Solvent Extraction of Boric Acid: Comparison of Five Different Monohydric Alcohols and Equilibrium Modeling with Numerical Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Stripping Experiments
2.2. Determination of Boric Acid Concentration in Aqueous Solutions
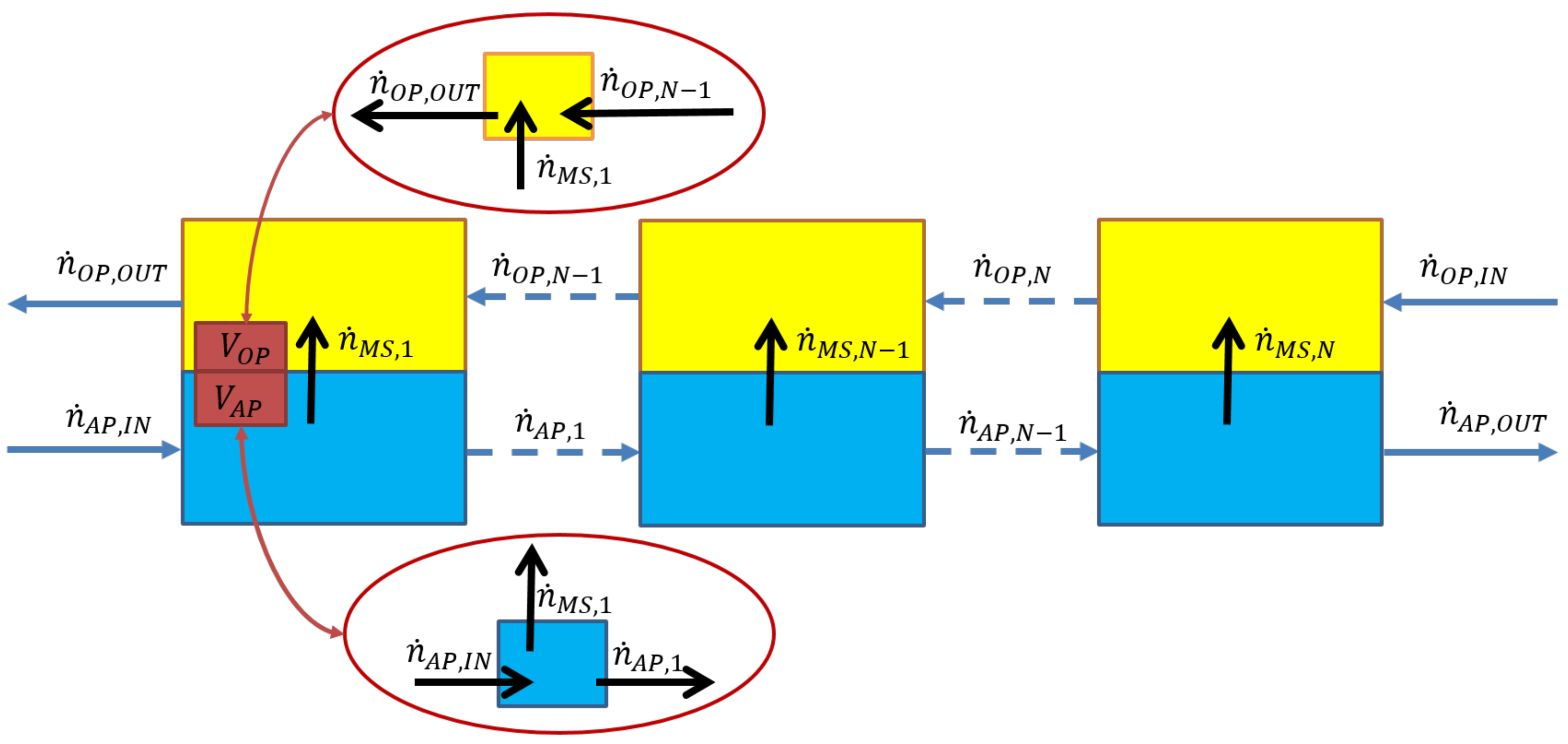
2.3. Determination of the Relationship between the Number of Theoretical Stages and the Phase Ratio with Numerical Methods
- The volume of organic and aqueous phase is much greater than volume flow (residence time V/ = V/ = 1000 h);
- The mass transfer is very fast (Ka = 1000/h);
- The volume of the organic and aqueous phase remains constant during the extraction process.
3. Results and Discussion
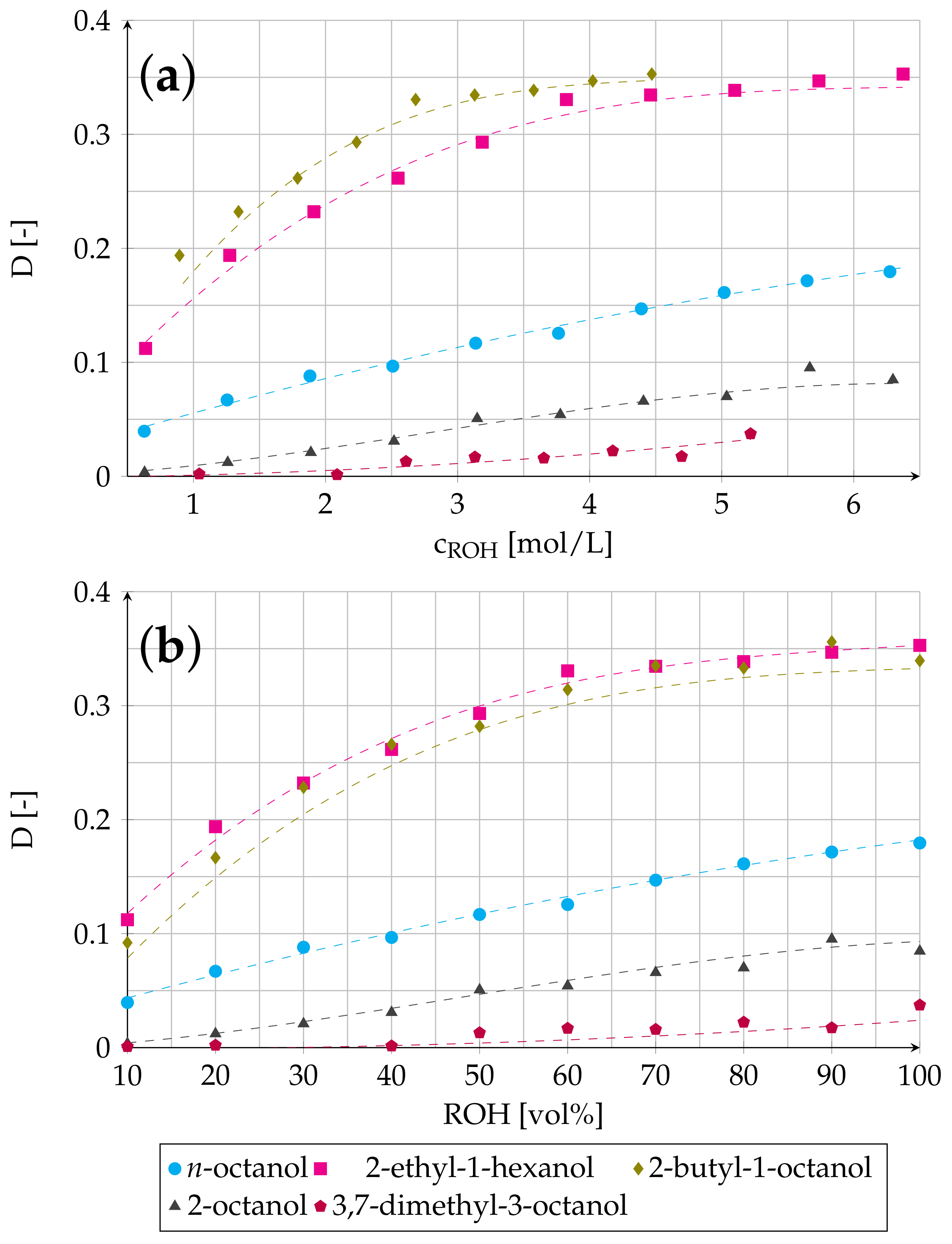
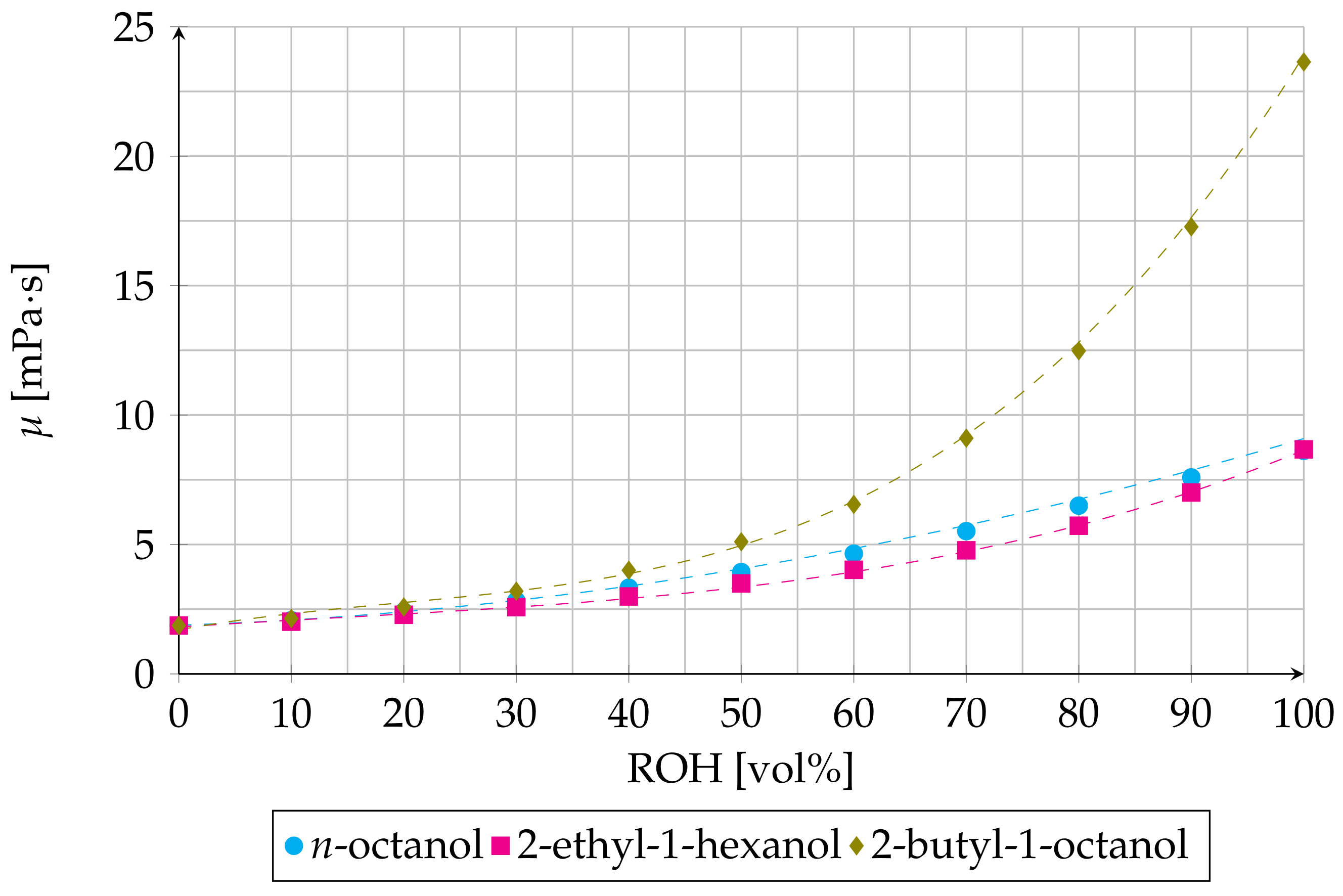
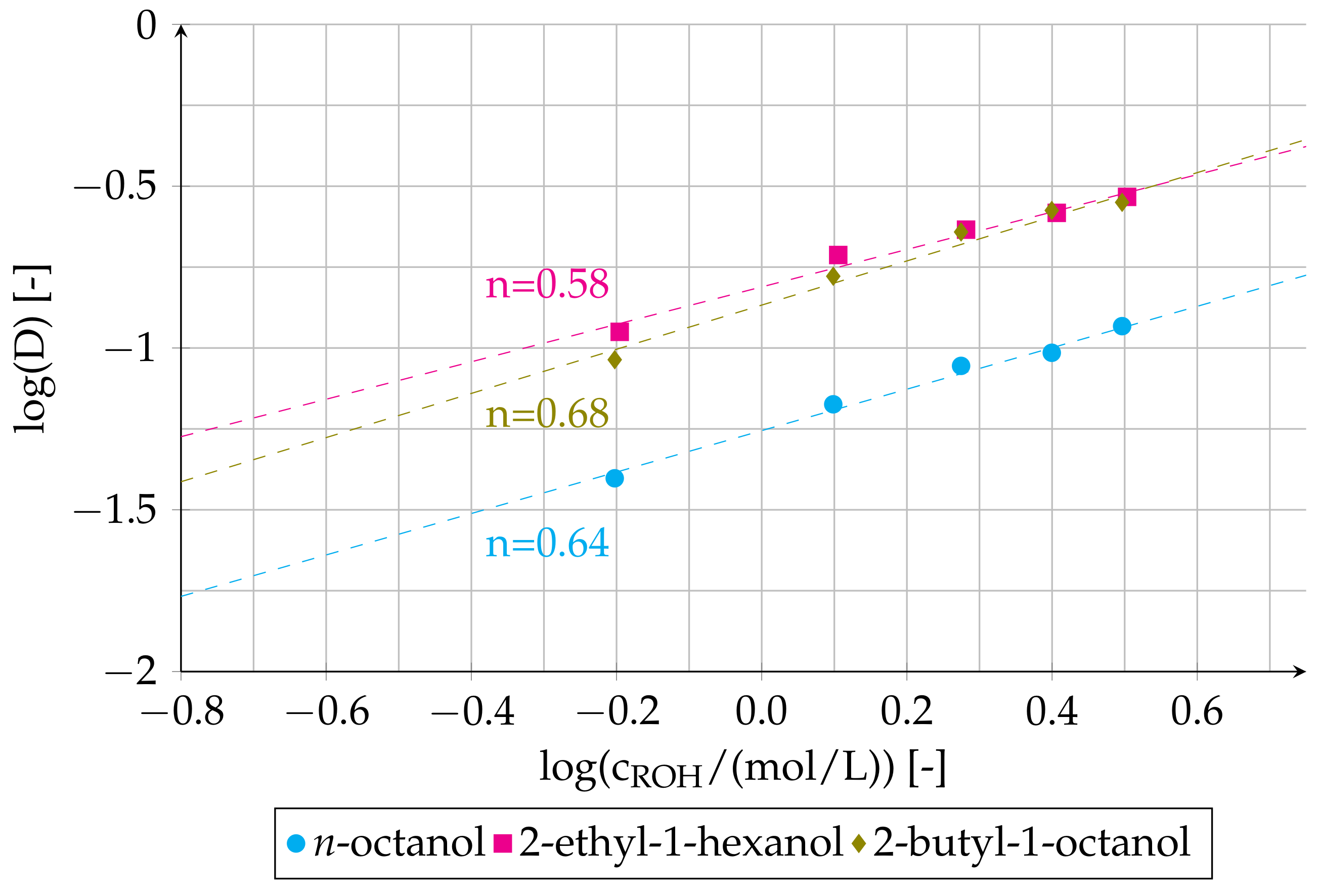
3.1. Effect of ROH Concentration on Distribution Ratio and on Viscosity of Organic Phase
3.2. Slope Analysis
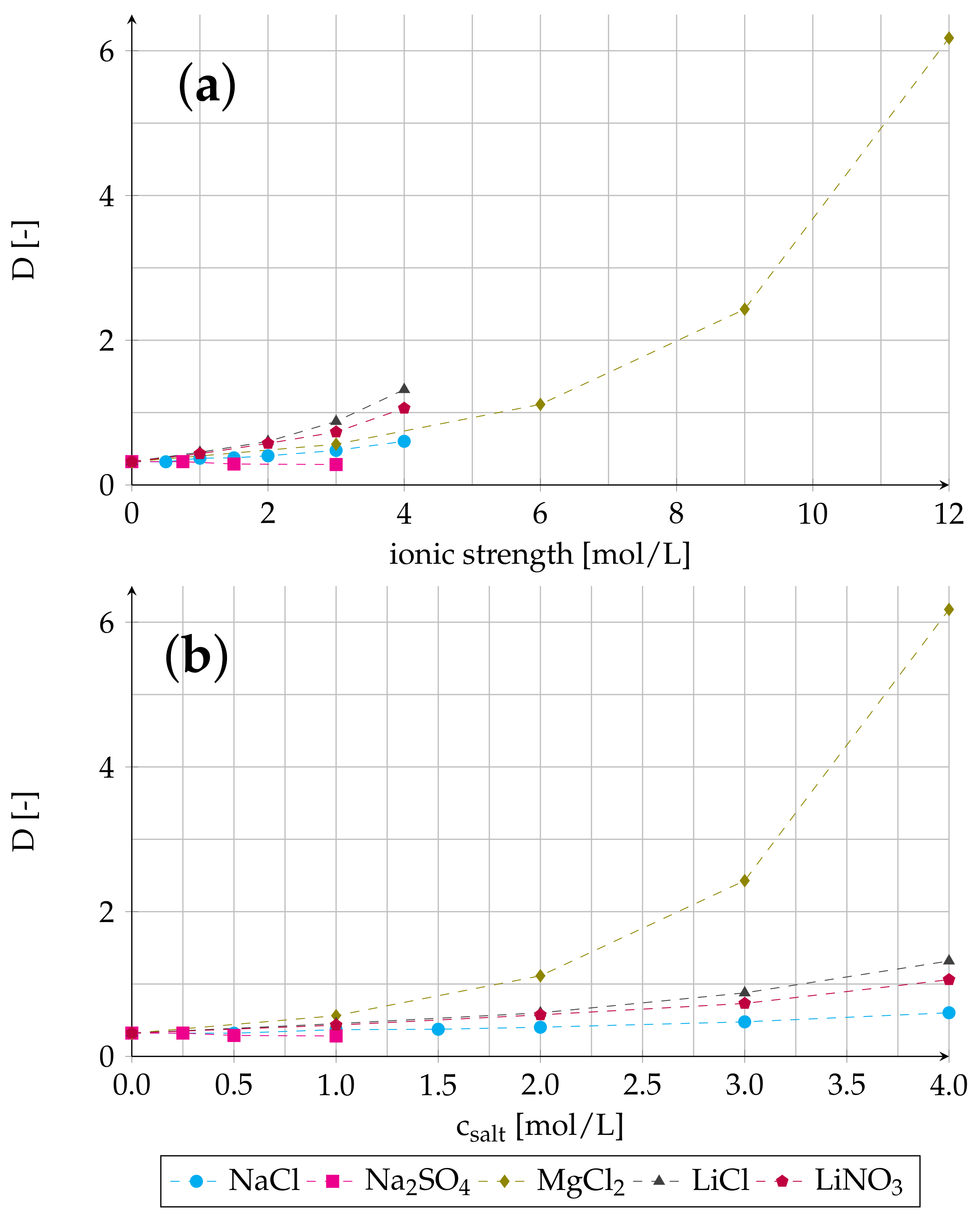
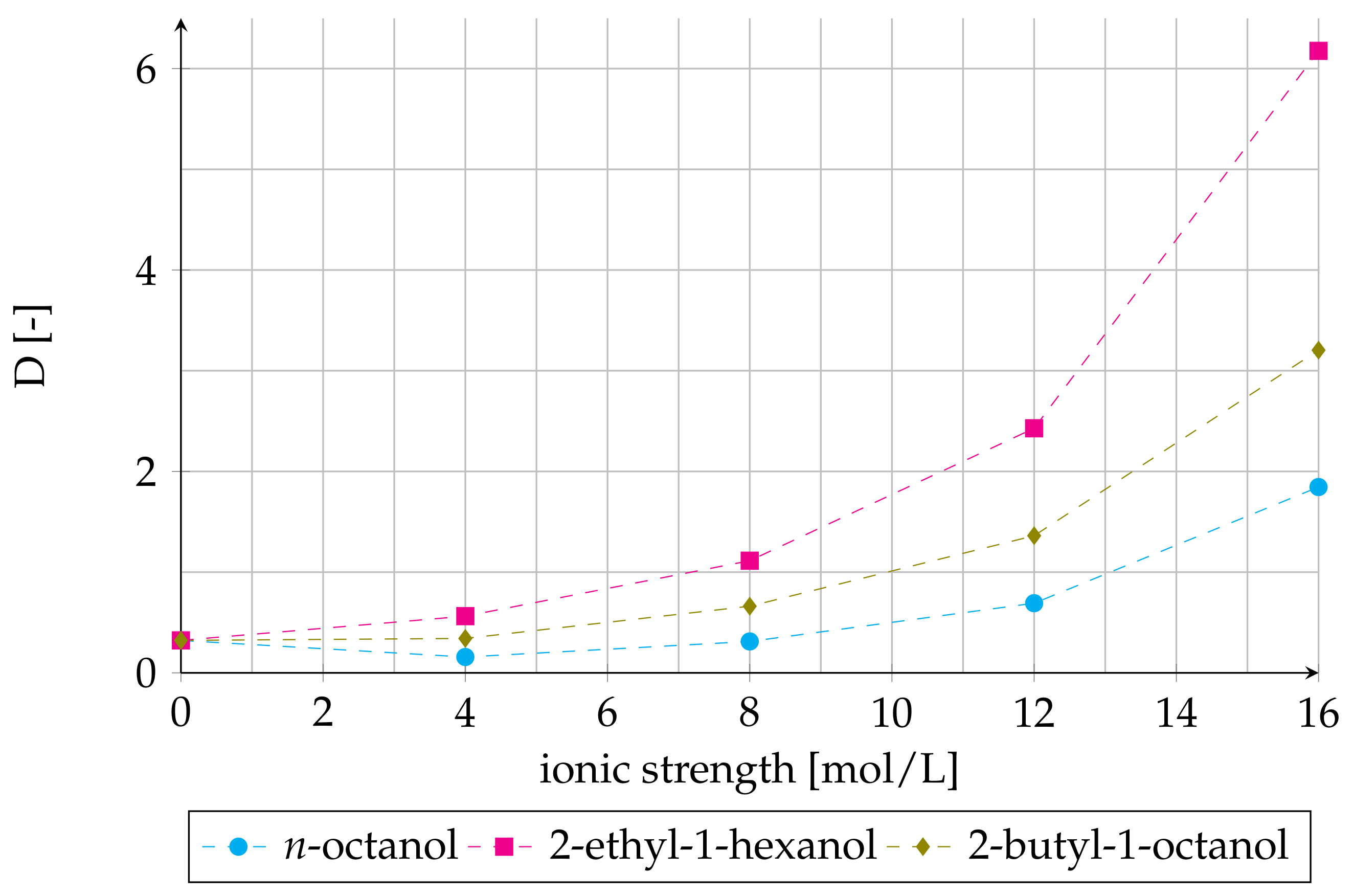
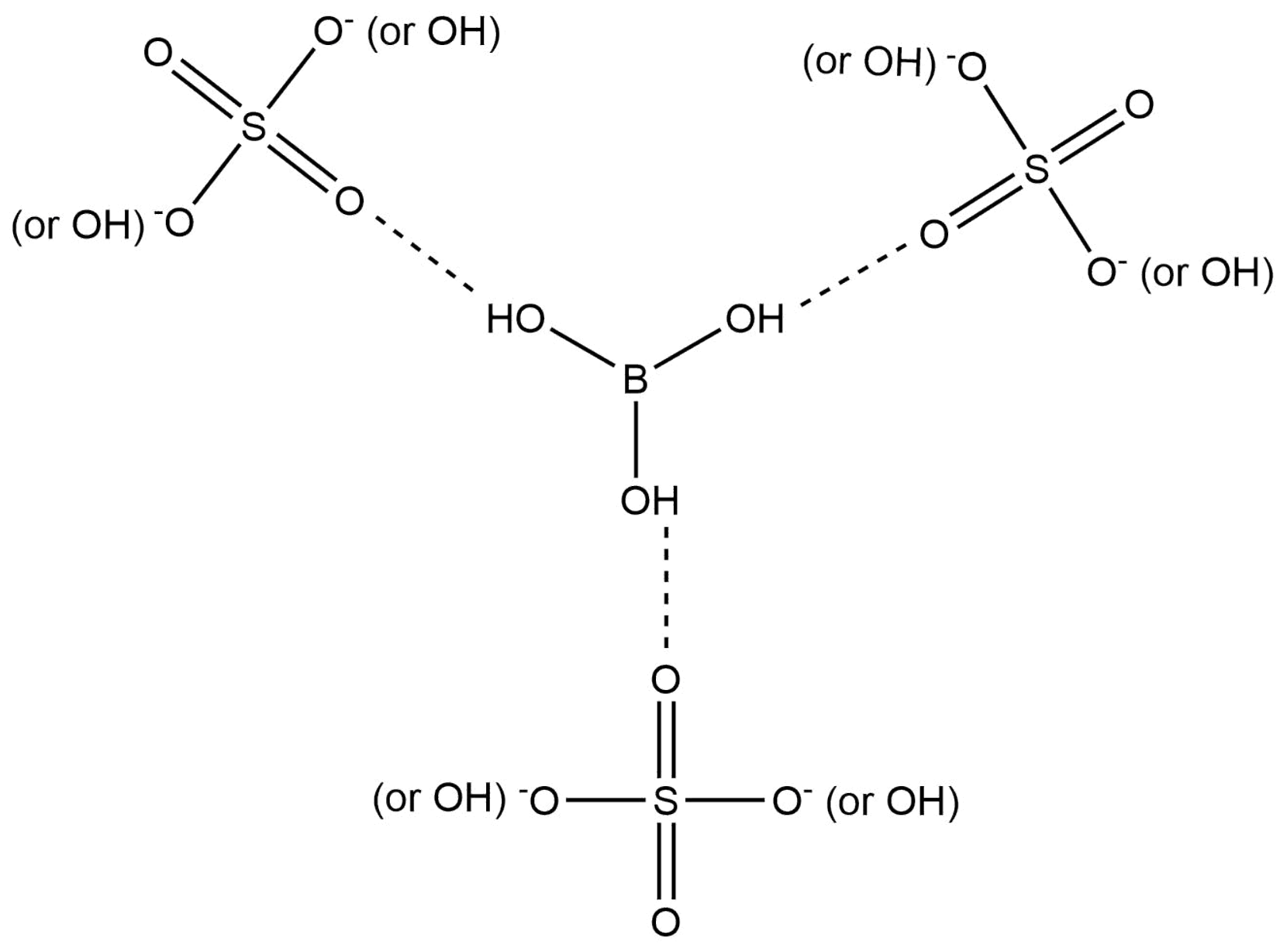
3.3. Effect of Ionic Strength on Distribution Ratio
- c—molar concentration of ion i [mol/L];
- z—charge number of ion i
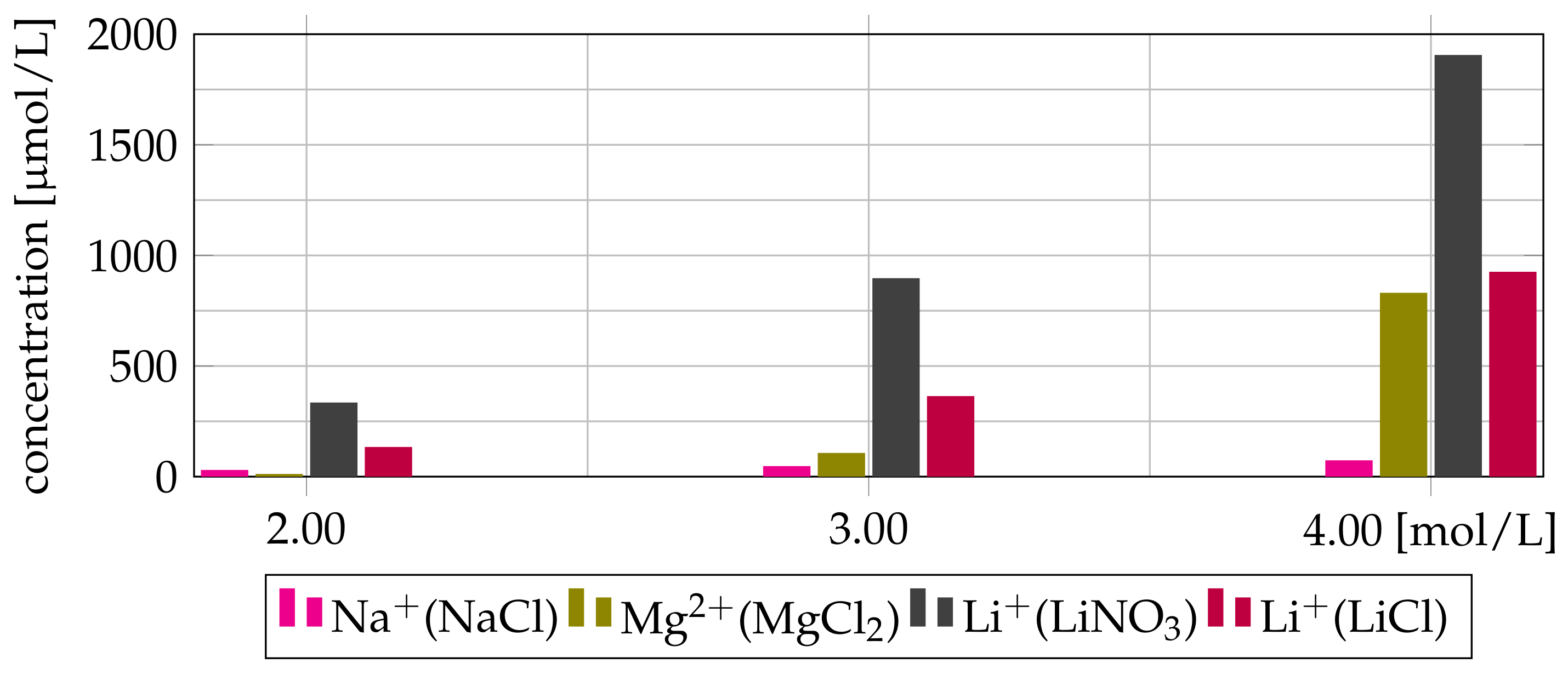
3.4. Co-Extraction of Salting-Out Agent
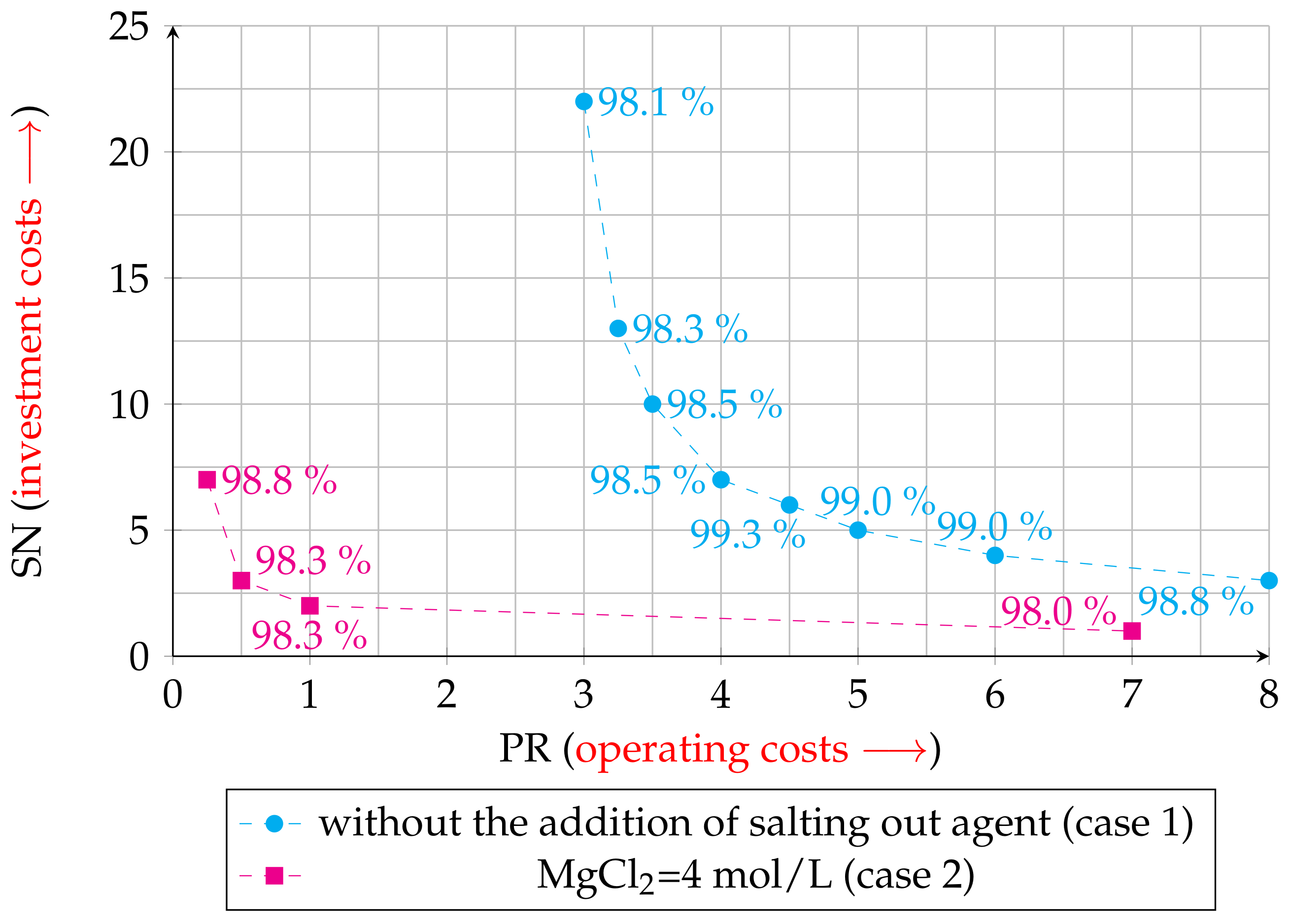
3.5. Determination of the Relationship between the Number of Theoretical Stages and the Phase Ratio with MATLAB
3.5.1. Case 1
3.5.2. Case 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAS | Atomic absorption spectroscopy |
| AP | Aqueous phase |
| c | Molar concentration |
| D | Distribution ratio |
| eq | Equilibrium |
| I | Ionic strength |
| ICP-OES | Inductively coupled plasma optical emission spectrometry |
| Ka | Volumetric mass transfer coefficient |
| MS | Mass transfer |
| Viscosity | |
| n | mol |
| OP | Organic phase |
| PR | Phase ratio |
| ROH | Alcohol |
| SN | Stage number |
| V | Volume |
| z | Charge number |
References
- Mergen, A.; Demirhan, M.H.; Bilen, M. Processing of boric acid from borax by a wet chemical method. Adv. Powder Technol. 2003, 14, 279–293. [Google Scholar] [CrossRef]
- Kistler, R.B.; Helvaci, C. Industrial Minerals and Rocks; Donald, D.C., Ed.; Society for Mining and Metallurgy and Exploration Inc.: Littleton, CO, USA, 1994; pp. 171–186. [Google Scholar]
- Brown, C.G.; Sanderson, B.R. Solvent extraction of boron. Chem. Ind. 1980, 68–73. [Google Scholar]
- Cramer, T.M. Production of Boric Acid and Sodium Nitrate. U.S. Patent US2965446A, 20 December 1960. [Google Scholar]
- ZareNezhad, B. Experimental and theoretical investigation of boric acid production through reactive dissolution of oxalic acid crystals in borax aqueous solution. Korean J. Chem. Eng. 2003, 20, 44–47. [Google Scholar] [CrossRef]
- ZareNezhad, B. Direct production of crystalline boric acid through heterogeneous reaction of solid borax with propionic acid: Operation and simulation. Korean J. Chem. Eng. 2004, 21, 956–962. [Google Scholar] [CrossRef]
- Kusaky, B.; Bulutcu, A.N. Design parameters of boric acid production process from colemanite ore in the presence of propionic acid. Chem. Eng. Process. Process. Intensif. 2011, 50, 377–383. [Google Scholar] [CrossRef]
- Bulutcu, A.N.; Ertekin, C.O.; Kuskay Celikoyan, M.B. Impurity control in the production of boric acid from colemanite in the presence of propionic acid. Chem. Eng. Process. Process. Intensif. 2008, 47, 2270–2274. [Google Scholar] [CrossRef]
- Polendo-Loredo, J. Process for the Preparation of Boric Acid from Colemanite and/or Howlite Minerals. U.S. Patent US4804524A, 14 February 1989. [Google Scholar]
- Elbeyli, İ.Y.; Turan, A.Z.; Kalafatoğlu, İ.E. The electrochemical production of boric acid. Chem. Ind. 2015, 90, 1855–1860. [Google Scholar]
- Erkmen, J.; Yapici, S. A environmentally friendly process for boric acid and sodium hydroxide production from borax; bipolar membrane electrodialysis. Desalin. Water Treat. 2016, 57, 20261–20269. [Google Scholar] [CrossRef]
- Zahid Turan, A.; Elbeyli, İ.A.; Bahar, T.; Kalafatoğlu, İ.E. The performance of Nafion®424 in the membrane electrolysis of borax solution. J. Ind. Eng. Chem. 2012, 18, 1102–1106. [Google Scholar] [CrossRef]
- An, J.W.; Kang, D.J.; Tran, K.T.; Kim, M.J.; Lim, T.; Tran, T. Recovery of lithium from Uyuni salar brine. Hydrometallurgy 2012, 117–118, 64–70. [Google Scholar] [CrossRef]
- Flexer, V.; Baspineiro, C.I. Lithium recovery from brines: A vital raw material for green energies with a potential environmental impact in its mining and processing. Sci. Total Environ. 2018, 639, 1188–1204. [Google Scholar] [CrossRef] [PubMed]
- Boryta, D.A.; Kullberg, T.F.; Thurston, A.M. Production of Lithium Compounds Directly from Lithium Containing Brines. U.S. Patent US7449161B2, 11 November 2008. [Google Scholar]
- Wilkomirsky, I. Process for Extracting the Boron Content in the Brine of Natural or Industrial Salt Mine. U.S. Patent US4804524A, 14 October 1997. [Google Scholar]
- Wilkomirsky, I. Extraction of Boron from Brines Having a pH of Less than about 1.7 Using Beta-Aliphatic Diols. U.S. Patent US3493349A, 3 February 1970. [Google Scholar]
- Grinstead, R.R. Removal of boron and calcium from magnesium chloride brines by solvent extraction. Ind. Eng. Chem. Prod. Res. Dev. 1972, 11, 454–460. [Google Scholar] [CrossRef]
- Fan, X.; Yu, X.; Guo, Y.; Deng, T. Recovery of boron from underground brine by continuous centrifugal extraction with 2-Ethyl-1,3-hexanediol (EHD) and its mechanism. J. Chem. 2018, 2018, 7530837. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Y.; Gao, X.; Yu, J. Boron extraction from lithium-rich brine using mixed alcohols. Hydrometallurgy 2020, 197, 105477. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, Y.; Song, J.; Xing, L.; Kong, D.; Li, X.-M.; He, T. Extraction of boron from salt lake brine using 2-ethylhexanol. Hydrometallurgy 2016, 160, 129–136. [Google Scholar] [CrossRef]
- Peng, X.; Li, L.; Shi, D.; Zhang, L.; Li, H.; Nie, F.; Song, F. Recovery of boric acid from salt lake brines by solvent extraction with 2-butyl-1-n-octanol. Hydrometallurgy 2018, 177, 161–167. [Google Scholar] [CrossRef]
- Schilde, U.; Uhlemann, E. Extraction of boric acid from brines by ion exchange. Int. J. Miner. Process. 1991, 32, 295–309. [Google Scholar] [CrossRef]
- Schilde, U.; Uhlemann, E. A simple method for the control of ion-exchange processes with boric acid using specific chelating resins. React. Polym. 1992, 18, 155–158. [Google Scholar] [CrossRef]
- Blasdale, W.C.; Slansky, C.M. The solubility curves of boric acid and the borates of sodium. J. Am. Chem. Soc. 1939, 61, 917–920. [Google Scholar] [CrossRef]
- Folkestad, I. Method of Removing Boric Acid and Boric Acid Salts from Aqueous Magnesium Chloride Solutions. U.S. Patent US3855392A, 17 December 1974. [Google Scholar]
- Ayers, P.; Dudeney, A.W.L.; Kahraman, K. Solvent extraction of boron with 2-ethyl-1,3-hexanediol and 2-chloro-4-(1,1,3,3-tetramethylbutyl)-6-methylol-phenol. J. Inorg. Nucl. Chem. 1981, 43, 2097–2100. [Google Scholar] [CrossRef]
- Mohapatra, D.; Chaudhury, G.R.; Park, K.H. Solvent extraction approach to recover boron from wastewater generated by the LCD manufacturing industry: Part 1. Min. Metall. Explor. 2008, 25, 175–180. [Google Scholar] [CrossRef]
- Garrett, D.E.; Weck, F.J.; Marsh, A.J.; Foster, H.R., Jr. Boron Extractants. U.S. Patent US3855392A, 19 November 1963. [Google Scholar]
- Wilkomirsky, I. Process for Removing Boron from Brines. U.S. Patent US5939038A, 17 August 1999. [Google Scholar]
- Tsuboi, I.; Kunugita, E.; Komasawa, I. Recovery and purification of boron from coal fly ash. J. Chem. Eng. Jpn. 1990, 23, 480–485. [Google Scholar] [CrossRef][Green Version]
- Matsumoto, M.; Kondo, K.; Hirata, M.; Kokubu, S.; Hano, T.; Takada, T. Recovery of boric acid from wastewater by solvent extraction. Sep. Sci. Technol. 1997, 32, 983–991. [Google Scholar] [CrossRef]
- Garrett, D.E. (Ed.) Part 1—Lithium. In Handbook of Lithium and Natural Calcium Chloride; Academic Press: Oxford, UK, 2004; pp. 1–235. [Google Scholar]
- John, M.K.; Chuah, H.H.; Neufeld, J.H. Application of improved azomethine-H method to the determination of boron in soils and plant. Anal. Lett. 1975, 8, 559–568. [Google Scholar] [CrossRef]
- Warade, A.; Gaikwad, R.; Sapkal, R.; Sapkal, V. Simulation of multistage countercurrent liquid-liquid extraction. Leonardo J. Sci. 2011, 20, 79–94. [Google Scholar]
- Hejda, J.; Jedináková, V. Separation of boric acid from radioactive wastes by liquid-liquid extraction. J. Radioanal. Chem. 1983, 80, 23–29. [Google Scholar] [CrossRef]
- GESTIS Substance Database. Available online: https://gestis-database.dguv.de/ (accessed on 29 October 2020).
- Fletcher, A.N.; Heller, C.A. Self-association of alcohols in nonpolar solvents. J. Phys. Chem. 1967, 71, 3742–3756. [Google Scholar] [CrossRef]
- Tural, B.; Tural, S.; Hosgören, H. Investigation of some 1,3-diols for the requirements of solvent extraction of boron: 2,2,6-trimethyl-1,3 heptanediol as a potential boron extractant. Turk. J. Chem. 2007, 31, 163–170. [Google Scholar]
- Uppström, L.R. The boron/chlorinity ratio of deep-sea water from the Pacific Ocean. Deep. Sea Res. Oceanogr. Abstr. 1974, 21, 161–162. [Google Scholar] [CrossRef]
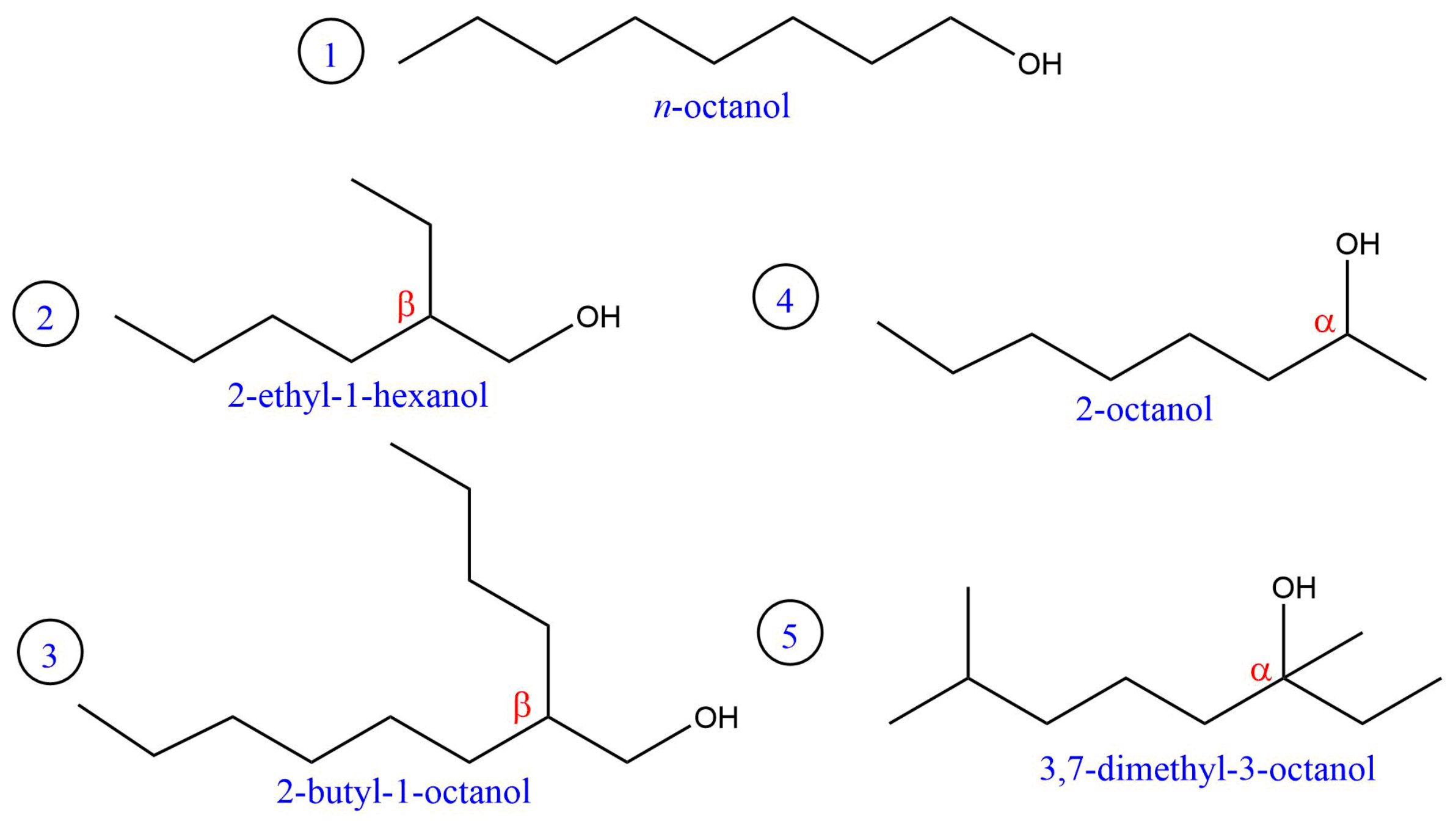


| Experiment | (1) | (2) | (3) | (4) |
|---|---|---|---|---|
| HBO in aqueous phase [mmol/L] | 185 | 185 | - (deionized water) | case 1: 46, 185, 738 case 2: 185 |
| Organic phase (vol% of extractant diluted in kerosene) | n-octanol | 2-ethyl-1-hexanol | Loaded organic | 2-ethyl-1-hexanol |
| (10–100) | (70) | phases from | (70) | |
| 2-ethyl-1-hexanol | 2-octanol | experiment (2) | ||
| (10–100) | (70) | |||
| 2-butyl-1-octanol | 2-butyl-1-octanol | |||
| (10–100) | (70) | |||
| 2-octanol | ||||
| (10-100) | ||||
| 3,7-dimethyl-3-octanol | ||||
| (10-100) | ||||
| Phase ratio [-] | 2 | 2 | 1 | 0.5–4 |
| pH [-] | 1.9–2.1 | 1.9–2.1 | approx. 7 | 1.9–2.1 |
| Extraction temperature [C] | 20 | |||
| Extraction time [h] | 2 | |||
| Rotation speed [rpm] | 60 | |||
| Compound | (1) | (2) | (3) | (4) | (5) |
|---|---|---|---|---|---|
| solubility in water [g/L] | 0.30 | 0.60 | <0.001 | 1.12 | 0.32 |
| Alcohol | (1) | (2) | (3) | (4) | (5) |
|---|---|---|---|---|---|
| Special feature of carbon chain | Tertiary -carbon | Secondary -carbon | Unbranched carbon chain | Secondary -carbon | |
| Relative extraction performance without salting out agent | 0.20 | 0.53 | 1.00 | 1.96 | 1.98 |
| Relative extraction performance at an ionic strength of 16 mol/L | Not determined | Not determined | 10.28 | 34.33 | 17.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balinski, A.; Recksiek, V.; Kelly, N. Solvent Extraction of Boric Acid: Comparison of Five Different Monohydric Alcohols and Equilibrium Modeling with Numerical Methods. Processes 2021, 9, 398. https://doi.org/10.3390/pr9020398
Balinski A, Recksiek V, Kelly N. Solvent Extraction of Boric Acid: Comparison of Five Different Monohydric Alcohols and Equilibrium Modeling with Numerical Methods. Processes. 2021; 9(2):398. https://doi.org/10.3390/pr9020398
Chicago/Turabian StyleBalinski, Adam, Volker Recksiek, and Norman Kelly. 2021. "Solvent Extraction of Boric Acid: Comparison of Five Different Monohydric Alcohols and Equilibrium Modeling with Numerical Methods" Processes 9, no. 2: 398. https://doi.org/10.3390/pr9020398
APA StyleBalinski, A., Recksiek, V., & Kelly, N. (2021). Solvent Extraction of Boric Acid: Comparison of Five Different Monohydric Alcohols and Equilibrium Modeling with Numerical Methods. Processes, 9(2), 398. https://doi.org/10.3390/pr9020398







