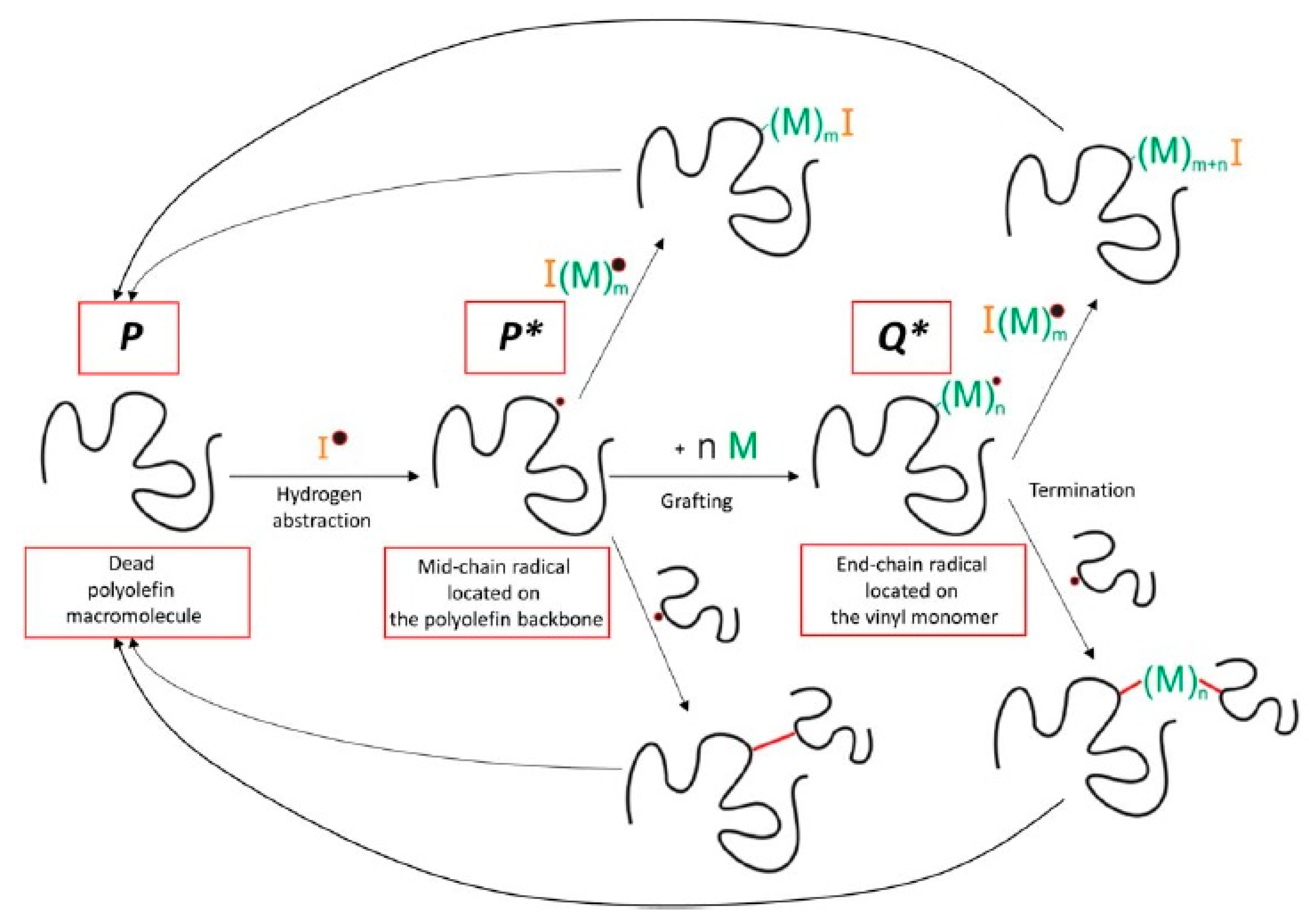
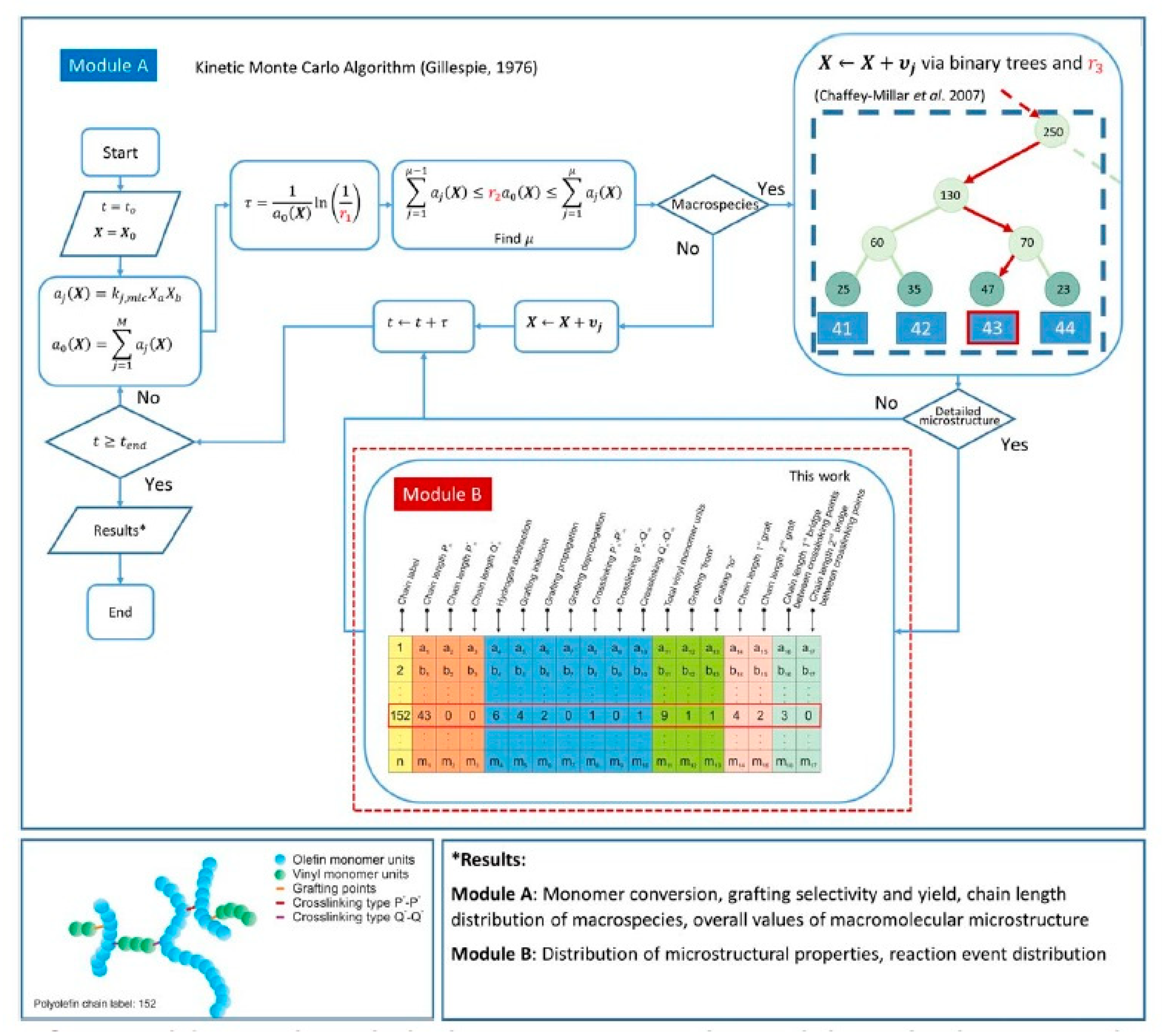
| Backbone | Functionalization Method | Graft Chains | Grafting Technique | Grafting Conditions | Measured Properties and Characterization Methods | Ref. |
|---|---|---|---|---|---|---|
| Rubber (GRS or natural) | Generation of internal free radicals by CTP. | PSty or PMMA | Grafting from | 95–180 °C; Mass FRP (rubber dissolved in monomer, in presence of initiator); solvent-non-solvent fractionation. | Determination of vinyl unsaturation (peracid and infrared methods); molecular weight by intrinsic viscosity; DMA; mechanical properties: tensile strength, elongation-at-break, hardness, modulus at 100 and 300% elongation, as well as tear at 20, 80, and 120 °C. | [8,9] |
| PMMA (a copolymer of MMA and small content of GMA) | Incorporation of mercaptan groups by reaction of GMA and hydrogen sulfide in presence of sodium ethoxide catalyst. | PSty, PBMA, PLMA, or PMA | Grafting from | Mass or solution polymerization of monomer in presence of PMMA with pendant mercaptan groups (grafting occurs by chain transfer to mercaptan groups). | Solvent extraction of ungrafted polymer, measured by UV analysis; grafting efficiency calculated with the aid of a kinetic model. | [10] |
| Either PAN or PAM | Two methods used: (a) CTP; (b) photolysis of a copolymer containing a few per cent of ACN. | PAM, PAN; PMMA (onto PAN) [12] | Grafting from | (a) SP in sodium perchlorate at 55 °C, using persulphate-bisulphite; (b) SP in sodium perchlorate at 25 to 35 °C in a quartz tube under a G.E. Sun Lamp. | Composition by IR; molecular weight by intrinsic viscosity calibrated from light scattering data; phase contrast microscopy; measurement of softening points; analysis of X-ray scattering curves [12]. | [11,12] |
| PE | UV irradiation of surface of sensitized PE. | PSty | Grafting from | (a) Sensitized PE irradiated one minute, stand a week, and then proceed to mass polymerization in Sty at 70 °C; (b) irradiation of plastic in presence of Sty. | Mass difference or ability of the surface to adhere to pressure-sensitive tape under load. | [13] |
| Cellulose | γ-ray pre-irradiation technique. | PSty | Grafting from | Pre-irradiation by Co60 γ-rays in water or in a H2O2 solution; grafting in a 20 vol. Sty solution (methanol/water) at 50 °C. | Degree of grafting by weight gain; estimation of active sites by the ferrous ion method; solubilization of material by acetylation and acetolysis, followed by IR spectroscopy. | [14] |
| Cellulose | Binding initiators or components of initiation systems by means of ion exchange with such materials. | Various polymers (PAN, PMMA, PSty, PAA, PVDC) | Grafting from | Starting material contacted with a dilute solution of catalyst cation salt; the exchanged cellulose was then placed in the monomer or monomer solution; the mixture was heated or irradiated for the required time. | Swelling by centrifuging; grafting efficiency by solvent extraction; mechanical properties (elongation, moduli; toughness); chemical properties (basic/acid dying and hydrolysis, bromination, oxidation, complex formation, etc.); wetting; rotproofing. | [15] |
| Cellulose | Formation of free radicals in cellulose by exposure to high energy electrons or to γ-rays from Co60. | PSty | Grafting from | Sty brought into intimate contact with cellulose by the inclusion technique; then, (a) perform irradiation with high energy electrons using a 2-M.e.v. Van de Graaff accelerator; or (b) induce grafting by γ-ray irradiation. | Degree of grating and grafting efficiency from extraction curves (mass determination); drastic hydrolysis of cellulose backbone and molecular weight determination of isolated PSty chains by intrinsic viscosity; calculation of grated chains per cellulose chain. | [16] |
| Terpolymer of ethylene– ethyl acrylate–maleic anhydride (EEAMA) | Step-growth polymerization (SGP) between maleic anhydride (MAnh) from EEAMA and amine groups from PDMS. | Polydi-methyl-siloxane (PDMS) | Grafting to | Melt reactive mixing proceeded in a Haake Rheocord 3000 batch mixer; T = 140 °C. | Composition and evidence of grafting by 1H and 13C nuclear magnetic resonance. Molecular weight distributions (MWDs) were determined using a GPC or SEC chromatograph (Alliance GPCV 2000, Waters) with refractive index viscometer detectors. Linear viscoelastic properties were determined using a rheometer (AR 2000, TA Instrument) with a cone-and-plate configuration. | [17] |
| Cores of different polymers: polystyrene substrates; arborescent poly(γ-benzyl l-glutamate) (PBG) | Typically, successive anionic grafting reactions of pre-formed side chains onto substrates randomly functionalized with coupling sites (e.g., alkyne-azide click chemistry coupling) | poly(2-vinyl pyridine), polyisoprene, poly(tert-butyl methacrylate), and poly(ethylene oxide); poly(γ-benzyl L-glutamate) (PBG); polyglycidol, poly(ethylene oxide) (PEO), or poly(l-glutamic acid) (PGA) | “Dendrigraft polymers” synthesized by different chemical routes. | MWD determined by SEC and static light scattering; morphology of arborescent polystyrene molecules determined by small-angle neutron scattering (SANS); film formation of isoprene copolymers on mica surfaces investigated using atomic force microscopy (AFM) after spin-casting from different solvents; morphologies of core-shell-corona copolymers studied by transmission electron microscopy (TEM). | [18,19,20,21,22,23,24,25,26] |
| Polymerization Method | General Description | Type of Reaction |
|---|---|---|
| Conventional free-radical polymerization (FRP) | Three steps involved: (1) initiation, with formation of free radicals; (2) propagation, where free radicals react with monomer; and (3) termination of polymer radicals by either combination, disproportionation, or chain transfer to small molecules. The simultaneous participation of these reactions leads to broad molar mass distributions. | Chain transfer reaction: free radicals generated in the system tend to react with backbones by CTP, thereby activating them. Direct generation of free radicals along the backbone: activators generate free radicals from reaction with functional groups placed along the backbone, which correspond to the initiating step of a SIP [27]. |
| Reversible deactivation radical polymerization (RDRP) | A group of polymerization techniques based on free radical technology that controls the growth of polymer molecules during the polymerization. Polymer radicals are reversibly deactivated by effect of controllers that act under some relatively new chemical routes. These techniques allow the development of advanced materials, with various architectures, and well-defined microstructures. Each technique has its own mechanism and conditions that favor them. SIP can proceed by any of the known RDRP techniques. | Atom transfer radical polymerization (ATRP): It is a catalytic process where an alkyl halide macromolecule reacts with the catalyst, allowing the formation of a radical that propagates until it reacts again with the catalyst, in a reversible way [71]. Nitroxide mediated polymerization (NMP): A stable nitroxide free radical acts as controller, reversibly deactivating the propagating and polymer radicals forming dormant polymer molecules with alkoxyamine end functionalities [50]. Reversible addition-fragmentation chain transfer (RAFT) polymerization: Thiocarbonilthio compounds are used as chain transfer agents which control molecular weight development by reversible activation-deactivation reactions [50]. |
| Reactive extrusion (REX) | REX is a set of techniques designed to produce and modify polymers, typically carried out in single or twin extruders. Five main types of reactive polymerizations caried out in extruders have been reported: bulk polymerization, polymer grafting, polymer functionalization, controlled degradation, and reactive blending [72]. Reactions proceed in melt phase. Examples of polymer grafting by REX include polyolefin [73] and starch modifications [74]. | Polymer modification by free-radical polymerization: Free radical initiators such as peroxides are used to generate activate sites within the backbone [73,75]. Polymer modification by insertion of active pendant groups: It consists of the copolymerization of monomers who have no functional groups with co-monomers possessing pendant which make polymer grafting easier to accomplish [49]. |
| Backbone | Functionalization Method | Grafted Chains | Grafting Technique | Grafting Conditions | Measured Properties and Methods | Refs. |
|---|---|---|---|---|---|---|
| Cotton fabric | FRP by a cellulose thiocarbonate-AIBN redox system. | PMMA, PAA, PAN, PAM | Grafting from | T = 60–80 °C. | Degree of grafting (GP), gravimetric method. | [95] |
| Cellulose | FRP by a KPS-FAS REDOX system. | PSty, PAN | Grafting from | T = 60 °C; t = 3 h; [STY] = 0.65 M [KPS] = 0.14 M; [FAS] = 0.01 M. | GP, gravimetric method; FTIR and TGA to corroborate GP. | [96] |
| Cellulose fabrics | FRP by KPS initiation. | PIA | Grafting from | T = 55–80 °C; t = 2.5–5 h; [KPS] = 0.05–0.5 M; [IA] = 0.5–4 M. | GP, gravimetric method; FTIR, XRD, TGA and SEM to corroborate grafting. | [97] |
| Cellulose | FRP by CAAC initiation. | PMBA, P(N-VP) | Grafting from | T = 30–70 °C; [CAAC] = 2 − 30 × 10−5 M. | Grafting yield (GY) and other grafting parameters; gravimetric method. | [98,99] |
| Cellulose | FRP by CAN-NAC REDOX system. | PEA, PNIPAAM, PAAM-PEA, (AAM-EMA), P(AAM-MA), P(AN-EMA) | Grafting from | T = 10–60 °C; t = 24 h; [CAN] = 1.5 − 32 × 10−3 M; [NAC] = 2.5−8 × 10−2 M. | GY and other grafting parameters, gravimetric method; FTIR and TGA to corroborate grafting. FTIR and EA for composition. MMass by viscometric method and GPC. | [100,101,102,103,104,105] |
| Cellulose microfibers | Redox initiation. | PAA | Grafting through | KPS = 0.1–0.4% respect to fiber weight. t = 3 h, T = 75 °C | GP, gravimetric method; FTIR and TGA to corroborate GP. | [106] |
| Cellulose powder | Co(acac)3 | N’N’-MBA [98], or N-VP [99] | Grafting from | Cellulose washed with CH3OH, C3H6O, and water; then dried. Reaction under nitrogen atmosphere. Different temperatures, 30–60 °C [98], or 40–50 °C [99]; reaction carried out in water. Kinetic data from 0–150 min [86], or 0–120 min [99]. | Percent grafting (% G), true grafting (% GT), grafting efficiency (% GE), homopolymer conversion (% CH), cellulose conversion (% CC), and total conversion (% CT) by gravimetric methods. | [98,99] |
| Cotton linter Cellulose powder | Ceric ammonium sulfate, 1% sulfuric acid. | AcN EA MMA | Grafting from | Cellulose treated with sodium hydroxide 5–30 % wt./vol at 25 °C. Grafting temperatures: 30, 40 and 60 °C, sodium bisulfite clay as initiator. Polymerization in diluted HCl for 2 h. | Percent grafting (% G), true grafting (% GT), grafting efficiency (% GE), by gravimetric methods. | [107] |
| Hydroxypropyl cellulose (HPC) | Steglich esterification of PABTC onto HPC using DCC and DMAP. | EA NIPAAM | Grafting from | Steglich esterification using DCC and DMAP in chloroform at 40 °C, in presence of HPC and PABTC. 6 days for 50% conversion. Polymerization of EA and NIPAAM at 60 °C using AIBN; 94% conversion with free polymer in DMAc. | Tg by DSC at 45, 55 and 135 °C for PNIPAAM, and Tm at 157 °C. TGA from ambient to 600 °C at 10 °C/min, nitrogen atmosphere for HPC, PINIPAAM and the grafted HPC-g-PNIPAAM. 1HNMR for degree of substitution. SEC for HPC Macro CTA and HPC-g-PNIPAAM. | [108] |
| Cellulose chloroacetate (CellClAc) | Macro initiator, Cu(I)Cl/2′2′BIPI catalytic system via ATRP controller. | 4NPA MMA | Grafting from | ATRP of 4NPA and MMA carried out in DMF at 130 °C for 24 h, in the presence of CellClAc as macro initiator, Cu(I)Cl/2,2′BIPI catalytic system. Grafting conversion under 15%. | NMR, FTIR, TGA, and elemental analysis. | [109] |
| Cellulosic Grewia optiva fibers | Redox initiation using FAS-H2O2 for grafting of MA, and KPS for polymerization of MA. | MA | Grafting from | 1 g of mercerized Grewia optiva fibers was set in distilled aqueous solution with NaOH for 24 h, followed by addition of redox initiator (FAS-H2O2). Solution was stirred for 10 min; MA was then added. Solution was polymerized using microwave irradiation at different times. | FTIR, SEM, TGA, swell index. | [110] |
| Cellulose acetate | Solvents: DMSO, PDX, DMAc, C3H6O. Initiators for grafting and polymerization: CAN, Sn(Oct)2 and BPO. | MMA | Grafting from | Focus on solvent effect. 1.25 g of cellulose acetate were dissolved in 125 mL of solvent. CAN or Sn(Oct)2 or BPO (0.3–0.5 g) were added with MMA (1.25–2 mL). Nitrogen atmosphere, 2–6 h, 30–80 °C, except acetone, which proceeded at 55 °C. | Grafting yield (GY), total monomer conversion (TC), grafting efficiency (GE) and number of grafting chains per cellulose acetate molecule were obtained by gravimetric methods. TGA, GPC, FTIR, 1HNMR. | [111] |
| Cellulose chloroacetate (CellClAc) | Macro initiator, Cu(I)Cl/2′2′BIPI catalytic system via ATRP controller. | NCA MMA | Grafting from | ATRP of NCA and MMA in DMF at 130 °C, in the presence of CellClAc. | FTIR, TGA, and elemental analysis. | [112] |
| Cellulose cotton fibers | Na2CO3 and thermal activation. | MTC-b-CD | Grafting to | Grafting of MCT-β-CD onto cotton fabric carried out in alkaline medium; 1 mg of cellulose fiber was impregnated with solutions of 50–150 g/L of MCTβ-CD and 20–80 g/L Na2CO3. Solvent was eliminated at room temperature before heating at 100–160 °C, for 10, 15 and 20 min. Sample were washed to obtain neutral pH. Silver nitrate solutions were added in situ for some samples. | FTIR and microbiological tests [113]. Gravimetric methods and analytical modeling [114]. MODDE software was used to study the relationship between 3 significant independent variables and degree of grafting. | [113,114] |
| Cotton linter cellulose | CTP using APS as initiator. | MMA | Grafting from in situ polymer formation (embedded) | Cellulose pre-swelling: Cellulose was pre-swollen in DMAc at 160 °C for 0.5 h. Pre-swollen cellulose was filtered. A solution of LiCl in DMAc (8%, w/w) was prepared. The pre-swollen cellulose was added to the DMAc/LiCl solution. The mixture was stirred at 100 °C for 2 h and purged with gaseous N2. MMA polymerization was carried out at 70–90 °C using APS and DMSO. | TGA-DTA, FTIR, SEM, XRD. | [115] |
| Microcrystalline cellulose | Ring-opening polymerization (ROP) of L-LA with DMAP in an ionic liquid AmimCl. | PLLA | Grafting from | A 4% (w/w) microcrystalline cellulose/AmimCl solution was prepared and stirred at 60 °C, in N2 environment for 1 h. L-LA and DMAP were then added. The sample was degassed 3 times in vacuum/N2 during 1 h cycles. ROP proceeds at 90 °C in presence of N2 atmosphere during 11 h. | Controlled release of vitamin C. Characterization by 1HNRM, UV analysis, XRD, TEM, and HPLC. | [116] |
| Cellulose (DP=1130) | CTP; APS and MMA embedded. | MMA | Grafting from | Cellulose is pre-swollen in DMAc during 30 min, at 160 °C. 5 g of pre-swollen cellulose are mixed with 95 g of water and proper amounts of APS and MMA. Grafting proceeds at 80 °C. Excess of PMMA was washed with acetone. | Characterization: FTIR, WARD-XRD, SEM, and TGA-DTA. | [117] |
| Cellulose nanocrystal (CNC) | Macromolecular initiator obtained from the reaction between Br-iBuBr and CNC using TEA as catalyst, via SI-ATRP. | STY. Cast in MMA | Grafting from | Macromolecular initiator: Br-iBuBr, CNC, and TEA were dissolved in DMF, and stirred under N2 at 70 °C for 24 h. CNC, 2-bromoisobutyrate, styrene and copper(I)bromide were dissolved in anisol; PMDETA was then added. The reaction proceeded at 100 °C for 12 h and N2 atmosphere via SI-ATRP. The remanent PS homopolymer was eliminated using methanol, and centrifuging. The modified crystals were dosed into PMMA nanocomposites by solution casting. | Characterization: TEM, SEM, FTIR, UV-Vis., XRD, 13C-NMR, TGA, DSC, and tensile test | [118] |
| Cellulose nanocrystal(CNC) | L-LA in situ polymerization using MgH2 as redox agent. PLLA-g-CNC particles were casted in PLLA. | PLLA | Grafting from | 1.50 g of L-LA and 0.05 g of CNCs were pre-mixed. 0.03 g of MgH2 were then added and stirred during 30 min. The mixture was heated to 110 °C for 3, 6, 10, 12, 18 and 24 h, under nitrogen atmosphere. Casting method: 1.0 g of PLLA and 0.1 g of CNC or 1.3 g of PLLA-g-CNC were dispersed in 10 mL of chloroform or toluene solution and mixed for 10 h. The mixtures/solutions were poured into Teflon® plates and let stand for 18 h at 80 °C. The films were dried under vacuum at 60 °C for 72 h at room temperature. | Characterization: FTIR, 1HNRM, 13C-NMR, XRD, XPS, TGA, DSC and DMA. | [119] |
| Cellulose cotton fiber pulp | (a) Esterification of maleic anhydride grafted onto PHA (through double bond); (b) PHA-g-MA is grafted onto cellulose cotton fiber pulp (through the anhydride group). | PHA-g-MA | Grafting to | Cotton fibers were defibered into pulp to 25°SR using a PL4-2 speed governing beater; 30 g/m2 paper film was then formed in a RK3-KWTjul Rapid-Koethen sheet former. PHA-g-MA was dissolved in refluxing dichloromethane at 60 °C, and the paper film was dipped into the solution of PHA. The PHA/CF composite film was then washed with dichloromethane and dried. | Characterization: FTIR; XRD; SEM; surface roughness; surface hydrophobicity, with contact angle; tensile test, and TGA. | [120] |
| Cellulosic filter papers | Radiation-induced graft copolymerization (RIGCP) of AcN. | Acrylonitrile | Grafting from | The reaction took place in a glass tube which contained a Whatman filter paper (W1) and a 45% AcN solution in DMF. The solution was irradiated with cobalt-60 g-rays at a dose rate of 4 kGy/h, in air atmosphere. The grafted material was washed with DMF and water, followed by drying (T = 80 °C, 2 h). | Degree of grafting (G%) was determined. Characterization: FTIR, XRD, XRF, TGA and SEM. | [121] |
| CellClAc | ATRP grafting of the studied monomers using CuCl, 2′2′BIPI as catalyst. | NCHA, 4VP, DA, DAAM. | Grafting from | Cellulose chloroacetate (ATRP macroinitiator) and any of the monomers (NCHA, 4VP, DAAM or DA) were added to 10 mL of DMF; an inert environment was created using argon. The grafting reaction proceeded at 130 °C for 24 h. The proportion of Cell.ClAc, CuCl, 2′2′BIPI and each monomer was 1:1:3:100. The reacting mass proceeded to filtering and washing using acetonitrile, DMF, chloroform, a mixture of water-ethanol-HCl, pure water, ethanol, acetone, and diethyl ether. The product was dried under vacuum. | Characterization: FTIR, UV-Visible, TGA, elemental analysis, and electrical conductivity. | [122] |
| Regenerated cellulose fibers (rayon) | Photo-chemical grafting of PETA without photoinitiator. | PETA | Grafting to | Fibers were washed with a solution of PETA in isopropanol. Monomer content of 1% and 5% was used. The cellulosic material was irradiated with a broadband Hg lamp (emission band of 200 and 300 nm), at 50 W/cm. Layer deposition from homo-polymerization and subsequent grafting-to process onto the fiber took place. | Fiber volume content; tensile and fatigue tests; SEM. | [123] |
| Cellulose nanofibrils | Nitroxide TEMPO insertion and NMP of HEMA | HEMA | Grafting from | Preparation of TEMPO-oxidized cellulose nanofibrils. A suspension was prepared using HEMA (0.15 g), H2O2 (0.0075 g), CaCl2 (0.0075 g) and TCNF (0.3 wt.%, 500 g), followed by stirring and heating at 50 °C for 4 h. Either freezer freezing, or gradient freezing in liquid nitrogen were used. The nanofibril suspension was set in a freezer (−25 °C). The frozen material pieces were 3 cm height. Drying in a lyophilizer to form aerogels then took place. | Characterization: XRD; FTIR; XPS; SEM; stress-strain under compression analyses; BET analysis and electrical resistivity. | [124] |
| Functionalization Method | Grafted Chains | Grafting Technique | Grafting Conditions | Refs. |
|---|---|---|---|---|
| Steglich esterification of RAFT controller onto organosolv lignin to produce a microcontroller. Subsequent polymerization of soybean oil derived methacrylate monomers. | Poly(soybean oil methacrylate) derivatives. PSBMA, PSBMAH, PSBMAEO | Grafting from | 4-cyano-4-(phenylcarbonothioylthio) pentanoic acid was coupled with lignin via Steglich esterification using DCC and DMAP at ambient conditions. [Monomer]/[Lignin-RAFT]/[AIBN] molar ratio = 100:1:0.3; Temperature: 70–80 °C; Time: 24–48 h; Conversion: 74–94%. | [134] |
| Phosphorylation. SN2 reaction mechanism. | Imidazole and POCl3 react with (1H-imidazol-1-yl) phosphonic group, which reacts with lignin. | Grafting to | Reacted imidazole-POCl3. DMF, 80 °C, 6h. Lignin-g- (1H-imidazol-1-yl)phosphonic group. 95 °C, 12 h, pH = 3–4 in DMF, yield = 92%. Composites were blended with PP-block-PE, MFI of 1.39 g/10 min (T = 230 °C; standard weight of 2.16 kg), ethylene content 17.8 wt %, from China Petrochemical Co. in a Thermo Haake torque rheometer (180 °C: 10 min; 60 rpm). | [125,135] |
| Phosphorus(V) oxide | Grafting to | P4O10. Temperature = 20–25 °C, time = 7–8 h. Solvent: THF. | [125,136,137] | |
| Hemicellulose grafting using TBD and ɛ-caprolactone monomer. | PCL - Hemicellulose (HC-g-PCL) | Grafting from | 5 g of dried hemicellulose (5.00 g) were introduced into a reactor containing 10 mL of DMSO at 80 °C. Agitation at 60 rpm took place until a homogeneous viscous solution was obtained. Grafting temperature was 110 °C. ε-caprolactone monomer was added to the hemicellulose solution and agitated during 30 min. TBD, 1% of the total mass, was added. The reaction proceeded during 4 hrs. in a system with flow of nitrogen. Drying at 60 °C under vacuum proceeded for 2 days. FTIR, 1HRM, 13CNMR, HSQC, GPC, DSC, TGA-FTIR, Tensile Test, Contact Angle, Biodegradation test. | [37] |
| Several techniques presented in the review. | Monomers or polymers used in: ATRP: NIPAAM, DMAEMA, STY, MMA, BA, EG; RAFT: AM, AA, others; ROP: PCL, L-LA, EG; Click chemistry: polymer having alkyne or azide groups for the azide-alkyne cycloaddition, EG, PCL, PLLA. | Grafting, from; grafting to; network copolymer grafting | Grafting from: ATRP, RAFT, ROP, and FRP. Grafting onto: Thiol-ene reaction with photo-redox catalysis, click chemistry, epoxide group-mediated reactions, condensation. Network copolymer grafting: Polycondensation, free radical crosslinking. | [9] |
| Insertion of ACX onto lignin to synthesize a RAFT macrocontroller which polymerized AM and AA. | RAFT polymerization of AM and AA. | Grafting from | 0.1 g of RAFT macroinitiator and 0.005 g of AIBN were added to a solution of monomer (0.3 g) in DMF (4 mL). The reactor was degassed with nitrogen for 30 min at ambient conditios. Reaction temperature was 70 °C, target conversion was 98% in 24 h for both monomers. The product was obtained by precipitation, filtration, washing and drying. Grafting was measured using 1H NMR. 0.2 g Lignin-g-Pam were dissolved in KOH solution (0.1 g in 1 mL of water), followed by heating (T = 70 °C, t = 12 hrs.), followed by neutralization with HCl. The solution was then precipitated into diethyl ether and dried in air. Ungrafted PAm was withdrawn by extraction with CH2Cl2. The product was vacuum dried, and analyzed by 1H NMR and GPC, using adequate solvents. | [138,139] |
| Several techniques described in the review. Monomers copolymerized with natural extracts. | Several procedures described. | Grafting from Grafting through | Monomers and polymers grafted by: FRP: Guaiacol-AM, Vainillin-LMA. RAFT: Syringyl methacrylate, STY, MMA, 4-propylsyringol, 4-propylguaiacol. ADMET (Acyclic Diene Metathesis Polymerization) and ROP: Ferulic acid, isorbide, butanediol. | [140] |
| ATRP | NIPAAM  PNIPAAM PNIPAAM | Grafting from | Backbone: Kraft lignin (alkali); Catalyst: CuBr/PMDETA; Solvent: water/DMF; T = 50 °C; Thermoresponsive material. | [140,141] |
| ATRP | DAEA  PDAEA PDAEA | Grafting from | Backbone: Organosolv lignin; Catalyst: CuBr/Me6TREN; Solvent: THF; T = 65 °C; Hydrophobic polymer composites. | [140,142] |
| ATRP | PEG-A; NIPAAM | Grafting from | Backbone: Kraft lignin (alkali); Catalyst: CuBr/HMTETA. Solvent: PDX; T = 60–70 °C; Thermogelling material. | [140,143] |
| ATRP | MMA  PMMA PMMABMA  PBMA PBMA | Grafting from | Backbone: Kraft lignin; Catalyst: CuBr/PMDETA; Solvent: water/DMF; T = 80 °C; Thermoplastic elastomers. | [140,144] |
| SI-ATRP | NIPAAM  PNIPAAM PNIPAAM | Grafting from | Backbone: Softwood Kraft lignin; Catalyst: CuCl/HMTETA; Solvent: water; Room temperature; Ionic responsive nanofibrous material. | [140,145] |
| ATRP | MMA  PMMA PMMASTY  PS PS | Grafting from | PMMA Backbone: Kraft lignin (alkali); Lignin + STY  lignin-g-PS; Catalyst: CuCl/2′2′BIPI; Solvent: DMF; T = 100 °C, 14 days. lignin-g-PS; Catalyst: CuCl/2′2′BIPI; Solvent: DMF; T = 100 °C, 14 days.PS (PSty) Backbone: Kraft lignin (alkali); Lignin + MMA  Lignin-g-PMMA; Catalyst: CuBr/PMDETA; Solvent: DMF; T = 70 °C, 24 h; Thermoplastic lignin composites. Lignin-g-PMMA; Catalyst: CuBr/PMDETA; Solvent: DMF; T = 70 °C, 24 h; Thermoplastic lignin composites. | [140,146] |
| ATRP | MMA  PMMA PMMA | Grafting from | Backbone: Kraft lignin (alkali); Lignin + MMA  Lignin-g-PMMA; Catalyst: CuBr/PMDETA; Solvent: DMF; T = 70 °C, 24 h; Thermoplastic lignin composites. Lignin-g-PMMA; Catalyst: CuBr/PMDETA; Solvent: DMF; T = 70 °C, 24 h; Thermoplastic lignin composites. | [140,147] |
| ATRP | PEGMA | Grafting from | Backbone: Kraft lignin (alkali); Lignin-Br + PEGMA  Lignin-g-PEGMA; Catalyst: CuBr/HMTETA; Solvent: Acetone; Room temperature, overnight; Supramolecular hydrogels, self-healing materials. Lignin-g-PEGMA; Catalyst: CuBr/HMTETA; Solvent: Acetone; Room temperature, overnight; Supramolecular hydrogels, self-healing materials. | [140,148] |
| ATRP | STY  PS PS | Grafting from | Backbone: Kraft lignin (alkali); Lignin-Br + STY  lignin-g-PS; Catalyst: FeCl3 6H2O/PPh3/ascorbic acid; Solvent: DMF; T = 110 °C, 24 h; Novel polymerization method. lignin-g-PS; Catalyst: FeCl3 6H2O/PPh3/ascorbic acid; Solvent: DMF; T = 110 °C, 24 h; Novel polymerization method. | [140,149] |
| ATRP | MMA  PMMA PMMA | Grafting from | Backbone: Kraft lignin (alkali); Lignin + MMA  lignin-g-PMMA; Catalyst: FeCl3 6H2O/PPh3/ascorbic acid; Solvent: DMF; T = 90 °C, 24 h; Novel polymerization approach. lignin-g-PMMA; Catalyst: FeCl3 6H2O/PPh3/ascorbic acid; Solvent: DMF; T = 90 °C, 24 h; Novel polymerization approach. | [140,149] |
| ATRP | DMAEMA  PDMAEMA PDMAEMA | Grafting from | Backbone: Kraft lignin (alkali); Lignin-Br + DMAEMA  lignin-g-PDMAEMA; Catalyst: CuBr/HMTETA; Solvent: PDX; T = 65 °C, 48 h; Gene delivery. lignin-g-PDMAEMA; Catalyst: CuBr/HMTETA; Solvent: PDX; T = 65 °C, 48 h; Gene delivery. | [150] |
| RAFT | AM  PAM PAM | Grafting from | Backbone: Kraft lignin from softwood sources, prepared using KEX, 2-Bromopropionic acid, and thionyl chloride in dry THF in N2 atmosphere at 70 °C, overnight. Lignin-KEX + AM  Lignin-KEX-g-PAM; RAFT controller: Lignin Macrocontroller; Initiator: AIBN; Solvent: DMF; T = 70 °C, overnight; Application: Pickering emulsions. Lignin-KEX-g-PAM; RAFT controller: Lignin Macrocontroller; Initiator: AIBN; Solvent: DMF; T = 70 °C, overnight; Application: Pickering emulsions. | [151] |
| RAFT | AM  PAM PAM | Grafting from | Backbone: Kraft lignin. Prepared with KEX, 2-Bromopropionic acid, and thionyl chloride in dry THF in N2 atmosphere at 70 °C, overnight. Lignin-KEX + AM  Lignin-KEX-g-PAM; RAFT controller: Lignin Macrocontroller; Initiator: AIBN. Solvent: DMF; T = 70 °C; Application: Plasticizer for Portland cement paste. Lignin-KEX-g-PAM; RAFT controller: Lignin Macrocontroller; Initiator: AIBN. Solvent: DMF; T = 70 °C; Application: Plasticizer for Portland cement paste. | [152] |
| Esterification via epoxy group | Lignin + GMA  Lignin-GMA + AM Lignin-GMA + AM  Lignin-GMA-g-PAM Lignin-GMA-g-PAM | Grafting from | Backbone: Kraft lignin was first functionalized by reacting with GM through the epoxide ring; Lignin + GMA  Lignin-GMA; Lignin-GMA + AM Lignin-GMA; Lignin-GMA + AM  Lignin-GMA-g-PAM; RAFT controller: Lignin Macrocontroller; Initiator: AIBN; Solvent: DMF; T = 70 °C; Application: Plasticizer for Portland cement paste. Lignin-GMA-g-PAM; RAFT controller: Lignin Macrocontroller; Initiator: AIBN; Solvent: DMF; T = 70 °C; Application: Plasticizer for Portland cement paste. | [153] |
| RAFT | Lignin + ACX  Lignin-ACX + AM Lignin-ACX + AM  Lignin-ACX-g-PAM Lignin-ACX-g-PAM | Grafting from | Backbone: Kraft lignin; Lignin + ACX  Lignin-ACX; Lignin-ACX + AM Lignin-ACX; Lignin-ACX + AM  Lignin-ACX-g-PAM; RAFT controller: Lignin Macrocontroller; Initiator: AIBN; Solvent: DMF; T = 70 °C; Application: Cationic flocculant. Lignin-ACX-g-PAM; RAFT controller: Lignin Macrocontroller; Initiator: AIBN; Solvent: DMF; T = 70 °C; Application: Cationic flocculant. | [153] |
| RAFT | Lignin + XCA  Lignin-XCA + DMC Lignin-XCA + DMC  Lignin-XCA-g-PDMC Lignin-XCA-g-PDMC | Grafting from | Backbone: Kraft lignin; Lignin + XCA  Lignin-XCA; Lignin-XCA + DMC Lignin-XCA; Lignin-XCA + DMC  Lignin-XCA-g-PDMC; RAFT controller: Lignin; Macrocontroller prepared by Steglich esterification using DCC and DMAP for 48 h; Initiator: AMBN. Solvent: DMF; T = 70 °C, under nitrogen atmosphere; Application: Cationic flocculant. Lignin-XCA-g-PDMC; RAFT controller: Lignin; Macrocontroller prepared by Steglich esterification using DCC and DMAP for 48 h; Initiator: AMBN. Solvent: DMF; T = 70 °C, under nitrogen atmosphere; Application: Cationic flocculant. | [154] |
| ROP | Lignophenol from Japanese cedar + EOX  Lignin-g-PEOX Lignin-g-PEOX | Grafting from | Backbone: Lignophenol from Japanese cedar; Solvent containing benzyl bromide groups; Monomer: EOX; T = 100 °C, 12 h; Application: Composite materials. | [155] |
| ROP | Sulfonated Lignin + MOX  Lignin-g-PMOX Lignin-g-PMOX | Grafting from | Backbone: Lignophenol from Japanese cedar; Initiator: Tosylated lignin; Monomer: MOX; Solvent: DMSO; T = 100 °C, 10 h; Application: Anti-ineffective ointment. | [156] |
| ROP | Indulin AT lignin + L-LA  Indulin AT lignin-g-PLLA Indulin AT lignin-g-PLLA | Grafting from | Backbone: Indulin AT lignin; Initiator: Triazabicyclodecene; Monomer: L-LA; Reaction carried out in bulk; T = 135 °C; Application: composites. | [157] |
| ROP | Biobutanol lignin + CL  Biobutanol lignin -g-PCL Biobutanol lignin -g-PCL | Grafting from | Backbone: Biobutanol lignin; Initiator: Triazabicyclodecene; Monomer: CL; Reaction carried out in bulk; T = 135 °C; Application: composites. | [158] |
| ROP | Lignin (organosolv) + CL + L-LA  Lignin-g-PCL/PLLA Lignin-g-PCL/PLLA | Grafting from | Backbone: Lignin orgaosolv; Initiator: Sn(Oct)2 (tin(II)2-ethylhexanoate) and alkyl alcohol functional groups on lignin; Monomer: CL and L-LA (ratio 2:1); Solvent: Toluene; T = 120 °C, under Ar atmosphere, during 45 h; Application: composites. | [159] |
| ROP | Alkali Lignin + B-BL  Lignin-g-PHB Lignin-g-PHB | Grafting from | Backbone: Lignin alkali; Initiator: Sn(Oct)2 (tin(II)2-ethylhexanoate) and alkyl alcohol functional groups on lignin; Monomer: B-BL; Reaction carried out in bulk; T = 130 °C, under nitrogen atmosphere, during 24 h; Application: Nano-fibers for medical applications. | [160] |
| ROP | Lignin alkali + CL  Lignin-g-PCL Lignin-g-PCL | Grafting from | Backbone: Lignin Alkali; Initiator: Sn(Oct)2 (tin(II)2-ethylhexanoate) and alkyl alcohol functional groups on lignin; Monomer: CL; Reaction carried out in bulk; T = 50 °C, under nitrogen atmosphere, during 14–62 h; Application: Nano-fibers for medical applications. | [161] |
| ROP | Lignin alkali + CL  Lignin-g-PCL Lignin-g-PCL | Grafting from | Backbone: Lignin alkali; Initiator: Sn(Oct)2 (tin(II)2-ethylhexanoate) and alkyl alcohol functional groups on lignin; Monomer: CL; Reaction carried out in bulk; T = 50 °C, under nitrogen atmosphere, during 14–62 h; Application: Nano-fibers for medical applications. | [162] |
| ROP | Softwood Kraft lignin from UPM BioPiva + EOX  Lignin-g-PEOX Lignin-g-PEOX | Grafting from | Backbone: Softwood Kraft lignin from UPM BioPiva; Initiator: P4-t-Bu; Monomer: CL; Solvent: Toluene; T = 180 °C, under Ar atmosphere, during 16 h; Application: Non-ionic surfactants. | [163] |
| Embedded polymerization and further crosslinking. | Chitosan; N-VP; 4VP; Crosslinking agent: N’N’-MBA | Grafting from | 1.5 g of chitosan were dried at 40–50 °C during 24 h and suspended in 150 mL of 2% CH3COOH for 1 to 2 h. 2 mL of monomers in a 1:1 ratio were incorporated and stirred for 30 min. 0.15 mol/L AIBN were then added and stirred for 2 more hours. T = 65 °C, under air environment. Product washed using several solvents, and dried at 50 °C. The yield was 78–86%. A solution of grafted chitosan in acetic acid (0.1 % v/v) was heated at 70 °C; N’N’-MBA was then added and allowed to react during 30 min. | [164] |
| RAFT; Steglich esterification of phthalic anhydride and 4,4-Azobis(4-cyanovaleric acid). | Chitosan + Phthalic Anhydride  (DCC/DMAP) (DCC/DMAP)  Chitosan-Phthaloylated Chitosan-Phthaloylated Chitosan-Phthaloylated + 4,4-Azobis(4-cyanovaleric acid)  (DCC/DMAP) (DCC/DMAP)  Chitosan-Phthaloylate-RAFT. Chitosan-Phthaloylate-RAFT.Chitosan-Phthaloylate-RAFT + NIPAAM  Chitosan-Phthaloaylte-RAFT-g-NIPAAM. Chitosan-Phthaloaylte-RAFT-g-NIPAAM. | Grafting from | Phthaloylation of chitosan: A solution of phthalic anhydride (5.5 mmol) in 6 mL of DMF/H2O (95/5 v/v %) was prepared, followed by the addition of chitosan powder in 1.86 mmol. The system was purged by N2 for 0.5 h. Heating at 120 °C was maintained during 8 h. The phthaloylated chitosan (1 mmol) was mixed with 1.2 mmol and 4,4-azobis(4-cyanovaleric acid).in 30 mL of dried DMF in the presence of carbonyl activating reagents of DCC (1 mmol) and 4-DMAP (0.12 mmol) as catalysts. Reaction at room temperature for 10 days. Chitosan-Phthaloylate-RAFT (0.0468 g) and dry DMF (5 mL) were mixed by stirring under nitrogen atmosphere. After complete dissolution, initiator 4,4-Azobis(4-cyanovaleric acid) (0.01 mmol) and NIPAAM monomer (0.50 g; 4.4 mmol) were incorporated. Polymerization conducted at 70 °C for 48 h. | [165] |
| Cerium (IV) attack | Chitin + acrylic monomers. | Grafting onto | Cerium (IV); Chitin + AM  Chitin-g-PAM; Chitin + AA Chitin-g-PAM; Chitin + AA  Chitin-g-PAA; Chitin + MA Chitin-g-PAA; Chitin + MA  Chitin-g-PMA; Chitin + MMA Chitin-g-PMA; Chitin + MMA  Chitin-g-PMMA Chitin-g-PMMA | [58] |
| γ-Radiation | Chitin + STY (radiated) | Grafting onto | Chitin + STY  Chitin-g-PS Chitin-g-PS | [58] |
| ROP | Cyclic and vinyl monomers. Modified Chitin | Grafting from | Surface-initiated ROP graft copolymerization of L-LA and CL with initiation by hydroxy groups on chitin nanofiber, catalyzed by tin(II) 2-ethylhexanoate was performed, obtaining chitin nanofiber-graft-PLA-co-PL, which is a known biodegradable polyester. Chitin was soaked in a solution of LA/CL in toluene; tin(II) 2-ethylhexanoate (ca. 5 mol%) was incorporated. Heating at 80 °C was maintained during 48 h. | [166] |
| γ-Radiation | Chitosan + AA +TiO2  Chitosan-g-PAA-TIO2 Chitosan-g-PAA-TIO2 | Grafting from | A solution of CS-PAAc (1:1 mass/vol) was prepared with different contents of TiO2; 0.0, 1.0, 2.0 and 3.0 wt.% of the total polymer concentration was incorporated. Each solution was sonicated in a bath for 15 min. The solutions were transferred into small glass vials and were subjected to 60 Co-gamma rays at irradiation dose of 30 kGy. | [167] |
| ROP | Chitosan + CL  Chitoan-g-PCL Chitoan-g-PCL | Grafting from | 0.5 g chitosan and 15 mL MeSO3H were placed in a round bottom flask under nitrogen atmosphere at 45 °C for 0.5 h. 4.19 g CL monomers were fed to the reactor, followed by stirring and reaction during 4.5 h. | [168] |
| Title a | Reference |
|---|---|
| Synthesis of diallyl dimethyl ammonium chloride grafted polyvinyl pyrrolidone (PVP-g-DADMAC) and its applications. | [190] |
| Synthesis and characterization of biocompatible hydrogel based on hydroxyethyl cellulose-g-poly(hydroxyethyl methacrylate). | [191] |
| Poly(vinylidene fluoride) (PVDF)/PVDF-g-polyvinylpyrrolidone (PVP)/TiO2 mixed matrix nanofiltration membranes: preparation and characterization. | [192] |
| Synthesis and characterization of poly (acrylonitrile-g-lignin) by semi-batch solution polymerization and evaluation of their potential application as carbon materials | [193] |
| High crosslinked sodium carboxyl methylstarch-g-poly (acrylic acid-co-acrylamide) resin for heavy metal adsorption: its characteristics and mechanisms. | [194] |
| Preparation and characterization of PLLA/chitosan-graft-poly (ε-caprolactone) (CS-g-PCL) composite fibrous mats: The microstructure, performance and proliferation assessment. | [168] |
| Synthesis of partly debranched starch-g-poly(2-acryloyloxyethyl trimethyl ammonium chloride) catalyzed by horseradish peroxidase and the effect on adhesion to polyester/cotton yarn. | [195] |
| Cellulose Acetate thermoplastics with high modulus, dimensional stability and anti-migration properties by using CA-g-PLA as macromolecular plasticizer. | [196] |
| Superwettable PVDF/PVDF- g-PEGMA ultrafiltration membranes. | [197] |
| Removal of malachite green using carboxymethyl cellulose-g-polyacrylamide/montmorillonite nanocomposite hydrogel. | [198] |
| Ultraviolet illumination responsivity of the Au/n-Si diodes with and without poly (linolenic acid)-g-poly (caprolactone)-g-poly (t-butyl acrylate) interfacial layer. | [199] |
| Synthesis of CO2-based polycarbonate-: G -polystyrene copolymers via NMRP. | [200] |
| Compatibilization of iPP/HDPE blends with PE-g-iPP graft copolymers. | [201] |
| Preparation, characterization of feather protein-g-poly(sodium allyl sulfonate) and its application as a low-temperature adhesive to cotton and viscose fibers for warp sizing. | [202] |
| Semi-Natural superabsorbents based on starch-g-poly(acrylic acid): Modification, synthesis and application. | [203] |
| Design and development of polymethylmethacrylate-grafted gellan gum (PMMA-g-GG)-based pH-sensitive novel drug delivery system for antidiabetic therapy. | [204] |
| Preparation and characterization of bioinert amphiphilic P(VDF-co-CTFE)-g-POEM graft copolymer. | [205] |
| Preparation, characterization of poly(acrylic acid)-g-feather protein-g-poly(methyl acrylate) and application in improving adhesion of protein to PLA fibers for sizing. | [206] |
| Antibacterial and pH-responsive quaternized hydroxypropyl cellulose-g-poly(THF-co-epichlorohydrin) graft copolymer: Synthesis, characterization and properties. | [207] |
| Thermally self-assembled biodegradable poly(casein-g-N-isopropylacrylamide) unimers and their application in drug delivery for cancer therapy. | [208] |
| Swelling capacity of sugarcane bagasse-g-poly(acrylamide)/attapulgite superabsorbent composites and their application as slow release fertilizer | [209] |
| Structure formation in pH-sensitive micro porous membrane from well-defined ethyl cellulose-g-PDEAEMA via non-solvent-induced phase separation process | [210] |
| Micelles with a loose core self-assembled from coil-g-rod graft copolymers displaying high drug loading capacity. | [211] |
| Synthesis and water absorbing properties of KGM-g-P(AA-AM-(DMAEA-EB)) via grafting polymerization method. | [212] |
| Preparation of hydrophilic woven fabrics: Surface modification of poly(ethylene terephthalate) by grafting of poly(vinyl alcohol) and poly(vinyl alcohol)-g-(N-vinyl-2-pyrrolidone). | [213] |
| The preparation, physicochemical and thermal properties of the high moisture, solvent and chemical resistant starch-g-poly(geranyl methacrylate) copolymers. | [214] |
| Reverse poly(ε-caprolactone)-g-dextran graft copolymers. Nano-carriers for intracellular uptake of anticancer drugs. | [215] |
| High-performance solid-state bendable supercapacitors based on PEGBEM-g-PAEMA graft copolymer electrolyte. | [216] |
| Design of well-defined polyethylene-g-poly-methyltrifluorosiloxane graft copolymers via direct copolymerization of ethylene with polyfluorosiloxane macromonomers. | [217] |
| Synthesis and characterization of poly(vinyl chloride-g-ε-caprolactone) brush type graft copolymers by ring-opening polymerization and “click” chemistry. | [218] |
| Adhesion of cornstarch-g-poly (2-hydroxyethyl acrylate) to cotton fibers in sizing. | [219] |
| Polynorbornene-g-poly(ethylene oxide) through the combination of ROMP and nitroxide radical coupling reactions. | [220] |
| Potent bioactive bone cements impregnated with polystyrene-g-soybean oil-AgNPs for advanced bone tissue applications. | [221] |
| Fabrication of cellulose nanocrystal-g-poly(acrylic acid-co-acrylamide) aerogels for efficient Pb(II) removal. | [222] |
| The compatibilization of PLA-g-TPU graft copolymer on polylactide/thermoplastic polyurethane blends. | [223] |
| Cellulose-g-poly-(acrylamide-co-acrylic acid) polymeric bioadsorbent for the removal of toxic inorganic pollutants from wastewaters | [224] |
| Performance improvement for thin-film composite nanofiltration membranes prepared on PSf/PSf-g-PEG blended substrates. | [225] |
| Synthesis and characterization of poly(vinyl chloride-g-methyl methacrylate) graft copolymer by redox polymerization and Cu catalyzed azide-alkyne cycloaddition reaction. | [226] |
| Encapsulation of NPK fertilizer for slow release using sodium carboxymethyl cellulose-g-poly (AA-C0-AM-C0-AMPS)/ Montmorillonite clay-based nanocomposite hydrogels for sustainable agricultural applications. | [227] |
| Engineering a “PEG-g-PEI/DNA nanoparticle-in- PLGA microsphere” hybrid-controlled release system to enhance immunogenicity of DNA vaccine. | [228] |
| Chitosan-g-oligo(L,L-lactide) copolymer hydrogel for nervous tissue regeneration in glutamate excitotoxicity: In vitro feasibility evaluation | [229] |
| Method | Property Measured |
|---|---|
| Nuclear magnetic resonance (NMR) | NMR identifies the nature of the group and the site where the graft is attached to the polymer backbone. 1H NMR and 13C NMR are utilized depending on the chemical nature of the material [73]. |
| Infrared spectroscopy (IR) | Identification of specific functional groups grafted to backbone. It can also be used to quantify grafting functionalities in modified polyolefins by determining the intensity of the characteristic reference bands in the sample, and compare them to a calibration curve of known concentrations of the same functional group [73]. |
| Scanning electron microscopy (SEM) | SEM is useful for the study of surface morphologies of grafted materials; also used as a tool to confirm that grafting has taken place [59]. |
| X-ray diffraction (XRD) | XRD is useful for the study of crystallinity of chemical substances. Polymer grafting is associated with changes in XRD patterns [59]. |
| Size exclusion chromatography (SEC) | It allows determination of molecular weight averages (MW) and molecular weight distributions (MWD) of grafted chains [230], which must be separated from the backbone before carrying out the analyses to focus on the grafts only. |
| Differential scanning calorimetry (DSC) | This is utilized to evaluate changes in crystallinity through the thermal behavior between backbones and grafted materials. |
| Thermogravimetric analysis (TGA) | This is used to study changes in thermal decomposition profiles between backbones and grafted materials. |
| Backbone | Grafts | Technique | Property Measured | Application | Refs. |
|---|---|---|---|---|---|
| Lignin-RAFT macrocontroller | Poly(soybean oil methacrylate) derivatives. PSBMA, PSBMAH, PSBMAEO | DSC | Tg of lignin-g-polymerized soybean oil derived methacrylate. Measured Tg: Lignin-g-PSBMA −5.6 °C, Lignin-g-PSBMAH 27.7 °C, Lignin-g-PSBMAEO −17.4 °C. DSC2000, TA Instruments. 10 mg samples were used with heating from 25 °C to 200 °C at 10 °C/min, and cooling to −70 °C at the same rate. Data was gathered from the second heating scan to 200 °C. Nitrogen flow: 50 mL/min. |   | [134] |
| TGA | Thermal stability of biocomposites. Two MDT values were found for all samples 380 °C and 430 °C. Q5000 TGA system (TA Instruments); 25 to 600 °C, at 10 °C/min, 25 mL/min nitrogen flow. | ||||
| Lignin | Imidazole and POCl3 react in to (1H-imidazol-1-yl)phosphonic group which reacts with lignin | TGA | MDT values for lignin derivatives and PP blends. Lignin MDT = 398 °C. Lignin-g-IPG MDT > 600 °C. PP =418°C, PP/Lignin = 472 °C, PP/20 °% Lignin-g-IPG = 479 °C, PP/30 °% Lignin-g-IPG = 483 °C. SDTQ600 thermal analyzer; 20 °C/min under nitrogen, from ambient to 600 °C. |  | [125,135] |
| FR | PHRR (kW/m2)//THR (MJ/m2) PP =1350 kW/m2//87.3 MJ/m2, PP/Lignin = 382/333 kW/m2//76 MJ/m2, PP/20%Lignin-g-IPG = 380 kW/m2//74.2 MJ/m2, PP/30%Lignin-g-IPG = 360 kW/m2//69.7 MJ/m2. Heat release rate measured using an FTT UK cone calorimeter following ISO 5660. Incident flux: 35 kW/m2. | ||||
| Hydroxypropyl cellulose (HPC) | Steglich esterification of PABTC over HPC using DCC and DMAP and grafting of PABTC and further polymerization of EA NIPAAM. | TGA | TGA 2950 analyzer; nitrogen atmosphere (60 cm3/min). Heating of samples (5–10 mg) from ambient temperature to 600 °C, at 10 °C/min. Calculations of the onset, the end decomposition temperature, and the residual mass. |  | [108] |
| DSC | Modulated DSC 2920 in nitrogen environment (60 cm3/min). The determination of glass transition temperature (Tg) required 5–10 mg samples. Heating from ambient temperature to 200 °C, followed by cooling to -100 °C, and reheating to to 200 °C, using 10 °C/min for both, heating and cooling was used. This sequence occured three times. | ||||
| CellClAc | ATRP of 4NPA and MMA using Cu(I)Cl/2′2′BIPI macro initiator. | TGA | Shimadzu TGA-50; heating rate: 10 °C/min; nitrogen flow: 10 mL/min. |  | [109,112] |
| Cellulosic Grewia optiva fibers | Redox initiator FAS-H2O2 for grafting MA and KPS for polymerization of MA. | TGA | Thermo gravimetric analyses of plain and grafted cellulosic fibers carried out in presence of nitrogen on a Perkin Elmer thermal analyzer. Heating from ambient temperature to 900 °C/min, at 10 °C/min. |  | [110] |
| Cotton linter cellulose | APS with MMA. | TGA-DTA | Samples were analyzed using a Pyris Diamond TG-DTA (STA449C/3/F, Germany). Sample weight: 14.2 mg. Heating rate: 10 °C/min. Flow of nitrogen 20 mL/min. |  | [115] |
| Cellulose (DP = 1130) | APS and MMA embedded. | TGA-DTA | Samples analyzed in a Pyris Diamond TG/DTA equipment (STA449C/3/F, German). Sample weight: 14.2 mg. Heating/Cooling rate: 10 °C/min. Flow of nitrogen: 20 mL/min. |  | |
| Cellulose nanocrystal (CNC) | Macromolecular initiator obtained from the reaction between Br-iBuBr and CNC with TEA as catalyst via SI-ATRP with sTY. Material was casted in PMMA. | TGA | Thermal degradation of studied materials analyzed in a TGA/SDTA 851e in nitrogen atmosphere. Heating from 30 to 550 °C, at 10 °C/min. |  | [118] |
| DSC | Tg determined in a Pyris 1 DSC equipment. Heating from 30 to 200 °C at 10 °C/min. | ||||
| Cellulose nanocrystal (CNC) | Macromolecular initiator obtained from the reaction between Br-iBuBr and CNC with TEA as catalyst via SI-ATRP with sTY. Material was casted in PMMA. | TGA | Analyses carried out in a TGA Q50 (TA Instruments), under nitrogen atmosphere (20 mL/min). Heating from 30 to 500 °C at 5, 10, 15, 20, 25 and 30 °C/min for determination of degradation activation energy. |  | [119] |
| DSC | Analyses performed in a Star 1 (Mettler- Toledo). Heating from 25 °C to 200 °C at 5 °C/min, remaining at 200 °C for 5 min. Cooling to −50 °C at 20 °C/min, remaining there for 5 min, followed by heating to 200 °C at 5 °C/min. Tg, Tm, Tc were measured. Crystallinity (v) calculated from melting enthalpy of fully crystalline PLLA. | ||||
| Cellulose cotton fiber pulp | Esterification of maleic anhydride grafted over PHA (by double bond). PHA-g-MA grafted over cellulose cotton fiber pulp (by the anhydride group). | TGA | Analyses carried out on a Q500 TGA (TA Instruments), in nitrogen environment. Heating from ambient to 600 °C at 20 °C/min. |  | [120] |
| Cellulosic filter papers | Radiation-induced graft (RIGCP) copolymerization of acrylonitrile. | TGA | Analyses obtained in TGA-50 (Shimadzu). Heating rate: 10 °C/min. N2 environment. |  | [121] |
| Hemicellulose | Hemicellulose grafting using TBD and e-caprolactona monomer | TGA | TGA/DSC 1 STARe System (Mettler Toledo). 5–15 mg of a sample. Nitrogen environment (85 mL/min). Heating from 30–500 °C at 10 °C/min. Compositions of produced gases measured in an FTIR (TA, Nicolet, iS); detector coupled to TGA. FTIR analyses from 4000 to 650 cm−1 with a 4 cm−1 resolution. |   | [37] |
| DSC | DSCQ10 (TA Instruments). Hermetic pan (T 090127). Heating from 25 to 165 °C at 10 °C/min. Cooling to 15 °C followed by heating to 165 °C at 10 °C/min. Tg obtained from second heating ramp. | ||||
| CellClAc | NCHA, 4VP, DA and DAAM grafted by atom transfer radical polymerization (ATRP) using CuCl, 2′2′BIPI as catalyst. | TGA | Thermal analyses carried out in a Shimadzu TGA-50 at 10 °C/min in nitrogen environment (10 mL/min). Cell-g-DA and Cell-g-4VP exhibited higher decomposition temperatures. |   | [122] |
| Chitosan | Embedded polymerization N-VP, 4VP. Crosslinking agent: N’N’-MBA | TGA | TGA analyses carried out using a NETZSCH, STA 409 PG.4.G Luxx. 50 mL/min nitrogen environment. Heating from 20 °C to 400 °C at 10 °C/min. |  | [164] |
| Backbone | Grafts | Technique | Property Measured | Application | Refs. |
|---|---|---|---|---|---|
| Lignin-RAFT macrocontroller | Poly(soybean oil methacrylate) derivatives. PSBMA, PSBMAH, PSBMAEO. | 1H-NMR | Amount of RAFT controller reacted (shifts 7.3, 7.6, 7.9 ppm) and nonattached monomer (shifts 5.3, 5.7 ppm). Varian Mercury 300 spectrometer, 300 MHz; tetramethyl silane as an internal reference. |   | [134] |
| FTIR | Amount of RAFT controller and polymer attached to lignin. Area of peak at 1760 cm−1. Perkin Elmer spectrum 100. ATR method, recorded at 4 cm−1, 32 scans. | ||||
| Lignin | Imidazole and POCl3 react in to (1H-imidazol-1-yl) phosphonic group which reacts with lignin. | FTIR | Lignin, lignin-OH, imidazole and lignin-g-IPG were compared at relevant bands (1662, 1260, 1080, 1050, 878, and 791 cm−1) for determination of C=N, P=O, P-O-C, P-O, -OH, and P-N groups, respectively. A Bruker VECTOR 22 spectrometer was used. |  | [125,135] |
| XPS | Surface elementary composition of samples (wt.%), were compared to determine the amount of functionalized or grafted groups in the surface. Elements analyzed: carbon, oxygen, phosphorous, nitrogen. Lignin, C: 68.2, O: 31.9; Modified lignin-OH, C: 67.5, O: 32.5; Lignin-g-IPG, C: 53.0, O: 31.7, P: 8.1, N: 7.2. A Thermo ESCALAB 250 spectrometer was used. Analyses were carried out from 50 up to 800 eV of binding energy. | ||||
| Hydroxypropyl cellulose (HPC) | Steglich esterification of PABTC over HPC using DCC and DMAP; grafting of PABTC and further polymerization of EA NIPAAM. | 1H NMR | NMR spectra obtained using a Bruker 300 or 200 MHz in deuterated chloroform or deuterated dimethyl sulfoxide (d6-DMSO). |  | [108] |
| CellClAc | Macro initiator, Cu(I)Cl/2′2′BIPI catalytic system via ATRP of 4NPA and MMA | 1H-NMR | NMR spectra obtained using a Bruker 400 MHz spectrometer at room temperature in DMSO-d6. |  | [109] |
| FTIR | FTIR data using solid samples as KBr pellets, from 4000 cm−1 to 450 cm−1. | ||||
| Cellulosic Grewia optiva fibers | Redox initiator FAS-H2O2 for grafting of MA, and KPS for polymerization of MA. | FTIR | Chemical structures of fibers before and after grafting were studied using FTIR (PERKIN ELMER RXI). Spectrum recorded from 4000 to 400 cm−1. |  | [110] |
| Cellulose acetate | Solvents: DMSO, PDX, DMAc, C3H6O. Initiators for grafting and polymerization: CAN, Sn(Oct)2 and BPO. | 1H-NMR | Spectra of studied materials obtained using a Bruker WM-400 apparatus, at 300 MHz. Tetramethyl silane (TMS) used as internal standard and DMSO-d6 as solvent. |  | [111] |
| FTIR | Grafted and ungrafted materials analyzed using a Spectrum RX1 PerkinElmer apparatus. Chloroform used as a solvent. KBr pellet technique used for grafted materials. | ||||
| CellClAc | Macro initiator, Cu(I)Cl/2′2′BIPI catalytic system via ATRP controller. | FTIR | FTIR spectrophotometer on solid samples as KBr pellets, from 4000 cm−1 to 450 cm−1. |  | [112] |
| Cellulose cotton fibers | Na2CO3 and thermal activation with MTC-b-CD and silver nitrate. | FTIR | MCT-β-CD grafted and ungrafted cotton fabrics were studied with attenuated total reflection (FTIR-ATR), which permits the study of solid surfaces using a diamond tipped device (SPECAC), connected to a Bruker Vertex 70 spectrophotometer. |  | [113] |
| Cotton linter cellulose | APS with MMA. | FTIR | A TENSOR27 FTIR apparatus (4500–400 cm−1) was used to analyze materials. |  | [115] |
| XRD | X-ray diffractometer (D/max-2500/PC, Rigaku Co) based on a reflection method using a Cu Ka target (40 kV and 60 mA). Diffraction angle: 5° to 60°. | ||||
| Microcrystalline cellulose | Ring-opening polymerization (ROP) of L-LA with DMAP in an ionic liquid AmimCl to form Cellulose-g-PLLA | 1H-NMR | Materials analyzed using 1H-NMR (Bruker AV400-MHz). Solvent: DMSO-d6 with a drop of trifluoroacetic acid-d; internal standard: tetramethyl silane (TMS). |  | [116] |
| XRD WAXD | WAXD carried out using an XRD-6000 X-ray diffractometer (Shimadzu, Japan); Ni-filtered Cu Ka radiation (40 kV, 30 mA); 4°/min at ambient temperature. | ||||
| UV spectroscopy | UV analyses performed in a UV2000 equipment (UNICO, China), to follow the load and controlled release of vitamin C. | ||||
| Cellulose (DP = 1130) | APS and MMA embedded. | FTIR | A TENSOR27 FTIR apparatus (4500–400 cm−1) was used to analyze materials. |  | [117] |
| WAXD-XRD | WAXD carried out in an X-ray diffractometer (D/max-2500/PC, Rigaku Co. Ltd., Tokyo); Cu Ka radiation (40 kV, 60 mA); 2q = 5°–60°. | ||||
| Cellulose nanocrystal (CNC) | Macromolecular initiator obtained from the reaction between Br-iBuBr and CNC with TEA as catalyst via SI-ATRP with STy. Material was casted in PMMA. | FTIR | Spectra obtained at ambient temperature on a NICOLET spectrometer 10 to study surface modification of studied materials. KBr pellet method used for samples. Resolution: 4 cm−1; measurement range; 4000–400 cm−1, and 30 scans. |  | [118] |
| UV-Vis | Transmittance of samples measured using a UV–vis spectrophotometer at wavelengths of 400 to 800 nm. | ||||
| XRD | XRD patterns of studied materials were obtained using a Bruker Siemens D8 X-ray apparatus with operation at 3 kW; CuKa radiation (l = 0.154 nm); 2q = 3–60°; 0.02° step. | ||||
| 13C-NMR | Chemical structures of studied materials obtained with the help of a Bruker 400 M solid-state 13C-NMR apparatus. | ||||
| Cellulose nanocrystal (CNC) | Redox agent: MgH2. L-LA polymerization in situ. PLLA-g-CNC particles were casted in PLLA. | FTIR | A Perkin Elmer Spectrum 100 apparatus in ATR mode, equipped with a Zn-Se crystal, was used. Modified and unmodified CNC samples. Range: 4000 to 650 cm−1. Accuracy: 4 cm−1 for 64 scans. |  | [119] |
| 1H-NMR 13C-NMR | A Bruker-300 apparatus with DMSO-d6 as solvent was used. Small amounts of CDCl3 were used to improve solubility of grafted product in DMSO-d6. | ||||
| XRD | XRD analyses carried out in a PAN analytical apparatus; Cu Ka radiation (l = 1.541 Å); incidence angle: 10 to 40°, 0.07° steps. | ||||
| XPS | XPS analyses performed in an ESCALAB 250 equipment (Thermo Scientific). Analyzed surface: 400 mm2. Atomic concentrations estimated from areas of photoelectron peaks considering atomic sensitivity factors. All binding energies were charge-corrected to 284.8 eV. | ||||
| Cellulose cotton fiber pulp | Esterification of maleic anhydride grafted onto PHA (through double bond). PHA-g-MA grafted onto cellulose cotton fiber pulp (through the anhydride group). | FTIR | ATR-FTIR analyses of ungrafted and grafted materials acquired using KBr discs on a Vector 33 Bruker apparatus. |  | [120] |
| XRD | XRD analyses carried out in a Bruker D8 ADVANCE X-ray apparatus with operation at 40 mA and 40 kV. Cu Kα filtered radiation (λ = 0.15418 nm). Scattering angle (2θ): 5° to 50°, 0.02° step. | ||||
| Cellulosic filter papers | Radiation-induced graft (RIGCP) copolymerization of acrylonitrile. | FTIR | FTIR analyses of studied materials (using KBr disks) were obtained using a Perkin Elmer-1650 FTIR apparatus. |  | [121] |
| XRD | XRD analyses of studied materials in an angle range 2θ = 5–50° were obtained in a Shimadzu XRD-610 equipment, with Cu Kα radiation (1.5418°A). Operating conditions: 8 °/min and 1.0 s; 30 kV and 20 mA. Peak height methods were used to estimate crystallinity index (CI). | ||||
| XRF | XRF analyses performed using a Philips wavelength dispersive equipment X’ Unique II, including flow and scintillation (fs) detectors. Qualitative analyses of chelating filter paper-metal complexes were obtained by X-ray fluorescence. | ||||
| Hemicellulose | Hemicellulose grafting using TBD and ε-caprolactone monomers | FTIR | A Nicolet Nexus spectrophotometer with single reflection ATR was used. Range: 4000 to 700 cm−1. Resolution: 4 cm−1; 60 scans. |   | [37] |
| 1H-NMR 13C-NMR HSQC | Apparatus: Bruker Advance III 400 MHz, containing a 5 mm multinuclear broad band probe (BBFO+) and z-gradient coil. 30 mg of sample dissolved in 1 mL of d6-DMSO. T = 60 °C. 128 scans for 1D 1H; 32 scans and 128 increments for 2D 1H–1H COSY. 16 scans and 256 increments for 2D 1H–13C HSQC. | ||||
| CellClAc | NCHA, 4VP, DA and DAAM grafted by ATRP using CuCl, 2′2′BIPI as catalyst. | FTIR | Apparatus: Perkin Elmer Spectrum One. Solid samples as KBr pellets. |   | [122] |
| UV-Visible | Apparatus: Shimadzu 3600 UV-VIR-NIR. | ||||
| Chitosan | Embedded polymerizations of N-VP and 4VP. Crosslinking agent: N’N’-MBA | FTIR | Apparatus: AVATAR 370 Thermo Nicolet. Range: 4000 to 500 cm−1, using the KBr pellet method. Spectral resolution: 4 cm−1. |  | [164] |
| XRD | Apparatus: Bruker Advance D8 equipment (Germany). Diffractograms comprised (2θ) 0.020 imaging; scattering rates: 3 to 800; scanning speeds: 2.0 min−1; accelerated tension of 40 kV; intensity of 35 mA. | ||||
| Cellulose nanofibrils | Nitroxide TEMPO insertion and nitroxide mediated polymerization of HEMA | XRD | XRD using a Bruker D8 Advance spectrometer. Analyses carried out from 5° to 40° at 4°/min, with a current of 40 mA. Measured property: CI. |   | [124] |
| FTIR | Apparatus: Bruker spectrometer, accumulation of 128 scans. Resolution: 4 cm−1. Range: 4000–400 cm−1; absorbance mode. | ||||
| XPS | XPS analyses using Thermo Electron Scientific Instruments were performed using a 1486.6 eV Al Kα X-ray source. | ||||
| Lignin | Insertion of ACX onto lignin to get a RAFT macrocontroller able to polymerize AM and AA. | Dynamic light scattering (Particle size distribution) | Particle size distributions (PSD) measured in an aqueous solution (1 mg/mL) using a DLS Zeta-sizer (Malvern Instruments). |  | [139] |
| 1H-NMR | Apparatus: Bruker 300 Advance; PFB used as internal standard to transform proton intensities into initiator concentration (μmol/(g lignin)). |
| Backbone | Grafts | Technique | Property Measured | Application | Refs. |
|---|---|---|---|---|---|
| Lignin | Imidazole and POCl3 react to produce (1H-imidazol-1-yl) phosphonic, which reacts with lignin. | SEM with EDAX | S4800 FEI SEM; 5 kV accelerating voltage. Comparison of morphologies of char residues after cone calorimeter analyses. PP/Lignin displayed loosely spheroidal structures with C: 86.5 wt.%, O: 13.5 wt.%. PP/30 °% Lignin-g-IPG formed a continuous and compact spheroidal structure. |  | [125,135] |
| Digital photo | Digital photos comparing PP composite sample test probes after cone calorimeter measurements were obtained. | ||||
| Cellulosic Grewia optiva fibers | FAS-H2O2 redox initiation for grafting of MA and KPS, for polymerization of MA. | SEM. | The morphologies of ungrafted and grafted fibers were studied using a SEM apparatus (LEO 435 VP). |  | [110] |
| Cotton linter cellulose | APS with MMA. | SEM | Apparatus: JSM-6700F scanning microscope. Samples coated with gold prior to study. Morphologies and differences between ungrafted and grafted cellulose materials were analyzed. |  | [115] |
| Microcrystalline cellulose | Ring-opening polymerization (ROP) of L-LA using DMAP in an ionic liquid (AmimCl) to produce Cellulose-g-PLLA | TEM | Apparatus: JEM-100CXa TEM at an acceleration voltage of 100 kV..Sample preparation involved dissolving them in DMSO 0.01% (w/v) and placement on a copper grid with formvar film. |  | [116] |
| Cellulose (DP = 1130) | Embedded APS and MMA. | SEM | Apparatus: JSM-6700F scanning microscope. Coating with a thin layer of gold required for samples. |  | [117] |
| Cellulose nanocrystal (CNC) | Macromolecular initiator obtained from Br-iBuBr and CNC with TEA as catalyst via SI-ATRP with Sty. Material was casted in PMMA. | SEM | Apparatus: su1510 (Hitachi Zosen Corporation) operating at 30 kV. Focus: measurement of homogeneity of PMMA composites. |  | [118] |
| TEM | Apparatus: JEM-2100 electron microscope. Operation involves acceleration voltage of 200 kV. Focus: Analysis of morphological features and distribution of cellulose nanocrystals. | ||||
| Cellulose cotton fiber pulp | Esterification of maleic anhydride grafted onto PHA (through double bond). PHA-g-MA grafted onto cellulose cotton fiber pulp (through the anhydride group). | SEM | Apparatus: Nova Nano SEM 430 (FEI Company); high-resolution field emission with operation at an acceleration voltage of 15 kV. Focus: Morphological analysis of PHA/CF composite films. |  | [108] |
| Cellulosic filter papers | Radiation-induced graft (RIGCP) copolymerization of acrylonitrile. | SEM | SEM micrographs of samples were obtained on a JEOL-SEM 5400 microscope. |  | [109] |
| Chitosan | Embedded polymerization of N-VP and 4VP; Crosslinking agent: N’N’-MBA | SEM | Morphological surface images of ungrafted and grafted chitosan with or without doxocyline were obtained using a JSM-6390 SEM microscope. |  | [153] |
| Regenerated cellulose fibres (rayon) | Photo-chemical grafting of PETA without photoinitiator. | SEM | Apparatus: JSM-6510 instrument (Joel GmbH, Freising, DE). Samples prepared following standard procedures. Micrographs obtained in SE mode; acceleration voltage: 5 and 10 kV. Purpose: Analyses of qualitative fracture. |    | [111] |
| Cellulose nanofibrils | Nitroxide TEMPO insertion and NMP of HEMA | SEM | Apparatus: Hitachi S-4800 SEM microscope. Purpose: Analysis of morphologies of materials at working distances of 15 mm. Operating voltage: of 2 kV. |   | [112] |
| Backbone | Grafts | Technique | Property Measured | Application | Refs. |
|---|---|---|---|---|---|
| Cellulose nanocrystal (CNC) | Redox agent: MgH2; in situ L-LA polymerization. PLLA-g-CNC particles were casted in PLLA. | DMA. Casted PLLA-g-CNC particles in PLLA matrix. | Apparatus: DMA 50, Metravib, tensile mode. Dimensions of sample specimens: 6.5 × 10.0 × 0.2 mm. Storage E’ and loss E” moduli were registered. Heating rate: 5 °C/min; -100–200 °C; 1 Hz. |  | [119] |
| Molten Rheometry; Casted PLLA-g-CNC particles in PLLA matrix. | Dynamic Anton Paar MCR 102 rheometer. Materials analyzed in molten state; T = 180 °C; Parallel plates geometry, 25-mm diameter; G’ and G” moduli were registered; 100 to10−3 rad/s. | ||||
| Cellulose nanofibrils | Nitroxide TEMPO insertion and NMP of HEMA | Stress-strain compression | Apparatus: Instron 5848; 50 N load cell. Test: cyclic compressive stress and strain; 5 mm/min for 70% strain, followed by the same releasing rate until zero loading, for 2 min. |   | [124] |
| Lignin | Insertion of ACX onto lignin to produce a RAFT macrocontroller to polymerize AM or AA. | Viscosity determination of an aqueous solution of grafted material. | Apparatus: Brookfield cone-and-plate viscometer. Steady shear measurements using 100 rpm. |  | [139] |
| Backbone | Grafts | Technique | Property Measured | Application | Refs. |
|---|---|---|---|---|---|
| Lignin-RAFT macrocontroller | Poly(soybean oil methacrylate) derivatives. PSBMA, PSBMAH, PSBMAEO. | GPC | Mn and MWD of different lignin-g-poly(soybean) synthesized materials: Lignin-g-PSBMA (31.9 M g/mol/2.7); Lignin-g-PSBMAH (57.1 M g/mol/4.4); Lignin-g-PSBMAEO (64.3 M g/mol/4.0). T = 35 °C; refractive index detector. Columns: HR1, HR3 and HR5E. Solvent: THF at 1 mL/min. Calibration with polystyrene standards. Samples prepared in THF (4 mg/mL) and filtered using a 0.2 mm mesh. |   | [134] |
| Hydroxypropyl cellulose (HPC) | Steglich esterification of PABTC over HPC using DCC and DMAP, and grafting of PABTC, with further polymerization of EA and NIPAAM | SEC | MWD by SEC; two PL polar gel M and one PL polar gel 5 mm guard columns; refractive index detector; Eluent: N,N-dimethylformamide/ 0.3 M LiBr, 0.5 mL/min. |  | [108] |
| Cellulose acetate | Solvents: DMSO, PDX, DMAc, C3H6O; initiators for grafting and polymerization: CAN, Sn(Oct)2 and BPO. | GPC | Number average molecular weights (Mn) and dispersity values (Ð) of grafted PMMA extracted from samples of graft copolymer. Apparatus: Agilent 1100 with 3 PSS GPC 8 300 mm, 5 mm, 106, 105, 103 A columns; eluent: THF, 0.8 mL/min, at 20°C. Calibration using polystyrene standards with molecular weights ranging 200−106 g/mol. |  | [111] |
| Microcrystalline cellulose | ROP of L-LA with DMAP in an ionic liquid (AmimCl) to produce Cellulose-g-PLLA. | HPLC | Apparatus: Agilent 1200 with an XDB-C18 phase column. Eluent: H2O—methanol (20:80 by vol.), 0.8 mL/min. |  | [116] |
| Hemicellulose | Hemicellulose grafting using TBD and e-caprolactone monomers. | GPC | Apparatus: Malvern Viscotek HT GPC 350; refractive index (RI) and viscometer detectors. PSS-GRAM columns covering a range of 100–1000,000 g/mol. Eluent: DMSO at 80 °C. Universal calibration using pullulan polysaccharide standards. |   | [37] |
| Lignin | Insertion of ACX onto lignin to produce a RAFT macro-controller which polymerizes AM and AA. | GPC | Apparatus: Waters Alliance 2695; eluent: aqueous solution of 0.1 M sodium phosphate buffer and 0.01% NaN3, at ambient temperature, 1 mL/min. |  | [139] |
| Backbone | Grafts | Technique | Property Measured | Application | Refs. |
|---|---|---|---|---|---|
| Lignin-RAFT macro-controller | Poly(soybean oil methacrylate) derivatives. PSBMA, PSBMAH, PSBMAEO. | Tensile strength | TT s/e values for Lignin-g-PSBMAH (1.5 MPa/220 %), PSBMAH (3.0 MPa/120 %) and epoxy resin prepared from grafted materials (17 MPa/22.5 %). Tensile tests were carried out using a crosshead speed of 20 mm/min at ambient temperature. Samples were casted to obtain films. THF polymer solution samples were dried for 12 h under vacuum at ambient temperature and for 6 h, at 60 °C. Films were cut into dog-bone tensile samples of 20 mm length and 5 mm width. |   | [134] |
| Cellulosic Grewia optiva fibers | FAS-H2O2 redox initiation for grafting of MA and KPS, followed by polymerization of MA. | Swell index | Swelling of raw and grafted fibers in different solvents (DMF, water, methanol, and isobutyl alcohol) was measured. |  | [110] |
| Cellulose nanocrystal (CNC) | Macromolecular initiator obtained from the reaction between Br-iBuBr and CNC using TEA as catalyst, via SI-ATRP with Sty. Material was casted in PMMA. | Tensile strength | The mechanical properties of nanocomposites were investigated through breaking strength and elongation at break tests. |  | [118] |
| Cellulose cotton fiber pulp | Esterification of maleic anhydride grafted over PHA (through double bonds). PHA-g-MA grafted onto cellulose cotton fiber pulp (through the anhydride group). | Tensile strength | Apparatus: Lloyd Instruments, Model LR5 K; 100 mm/min (ASTM D638); pieces sized 150 × 15 mm (length × width). Mean values were determined from measurements from four specimens. |  | [120] |
| Hemicellulose | Hemicellulose grafting using TBD and ɛ-caprolactone monomer | Tensile strength | Apparatus: Shimadzu Autograph AG-500A. Method: ISO 527-2. Specimen size: 35 × 4 × 0.3 mm. Tests carried out at ambient conditions; 10 mm/min, 12 mm distance between grips. Properties measured: ultimate strength, Young’s modulus, and elongation at break (5 repeats). |   | [37] |
| Regenerated cellulose fibers (rayon) | Photo-chemical grafting of PETA without photoinitiator. | Tensile strength | Apparatus: Z020 universal testing device with 20 kN load cell. Clamping length: 25 mm, 1 mm/min, pre-load stress of 5 N, and a clamping pressure of 2.5 bar. Specimen: 100 mm × 10 mm × 2 mm. |    | [123] |
| Fatigue | Apparatus: 858 Mini Bionix II, MTS testing machine, Systems Corporation. Cyclic tests under tensile stresses (R = 0.1) were performed. Experiments reaching total cycle numbers of 1 million were considered as runouts. Fatigue tests performed uniformly at 10 Hz. Experiments proceeded until breakage or reaching runout conditions. |
| Backbone | Grafts | Technique | Property Measured | Application | Refs. |
|---|---|---|---|---|---|
| CellClAc | Macro initiator, Cu(I)Cl/2′2′BIPI catalytic system via ATRP of 4NPA and MMA | Elemental analysis | Apparatus: Leco CHNS-932. Elements determined: C, N and H elements. |  | [109] |
| CellClAc | Macro initiator, Cu(I)Cl/2′2′BIPI catalytic system via ATRP controller. | Elemental analysis | Apparatus: Leco CHNS-932. Elements determined: C, N and H elements. |  | [112] |
| Cellulose cotton fibers | Na2CO3 and thermal activation using MTC-b-CD and silver nitrate | Microbiological investigations | Materials treated with Ag0 and Ag+ were evaluated for antibacterial activity. Gram-positive coccus (Staphylococcus aureus ATCC 25923) and Gram-negative bacillus (Escherichia coli ATCC 25922) microorganisms were used. The Kirby-Bauer diffusimetrical method was used to achieve this purpose. Bacterial cultures were standardized according to McFarland scale for 18 h, yielding 107–108 CFU/mL. After inoculation, sample discs were placed on the surface of the medium. Antibacterial activity was assessed after 24 h of incubation, at 37 °C. |  | [113] |
| Microcrystalline cellulose | ROP of L-LA with DMAP in an ionic liquid (AmimCl) to produce Cellulose-g-PLLA | Load and controlled release | A solution of grafted material (60.0 mg) and vitamin C (60.0 mg) in 2 mL of PBS was prepared and then transferred to a dialysis bag. Dialysis against 1 L distilled water for 24 h (water refreshment after 12 h) followed. Afterwards, the dialysis bag was immersed into a 400 mL phosphate buffer solution, at conditions similar to those of intestinal fluid (pH 7.40) at 37 °C. 2 mL samples were taken at predetermined times, and analyzed at lmax = 245 nm for vitamin C. |  | [116] |
| Cellulose cotton fiber pulp | Esterification of maleic anhydride grafted onto PHA (through double bonds). PHA-g-MA grafted onto cellulose cotton fiber pulp (through the anhydride group). | Surface roughness | Measurement of surface roughness accomplished using an L&M CE165 PPS tester. |  | [120] |
| Contact angle (hydrophobicity) | Surface hydrophilicity was analyzed by contact angle measurements. Tests carried out at ambient conditions using a Dataphysics OCA40 Micro instrument. Surface free energy parameters were estimated from these data. | ||||
| Cellulosic filter papers | Radiation-induced graft (RIGCP) copolymerization of acrylonitrile. | Chelating rare elements | Chelation of uranium, thorium, and lanthanides by the batch procedure took place. |  | [121] |
| Hemicellulose | Hemicellulose grafting using TBD and ε-caprolactone monomers. | Contact angle | Measurements carried out using an NRL Contact Angle Goniometer by Rame-Hart, model 100–00. |   | [37] |
| Biodegradation test | Method: ISO 14851. Biochemical Oxygen Demand (BOD) measurements were obtained under aerobic conditions. | ||||
| CellClAc | NCHA, 4VP, DA and DAAM grafted by atom transfer radical polymerization (ATRP) using CuCl, 2′2′BIPI as catalyst. | Elemental analysis | Elemental analyses carried out in a Leco CHNS-932 apparatus. |   | [122] |
| Electrical conductivity | Electrical conductivity determined with a Keithley 6517A electrometer. Cell-g-4VP was the only material that behaved as semiconductor. | ||||
| Chitosan | Embedded polymerization of N-VP and 4VP. Crosslinking agent: N’N’-MBA | Swell index | Swelling measurements were carried out in distilled water, in simulated intestinal buffer of pH 6.8 and in simulated gastric buffer of pH 1.4 (7 mL of HCl, 2 g of NaCl and 0.1 L of distilled water) at ambient conditions. A known weight of dry sample was placed into a teabag and immersed into aqueous medium. After a while, the bag was taken out and hung for 5–10 min to eliminate excess unabsorbed water, and then weighed. |  | [164] |
| Load and controlled release | The loading capacity (milligrams of doxocyline entrapped per 100 milligrams of dried drug-loaded gels, %) of CS graft gels (CS-g) was determined as follows: the drug loaded CS-g gel was ground to fine power followed by immersion of a known amount of it into 0.1 L of HCl aqueous solution for 24 h under magnetic stirring. Then, the solution was filtered and used to determine the absorbance (A) of the drug contained in it using a Perkin Elmer Lambda 35 apparatus at 280 nm. Release profiles of the model drug (doxocyline) from the drug-loaded polymers were determined in distilled water (pH = 1.5) and in buffer solutions (pH = 6.8). All the studies were carried out in triplicate. | ||||
| Regenerated cellulose fibres (rayon) | Photo-chemical grafting of PETA without photoinitiator. | Fiber volume content | Volume of materials was determined by measuring the dimensions of samples (calliper gauge 500-171-20, Mitutoyo), and from grammage and density of fibers, referred to the total mass determined using a type 440 35N fine-scale. |    | [123] |
| Cellulose nanofibrils | Nitroxide TEMPO insertion and NMP of HEMA | BET | Apparatus: NOVA 1200e Quantachrome. Method: Brunauer–Emmett–Teller (BET) method for analysis of N2 gas adsorption isotherms. Estimated properties: specific surface area (SBET) and average pore volumes. BET surface area improved from 9.87 m2/g for TEMPO-Cellulose to 19.7283 m2/g for HEMA–TEMPO-Cellulose. The microporous volume (Vtotal) increased from 0.0484 cm3/g to 0.0705 cm3/g, for HEMA–TEMPO-Cellulose. |   | [124] |
| Electrical conductivity | Electrical resistivity determined using a four-point probe system (ST 2253, Suzhou Jingge Electronic Co.). The direct freezing method was compared against the unidirectional gradient freezing method. Samples produced using the direct freezing method exhibited higher resistivity values. | ||||
| Lignin | Insertion of ACX onto lignin to produce a RAFT macro-controller that polymerizes AM and AA | Surface tension | Apparatus: De Nouy ring-type tensiometer (Krüss), at 25 °C. |  | [139] |
| Emulsification test | Absorbance of solutions of dissolved grafted material in deionized water and mixtures of hexanes (ultrasonication required for disolution) was measured using a Cary 300 spectrophotometer. Comparison of this value with that of the starting aqueous solution allowed calculation of lignin content in the emulsion phase. |
| Backbone | Functionalization Method | Graft Chains | Modeling Approach | Highlights/Comments | Ref. |
|---|---|---|---|---|---|
| Pre-polymer containing highly active chain transfer sites (pendant mercaptan groups). | CTP between polymer radicals and pendant mercaptan groups. | Vinyl polymer | Kinetic model for FRP including CTP; model equations solved numerically and approximate closed form equations were also used. | Calculated properties/variables: grafting efficiency (Equation (1)) and number average molecular weights of the different polymer molecules (Equations (2)–(6)). | [39] |
| Solid polymeric substrates. | Irradiation of polymer/monomer mixture. | Vinyl polymer | Calculation of rn, rw and number full chain length distributions (nx) from kinetic equations. | Two limiting cases were considered in the calculation of nx: (a) radicals terminating by disproportion, no significant chain transfer and complete conversion; and (b) no chain transfer and incomplete conversion (see Equation (7)). | [40] |
| Polyolefins | High temperature CTP. | Vinyl polymer | Kinetic modeling from a given polymerization scheme. | Review paper considering chemical modification of polyolefins by FRP in extruders. | [231] |
| Silica substrate | Formation of a growing grafted polymer chain from reaction between an initiator derived primary free radical and a “surface site.” | Poly(vinylpyrrolidone) | Use of a “conservational polymerization and molecular-weight distribution” (CPMWD) numerical method to solve the kinetic equations. | The proposed numerical approach provides monomer conversion, grafting yield, and full MWDs of active and inactive homopolymer and graft polymer species. | [232] |
| Porous silica beads | Chain transfer reaction to fixed mercapto groups present in the pre-functionalized silica beads. | Polystyrene | Kinetic model that allows calculation of full MWD and Ð; no details on numerical approach or software used were provided. | MWDs were approximated assuming them, as a first approach, to follow a normal distribution function. | [233] |
| PE | CTP by FRP in melt, in a twin-screw extruder (TSE). | Poly(glycidyl methacrylate) (PGMA) | Coupling of flow characteristics from a TSE simulation program and a reactor model consisting of plug flow and “axial dispersion” reactor cells. | Overall GMA conversion was predicted, showing general good agreement with experimental data. | [234] |
| PE | CTP by FRP in melt, in a twin-screw extruder (TSE). | Poly(maleic anhydride) (PMAH) | Coupling of reaction kinetics to flow equations in a twin-screw extruder. | Satisfactory agreement between calculated and experimental data. | [235] |
| Polypropylene (PP) | CTP by FRP in melt | PMAH | A kinetic model which captures the effect of MAH and initiator concentrations on grafting yield and MWDs during the grafting process was derived. | Model performance assessed by comparing calculated and experimental data from different sources, for batch (internal mixer, static film) and continuous systems (twin-screw extruder), under a wide window of operating conditions. | [236] |
| Cellulose or cellulose containing fibers | Not specified (empirical approach) | Monochlorotriazinyl- β-cyclodextrin | Neural network modeling (semi-empirical approach). | A good approximation of the studied grafting process was obtained. | [237,238,239] |
| PP | CTP by FRP in solid state, in presence and absence of supercritical CO2 (scCO2). | PMAH | A kinetic model focused on polymerization and grafting rates was used. | The effect of presence or absence of scCO2 on grafting rate was analyzed. | [240] |
| PP | CTP by FRP in solid state, with and without scCO2. | Not specified | The kinetic model and grafting mechanism of the scCO2 assisted grafting of PP in the solid state were reviewed. | Grafting rate controlled by diffusion in absence of scCO2 and by the chemical grafting step in presence of scCO2. | [241] |
| Polybutadiene (PB) | CTP by FRP in production of high-impact PSty (HIPS). | PSty | An existent heterogeneous model is generalized to consider bivariate distributions of graft copolymer topologies; each topology is determined by the number of trifunctional branching points per molecule. | Polymerization in two phases is assumed. Adjustment of a single kinetic parameter is required. Grafting efficiencies obtained from solvent extraction/gravimetry are contrasted against estimates obtained by the deconvolution of size exclusion chromatograms for total polymer. | [242] |
| Poly[ethylene-co-(1- octene)] | CTP by FRP in molten state | PMMA | A model compound approach was used. | The focus was on assessing the effect of temperature, polymerization time and initiator content on MMA polymerization rate and number average chain length, in the presence of alkoxyl radicals and alkanes. | [243] |
| PSty | Use of a Friedel-Crafts reaction, which gives rise to a mixture of unmodified PE, modified PS and a PE-g-PS copolymer. | PE | A model for predicting the complete MWD of the homopolymers, and the chemical composition distribution (CCD) of the graft copolymer was presented. Probability generating functions were used to carry out the calculations. | The model takes into account the opposing effects of rival reactions that occur in the studied grafting reaction. | [244,245] |
| PE | CTP by FRP | PSty | A kinetic mechanism for the whole process and application of the method of moments to solve the mass balance equations are proposed. | The model is able to calculate average molecular weights and the amount of grafted PS. | [246] |
| PP | CTP by FRP | PMAH | A kinetic model for the free-radical grafting of maleic anhydride MAH onto PP, considering that the reaction medium is heterogeneous due to the incomplete solubility of MAH in the polymer melt was developed. | Two different approaches are considered that derive from the limiting cases of very fast mass transfer (equilibrium) and no mass transfer between the two phases. The physical parameters considered in the model include the solubility of MAH in PP and the partition coefficient of the initiator between the two phases. | [247] |
| Carboxyl functionalized hydrophilic Sephadex (a cross-linked dextran gel used for gel filtration) derivatives. | Covalent amine conjugation method analyzed using XPS. | Poly(ethylene glycol) (PEG) | Langmuir and Langmuir-Freundlich isotherm models are used to study PEG-grafting kinetics. Fractional C-O intensities obtained from high-resolution C 1s scans are correlated with grafting. | The models are assessed by comparing calculated profiles and experimental data. | [248] |
| Various surfaces | Surface-initiated controlled radical polymerization. | Polymers from 2-methacryloyloxythyl phosphorylcholine (MPC), methyl acrylate, acrylamide, and N-isopropylacrylamide. | A kinetic model for surface-initiated atom transfer radical polymerization (SI-ATRP) with addition of excess deactivator in solution was presented. | Polymer layer thickness, and concentrations of radical, dormant and dead polymer molecules can be calculated. A simple but reliable analytical solution is proposed for estimation of the evolution of polymer layer thickness. | [249] |
| Partially fluorinated polymers such as poly(vinylidene fluoride) and poly(ethylene-co-tetrafluoro ethene). | Radiation induced grafting | Poly(4-vinylpyridine) | Box-Behnken factorial designs and response surface method (RSM) were used. | A quadratic model was used to obtain the optimal set of conditions (absorbed dose, monomer concentration, grafting time and reaction temperature) to maximize the degree of grafting (G%), using a statistical software. Adequate agreement between model predictions and experimental data supported the approach proposed. | [250,251] |
| Poly(propylene glycol) (PPG) | CTP by FRP | Poly(styrene-co-acrylonitrile) (SAN) | A batch kinetic model for grafting copolymerization of Sty and acrylonitrile (AN) in the presence of PPG is modified to include semi-continuous and continuous processes. | The semi-continuous model is validated using experimental data, although molecular weight agreement is poor. The continuous process is simulated but not contrasted against experimental data. According to the model, graft efficiency increases drastically, and Mw decreases with decreasing St/AN weight ratio. | [252] |
| Starch | (Mechanically activated) CTP by FRP | Polyacrylamide | A kinetic model for an inverse emulsion copolymerization system was used. The effects of initiator, monomer, starch, and emulsifier content on reaction rate of graft co-polymerization (Rg) were determined. The kinetic model was modified to provide acceptable agreement with experimental data. | It was observed that the relationship between Rg and the concentration of components in the polymerization system can be expressed for all of the four components by the following equation: Rg∞[mSt]1.24 [I]0.76[M]1.54[E]0.33, which is consistent with the kinetic equation from theoretical studies: Rp∞[mSt]0.5~1 [I]0.5~1[M]1~1.5[E]0.6. | [253] |
| PB seed latex particles | CTP by emulsion FRP | SAN | Emulsion grafting copolymerization is model using a two-phase kinetic model. Monomer concentrations in the different phases is calculated. | Partition of components between phases calculated as in previous models. The model is extended to account for calculation of grafting efficiency and copolymer composition of free and grafted molecules. | [254] |
| PE | CTP by FRP in REX | PAA | Monomer conversion to homo- and grated-polymers are calculated separately using an incremental theory. The approach allows calculation of degree of grafting, mass of homopolymer and grafting efficiency. | The focus was on analyzing the effect of temperature and initial concentrations of monomer and initiator on degree of grafting. Both grafting and amount of homopolymer increase with temperature and monomer concentration. | [255] |
| Natural rubber latex (core-shell particles) | CTP by emulsion FRP | PMMA | The model consists on rate expressions of polymer chain formation. A decrease of CHPO by TEPA and a population event of radicals between core/shell phases are considered in the model. | Grafting efficiency and polymer composition for free and grafted polymer populations can be calculated in terms of initial conditions (monomer and initiator content, and rubber weight ratio and temperature). | [256] |
| Vinyl/divinyl copolymer synthesized by RAFT chemistry. | CTP by FRP | Non-specified RAFT synthesized graft | A RAFT crosslinking model was used to address the molecular imprinting step. The size of grafted brushes was estimated from MWD calculations for a RAFT homo-polymerization. | The model includes the two steps involved in the production of MIP responsive particles. Limitations and ways to improve the model are discussed. | [257] |
| High-density PE (HDPE) | Melt free-radical grafting (semi-empirical approach) | GMA | Response surface, desirability function, and artificial intelligence approaches were combined to account for polymer grafting. Input variables: monomer content, initiator concentration, and melt-processing time. | An in-house code that learns and mimics processing torque and grafting of GMA onto HDPE was developed. Optimization of the process and quantification of the competition between grafting and crosslinking was carried out. | [258] |
| PE | CTP by FRP in post-polymerization modification | Vinyl polymers | A kinetic Monte-Carlo (kMC) modeling approach for free radical induced grafting of vinyl monomers onto polyethylene (PE), assuming isothermicity, perfect macromixing and diffusion-controlled effects was developed. | Calculated variables: monomer conversion, grafting selectivity and yield, MWDs of all macromolecular species involved, average grafting (from/to) and crosslink densities, number of grafts and crosslinks per individual polymer molecule, and chain length of every graft. | [259] |
| PE | CTP by FRP (batch operation) | Vinyl polymers | A kMC model was used. Reactions included: initiator dissociation, hydrogen abstraction, graft chain initiation, graft (de)propagation, crosslinking, and homo (de) polymerization. | Assumptions: isothermicity and single-phase polymerization. It is found that the grafting kinetics is affected by hydrogen abstraction and depropagation kinetic rate constants, equilibrium coefficient Keq, and initial content of monomer, initiator, and polyolefin. | [260] |
| PE | CTP by FRP (tubular reactor) | Vinyl polymers | kMC, as in [260] | They found that functionalization, selectivity and grafting density are able to reach remarkably better values (e.g., 100%) if multiple injection points are considered for monomer, initiator and/or a temperature profile. | [261] |
| PE | CTP by FRP (two-phase system) | Vinyl polymers | kMC; the model of [260,261] is further extended to include two phases. | The partition of monomer and initiator between the two phases is addressed by introducing overall mass transfer coefficients. The model is tested with a monomer with low to negligible homo-propagation. Positive agreement with industrial experimental data was obtained. | [41] |
| Reaction Step | Kinetic Expression | Remarks |
|---|---|---|
| Initiator decomposition | ||
| First propagation | ||
| Propagation | n = 1, …∞ | |
| Termination by disproportionation | n,m = 1, …∞ | |
| Propagation through intermediate free radicals | n = 1, …∞ b = 0, …∞ | |
| Chain transfer to polymer | n, m = 1, …∞ b, c = 0, …∞ | |
| Propagation through pendant double bonds (crosslinking), assuming a pseudo-kinetic rate constants method (pseudo-homopolymer) approach | r, s = 1, …∞ |
| Symbol of Corresponding Application | Meaning |
|---|---|
 | Biocomposites and biomaterials. |
 | Green chemistry and innovative processes. |
 | Electronic materials, electrical properties, and conjugated polymers. |
 | Surface modification, hydrophilic or hydrophobic surfaces. |
 | Flame retardancy, additives for flame retardancy in polymer blends, thermal resistance materials. |
 | Antimicrobial applications. |
 | Adhesives, polymer networks, crosslinked polymers, gels. |
 | Controlled release of drugs and chemicals. |
 | Polymer composites and blends, by extrusion, casting or co-precipitation. |
 | Chelating properties, membranes, effluent remediation. |
 | Mechanical properties improvement, micro and nano reinforcement. |
 | Application in polyolefins, polyethylene, polypropylene. |
 | Aerogels, light, or porous materials. |
| Abbreviation | Meaning |
|---|---|
| FTIR | Fourier transformed mid-infrared spectroscopy. |
| ATR | Attenuated total reflection. |
| 1H-NMR | Proton nuclear magnetic resonance. |
| 13C-NMR | Carbon nuclear magnetic resonance. |
| TT | Tensile test. |
| DMA | Dynamical mechanical analysis. |
| GPC | Gel permeation chromatography. |
| TGA | Thermogravimetric analysis. |
| DSC | Differential scanning calorimetry. |
| FR | Flame retardancy. |
| XPS | X-ray photoelectron spectroscopy. |
| EDAX | Energy-dispersive X-ray analysis/spectroscopy. |
| WAXD | Wide Angle X-ray Scattering. |
| XRF | X-ray fluorescence. |
| Technique | Abbreviation | Explanation | Units |
|---|---|---|---|
| Chromatography | MWD | Molecular weight distribution of polymers. | - |
| Mn | Number average molecular weight. | g/gmol | |
| Thermal | Tg | Glass transition temperature. | °C |
| MDT | Maximum degradation temperature. | °C | |
| MFI | Melt flow index. | g/10 min | |
| THR | Total heat release. | MJ/m2 | |
| PHRR | Peak heat release rate. | kW/m2 | |
| Mechanical | σ | Maximum stress measured in tensile test. | MPa |
| ɛ | Maximum elongation observed in tensile test. | % |
| Abbreviation | Compound | Chemical Structure | Ref. |
|---|---|---|---|
| “P” | Before the name or abbreviation of a monomer means polymer of that monomer. | ||
| “B-g-C” | Means “C” chains grafted to “B” backbone. | ||
| THF | Tetrahydrofuran. |  | [17,18] |
| DMF | Dimethylformamide. |  | [121,125,139,141,144,146,147,151,152,165] |
| DMSO | Dimethyl Sulfoxide. |  | [37,111] |
| PDX | 1,4-dioxane. |  | [111,140,142,150] |
| PP | Polypropylene. |  | [135] |
| PE | Polyethylene. |  | [135] |
| DCC | Dicyclohexylcarbodiimide. |  | [108,165] |
| DMAP | 4-Dimethylaminopyridine. |  | [108,116,165] |
| SBMA | (E)-2-(N-methyloctadec-9-enamido)ethyl methacrylate. |  | [134] |
| SBMAH | (E)-3-(octadec-9-enamido)propyl methacrylate. | 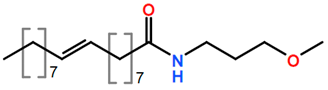 | [134] |
| SBMAEO | 2-(N-methyl-8-(3-octyloxiran-2-yl)octanamido)ethyl methacrylate. | 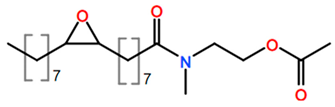 | [134] |
| 4-cyano-4-(phenylcarbonothioylthio) pentanoic acid. |  | [134] | |
| POCl3 | Phosphoryl trichloride. |  | [125,135] |
| P4O10 | Phosphorus(V) oxide. |  | [125,136,137] |
| Imidazole. | 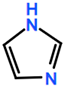 | [125,135] | |
| IPG | (1H-imidazol-1-yl) phosphonic group. |  | [125,135] |
| N’N’-MBA | N’N’-methylenebisacrylamide. | 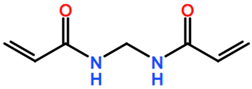 | [98,164] |
| N-VP | N-vinyl pyrrolidone. | 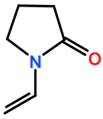 | [99,164] |
| Co(acac)3 | Cobaltacetylacetonate complex. | [99,103] | |
| MMA | Methyl methacrylate. | 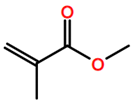 | [58,107,111,117,140,144,146,149] |
| PMMA | Poly(methyl methacrylate). | 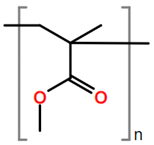 | [58,107,111,117,140,144,146,149] |
| AcN | Acrylonitrile. | 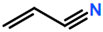 | [107,121] |
| EA | Ethyl acrylate. | 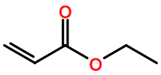 | [107,108] |
| NIPAAM | N-isopropylacrylamide. |  | [108,138,140,141,143,145,165] |
| PNIPAAM | Poly(N-isopropylacrylamide). | 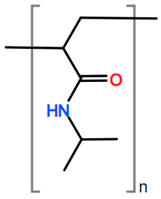 | [108,138,140,141,143,145,165] |
| PABTC | Propionic acidyl butyl trithiocarbonate. |  | [108] |
| HPC | Hydroxypropyl cellulose. |  | [108] |
| DMAc | Dimethylacetamide. |  | [108] |
| CellClAc | Cellulose chloroacetate. | [109,122] | |
| 2′2′BIPI | 2,2′-bipyridine. |  | [109,122,140,146,147] |
| 4NPA | N-(4-nitrophenyl) acrylamide. |  | [109] |
| MA | Methyl acrylate. |  | [110] |
| FAS | Ferrous ammonium sulphate. | (NH4)2Fe(SO4)2 6H2O | [110] |
| KPS | Potassium persulphate. |  | [110] |
| CAN | Ceric ammonium nitrate. |  | [111] |
| BPO | Benzoyl peroxide. |  | [111] |
| Sn(Oct)2 | Tin(II) 2-ethyl hexanoate. |  | [111] |
| NCA | N-cyclohexylacrylamide. |  | [112] |
| AgNPs | Silver nanoparticles. | Ag | [113] |
| b-CD | β-cyclodextrin. |  | [113,114] |
| MTC-b-CD | Monochlorotriazinyl-β-cyclodextrin. | 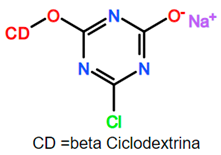 | [113,114] |
| APS | Ammonium persulfate. |  | [115,117] |
| AmimCl | 1-allyl-3-methylimidazolium chloride. |  | [116] |
| PBS | Phosphate-buffered saline solutions. | [116] | |
| DMAc | N,N-dimethyl acetamide. |  | [111,117] |
| Br-iBuBr | 2-Bromoisobutyryl bromide. |  | [118] |
| L-LA D-LA | L-lactide. D-lactide. |  | [116,119,157,159] |
| PLLA PDLA | Poly(L-lactide). Poly(D-lactide). |  | [116,119,157,159] |
| STY or Sty | Styrene. |  | [58,118,140,146,147,149] |
| PS or PSty | Polystyrene. |  | [58,118,140,146,147,149] |
| TEA | Triethylamine. |  | [118] |
| PMDETA | N, N, N′, N′, N″-Pentamethyldiethylenetriamine. | 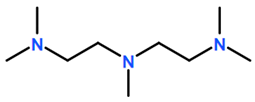 | [118,140,146] |
| PHA | Polyhydroxyalkanoate. | 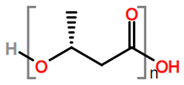 | [120] |
| PHA-g-MA | Poly(hydroxyalkanoate-grafted-maleic anhydride). | 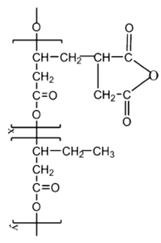 | [120] |
| TBD | 1,5,7- triazabicyclodecene [4.4.0]. | 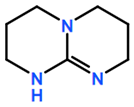 | [37] |
| NCHA | N-cyclohexylacrylamide. |  | [122] |
| CL | ε-Caprolactone.. |  | [37,158,159,161,162] |
| PCL | Poly-(ε-caprolactone). |  | [37,158,159,161,162] |
| 4VP | 4-vinylpyridine. |  | [122,164] |
| DAAM | Diacetone acrylamide. |  | [122] |
| DA | Diallylamine. |  | [122] |
| PETA | Pentaerythritol triacrylate. | 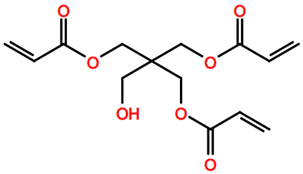 | [123] |
| TEMPO | TEMPO nitroxide. |  | [124] |
| HEMA | 2-hydroxyethyl methacrylate. | 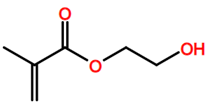 | [124] |
| DMAEMA | 2-dimethylaminoethyl methacrylate. |  | [138,150] |
| BA | Butyl acrylate. | 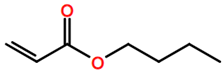 | [138] |
| EG | Ethylene glycol. |  | [138] |
| AM | Acrylamide. |  | [58,138,139,151,153] |
| AA | Acrylic Acid. | 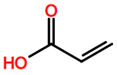 | [58,138,139] |
| ACX | Acyl chloride xanthate. |  | [139,153] |
| LMA | Lauryl methacrylate. |  | [140] |
| Guaiacol. |  | [140] | |
| Vainillin. |  | [140] | |
| Syringyl methacrylate. |  | [140] | |
| 4-propylsyringol. |  | [140] | |
| 4-propylguaiacol. |  | [140] | |
| Ferulic acid. |  | [140] | |
| Isorbide. |  | [140] | |
| CuBr/PMDETA | N,N,N′,N′′,N″-Pentamethyldiethylenetriamine Cu(I)Br complex. |  | [140,141,146,147] |
| CuBr/HMTETA | 1,1,4,7,10,10-Hexamethyltriethylenetetramine Cu(I)Br complex. |  | [140,143,150] |
| PEG-A | Poly(ethylene glycol) acrylate.n = 9 |  | [140,143] |
| PPG-A | Poly(propylen glycol) acrylate.n = 5 |  | [140,143] |
| DAEA | Dehydroabietic ethyl acrylate. |  | [140,142] |
| PDAEA | Poly(dehydroabietic ethyl acrylate). |  | [140,142] |
| BMA | Butyl methacrylate. |  | [140,144] |
| PBMA | Poly(butyl methacrylate). |  | [140,144] |
| PEGMA | Poly(ethylene glycol) methacrylate. |  | [140,148] |
| PPh3 | Triphenyl phosphine. |  | [149] |
| KEX | Potassium ethyl xanthate. |  | [151,152] |
| 2-Bromopropionic acid. |  | [151,152] | |
| GMA | Glycidyl methacrylate. |  | [153] |
| XCA | Xanthate carboxylic acid. |  | [154] |
| DMC | [2-(Methacryloyloxy)ethyl] trimethyl-ammonium chloride. |  | [154] |
| HONB | N-Hydroxy-5-norbornene-2,3-dicarboxylic. |  | [154] |
| AMBN | 2,2′-azobis (2-methylbutyronitrile). |  | [154] |
| EOX | 2-ethyl-2-oxazoline. |  | [155] |
| PEOX | Poly(2-ethyl-2-oxazoline). |  | [155] |
| MOX | 2-methyl-2-oxazoline. |  | [156] |
| PMOX | Poly(2-methyl-2-oxazoline). |  | [156] |
| B-BL | β-Butyrolactone. |  | [160] |
| PHB | Poly(3-hydroxybutyrate). | 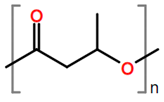 | [160] |
| EOX | Ethylene oxide. |  | [163] |
| PEO | Poly(ethylene oxide). | 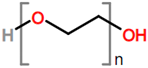 | [161] |
| P4-t-Bu | 1-tert-butyl-4,4,4-tris (dimethylamino)-2,2-bis[tris(dimethylamino)- phosphoranylidenamino]-2l5,4l5-catenadi (phosphazene) solution in hexane. | [163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Hernández, M.Á.; Cano-Díaz, G.S.; Vivaldo-Lima, E.; Rosas-Aburto, A.; Hernández-Luna, M.G.; Martinez, A.; Palacios-Alquisira, J.; Mohammadi, Y.; Penlidis, A. A Review on the Synthesis, Characterization, and Modeling of Polymer Grafting. Processes 2021, 9, 375. https://doi.org/10.3390/pr9020375
Vega-Hernández MÁ, Cano-Díaz GS, Vivaldo-Lima E, Rosas-Aburto A, Hernández-Luna MG, Martinez A, Palacios-Alquisira J, Mohammadi Y, Penlidis A. A Review on the Synthesis, Characterization, and Modeling of Polymer Grafting. Processes. 2021; 9(2):375. https://doi.org/10.3390/pr9020375
Chicago/Turabian StyleVega-Hernández, Miguel Ángel, Gema Susana Cano-Díaz, Eduardo Vivaldo-Lima, Alberto Rosas-Aburto, Martín G. Hernández-Luna, Alfredo Martinez, Joaquín Palacios-Alquisira, Yousef Mohammadi, and Alexander Penlidis. 2021. "A Review on the Synthesis, Characterization, and Modeling of Polymer Grafting" Processes 9, no. 2: 375. https://doi.org/10.3390/pr9020375
APA StyleVega-Hernández, M. Á., Cano-Díaz, G. S., Vivaldo-Lima, E., Rosas-Aburto, A., Hernández-Luna, M. G., Martinez, A., Palacios-Alquisira, J., Mohammadi, Y., & Penlidis, A. (2021). A Review on the Synthesis, Characterization, and Modeling of Polymer Grafting. Processes, 9(2), 375. https://doi.org/10.3390/pr9020375









