Effect of Air-Frying on the Bioactive Properties of Eggplant (Solanum melongena L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Microwave Cooking and Sample Preparation
2.2. Extraction
2.3. Total Polyphenol Content
2.4. Total Flavonoid Content
2.5. DPPH Scavenging
2.6. Reducing Power
2.7. HPLC Analysis of Phenolic Compounds
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Air-Frying and Extraction Solvents on the Total Polyphenol Content of Eggplant
3.2. Effect of Air-Frying and Extraction Solvents on the Total Flavonoid Content of Eggplant
3.3. Effect of Air-Frying and Extraction Solvents on the DPPH Scavenging of Eggplant
3.4. Effect of Air-Frying and Extraction Solvents on the Reducing Power of Eggplant
3.5. Correlation among Total Phenols, Total Flavonoids, and the Antioxidant Activities of Eggplant
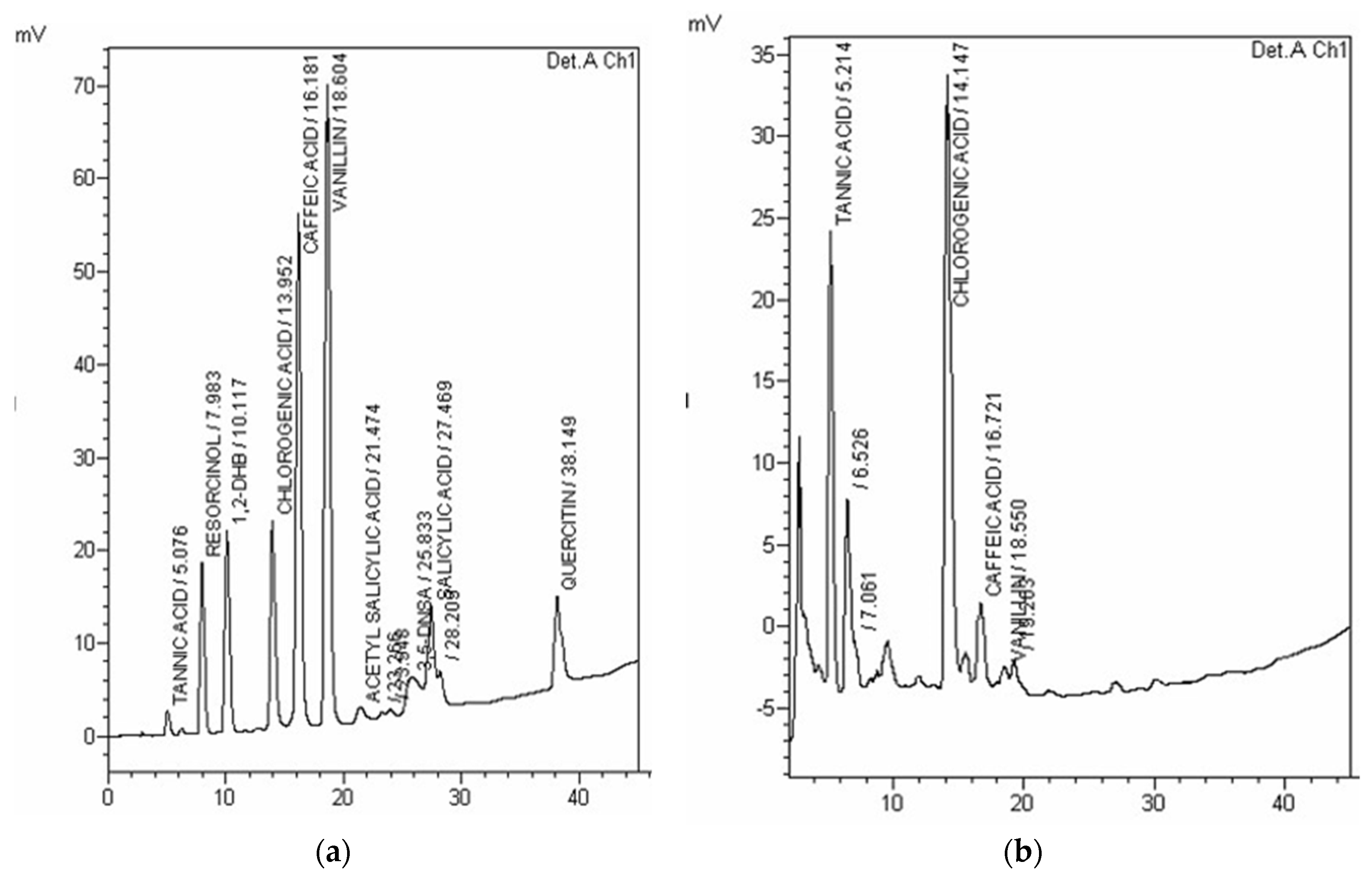
3.6. HPLC Analysis of Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taher, D.; Solberg, S.O.; Prohens, J.; Chou, Y.; Rakha, M.; Wu, T. World vegetable center eggplant collection: Origin, composition, seed dissemination and utilization in breeding. Front. Plant Sci. 2017, 8, 1484–1495. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 3 November 2020).
- Docimo, T.; Francese, G.; Ruggiero, A.; Batelli, G.; De Palma, M.; Bassolino, L.; Toppino, L.; Rotino, G.L.; Mennella, G.; Tucci, M. Phenylpropanoids accumulation in eggplant fruit: Characterization of biosynthetic genes and regulation by a MYB transcription factor. Front. Plant Sci. 2016, 6, 1233–1251. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.J.; Bae, M.S.; Jo, E.K.; Jo, Y.H.; Lee, S.C. Antioxidant activity of different parts of eggplant. J. Med. Plant Res. 2011, 5, 4610–4615. [Google Scholar]
- Akanitapichat, P.; Phraibung, K.; Nuchklang, K.; Prompitakkul, S. Antioxidant and hepatoprotective activities of 5 eggplant varieties. Food Chem. Toxicol. 2010, 48, 3017–3021. [Google Scholar] [CrossRef]
- Stommel, J.R.; Whitaker, B.D.; Haynes, K.G.; Prohens, J. Genotype × environment interactions in eggplant for fruit phenolic acid content. Euphytica 2015, 205, 823–836. [Google Scholar] [CrossRef]
- Singh, A.P.; Luthria, D.; Wilson, T.; Vorsa, N.; Singh, V.; Banuelos, G.S.; Pasakdee, S. Polyphenols content and antioxidant capacity of eggplant pulp. Food Chem. 2009, 114, 955–961. [Google Scholar] [CrossRef]
- Mennella, G.; Lo Scalzo, R.; Fibiani, M.; D’Alessandro, A.; Francese, G.; Toppino, L.; Acciarri, N.; de Almeida, A.E.; Rotino, G.L. Chemical and bioactive quality traits during fruit ripening in eggplant (S. melongena L.) and allied species. J. Agric. Food Chem. 2012, 60, 11821–11831. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Edwards, D.; Hamernig, I.; Jian, L.; James, A.P.; Johnson, S.K.; Tapsell, L.C. Vegetables containing phytochemicals with potential anti-obesity properties: A review. Food Res. Int. 2013, 52, 323–333. [Google Scholar] [CrossRef]
- Liu, R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Nayak, B.; Rui, H.L.; Tang, J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains-a review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–919. [Google Scholar] [CrossRef]
- Ferreira, S.F.; Sampaio, G.R.; Keller, L.M.; Sawaya, A.C.H.F.; Ch´avez, D.W.H.; Torres, E.A.F.S.; Saldanha, T. Impact of air Frying on cholesterol and fatty acids oxidation in sardines: Protective effects of aromatic herbs. J. Food Sci. 2017, 82, 2823–2831. [Google Scholar] [CrossRef]
- Donado-Pestana, C.M.; Salgado, J.M.; Rios, A.O.; Santos, P.R.; Jablonski, A. Stability of carotenoids, total phenolics and in vitro antioxidant capacity in the thermal processing of orange-fleshed sweet potato (Ipomoea batatas Lam.) cultivars grown in Brazil. Plant Foods Hum. Nutr. 2012, 67, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Arkoub-Djermoune, L.; Boulekbache-Makhlouf, L.; Zeghichi-Hamri, S.; Bellili, S.; Boukhalfa, F.; Madani, K. Influence of the thermal processing on the physicochemical properties and the antioxidant activity of a solanaceae vegetable: Eggplant. J. Food Qual. 2016, 39, 181–191. [Google Scholar] [CrossRef]
- Faller, A.L.K.; Fialho, E. The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking. Food Res. Int. 2009, 42, 210–215. [Google Scholar] [CrossRef]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, N.; Uluişik, S.; Frary, A.; Frary, A.; Doğanlar, S. Health benefits and bioactive compounds of eggplant. Food Chem. 2018, 268, 602–610. [Google Scholar] [CrossRef]
- Naeem, M.Y.; Ugur, S. Nutritional content and health benefits of eggplant. Turk. J. Agric.- Food Sci. Technol. 2019, 7, 31–36. [Google Scholar]
- Krokida, M.K.; Oreopolou, V.; Maroulis, Z.B. Water loss and oil uptake as a function of frying time. J. Food Eng. 2000, 44, 39–46. [Google Scholar] [CrossRef]
- Moreira, R.G.; Sun, X.; Chen, Y. Factors affecting oil uptake in tortilla chips in deep-fat frying. J. Food Eng. 1997, 31, 485–498. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, B.; Snetselaar, L.G.; Robinson, J.G.; Wallace, R.B.; Peterson, L.L.; Bao, W. Association of fried food consumption with all cause, cardiovascular, and cancer mortality: Prospective cohort study. BMJ 2019, 364, 5420–5429. [Google Scholar] [CrossRef]
- Payab, M.; Kelishadi, R.; Qorbani, M.; Motlagh, M.E.; Ranjbar, S.H.; Ardalan, G.; Zahedi, H.; Chinian, M.; Asayesh, H.; Larijani, B.; et al. Association of junk food consumption with high blood pressure and obesity in Iranian children and adolescents: The CASPIAN-IV Study. J. Pediatr. (Rio J) 2015, 91, 196–205. [Google Scholar] [CrossRef]
- Shaker, M. Air Frying a New Technique for Produce of Healthy Fried Potato Strips. J. Food Nutr. Sci. 2014, 2, 200–2006. [Google Scholar]
- Joshy, C.G.; Ratheesh, G.; Ninan, G.; Kumar, K.A.; Ravishankar, C.N. Optimizing air-frying process conditions for the development of healthy fish snack using response surface methodology under correlated observations. J. Food Sci. Technol. 2020, 57, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K. Impact of drying methods on the functional properties of peppermint (Mentha piperita L.) leaves. Sci. Lett. 2020, 8, 36–42. [Google Scholar]
- Aoshima, H.; Tsunoue, H.; Koda, H.; Kiso, Y. Aging of whiskey increases 1, 1-diphenyl-2-picrylhydrazyl radical scavenging activity. J. Agric. Food Chem. 2004, 52, 5240–5244. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Vig, A.P. Evaluation of in vitro antioxidant properties of methanol and aqueous extracts of Parkinsonia aculeata L. Leaves. Sci. World J. 2013, 604865, 1–7. [Google Scholar]
- He, J.; Yin, T.; Chen, Y.; Cai, L.; Tai, Z.; Li, Z.; Liu, C.; Wang, Y.; Ding, Z. Phenolic 374 compounds and antioxidant activities of edible flowers of Pyrus pashia. J. Funct. 2015, 17, 371–379. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The content of polyphenols in coffee beans as roasting, origin and storage effect. Eur. Food Res. Technol. 2020, 246, 33–39. [Google Scholar] [CrossRef]
- Diviš, P.; Pořízka, J.; Kříkala, J. The effect of coffee beans roasting on its chemical composition. Potravinarstvo Slovak J. Food Sci. 2019, 13, 344–350. [Google Scholar] [CrossRef]
- Das, S.; Raychaudhuri, U.; Falchi, M.; Bertelli, C.A.; Bragac, P.C.; Das, D.K. Cardioprotective properties of raw and cooked eggplant (Solanum melongena L). Food Funct. 2011, 2, 395–399. [Google Scholar] [CrossRef]
- Lo Scalzo, R.; Fibiani, M.; Mennella, G.; Rotino, G.L.; Dal Sasso, M.; Culici, M.; Spallino, A.; Braga, P.C. Thermal treatment of eggplant (Solanum melongena L.) increases the antioxidant content and the inhibitory effect on human neutrophil burst. J. Agric. and Food Chem. 2010, 58, 3371–3379. [Google Scholar] [CrossRef]
- Ramírez-Anaya, J.D.P.; Samaniego-Sánchez, C.; Castañeda-Saucedo, M.C.; Villalón-Mir, M.; de la Serrana, H.L.-G. Phenols and the antioxidant capacity of Mediterranean vegetables prepared with extra virgin olive oil using different domestic cooking techniques. Food Chem. 2015, 188, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Moreno, E.L.; Chávez-Jáuregui, R.N.; Plaza, M.D.L.; Wessel-Beaver, L. Phenolic content and antioxidant capacity in organically and conventionally grown eggplant (Solanum melongena) fruits following thermal processing. Food Sci. Technol. Campinas. 2015, 5, 414–420. [Google Scholar] [CrossRef]
- Pekič, B.; Kovač, V.; Alonso, E.; Revilla, E. Study of the extraction of proanthocyanidins from grape seeds. Food Chem. 1998, 61, 201–206. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J. Food Compos. Anal. 2006, 19, 41–44. [Google Scholar] [CrossRef]
- Biscaia, D.; Ferreira, S.R.S. Propolis extracts obtained by low-pressure methods and supercritical fluid extraction. J. Supercrit. Fluids 2009, 51, 17–23. [Google Scholar] [CrossRef]
- Chumyam, A.; Whangchai, K.; Jungklang, J.; Faiyue, B.; Saengnil, K. Effects of heat treatments on antioxidant capacity and total phenolic content of four cultivars of purple skin eggplants. Sci. Asia 2013, 39, 246–251. [Google Scholar] [CrossRef]
- Uthumporn, U.; Laila, D.L.; Rabeta, M.S.; Aida, H.; Ruri, A.S. Effects of different cooking methods on the physico-chemical and quality attributes of eggplants. Int. J. Adv. Sci. Eng. Inf. Technol. 2016, 6, 460–464. [Google Scholar]
- Juániz, I.; Ludwig, I.A.; Huarte, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; De Peña, M.P. Influence of heat treatment on antioxidant capacity and (poly)phenolic compounds of selected vegetables. Food Chem. 2016, 197, 466–473. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Šilarová, P.; Boulekbache-Makhlouf, L.; Pellati, F.; Česlová, L. Monitoring of chlorogenic acid and antioxidant capacity of Solanum melongena L. (eggplant) under different heat and storage treatments. Antioxidants (Basel) 2019, 8, 234. [Google Scholar]
- Chung, K.-T.; Wong, T.Y.; Wei, C.-I.; Huang, Y.-W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar]
- Alkurd, A.; Takruri, H.R.; Al-Sayyed, H. Tannin contents of selected plants used in Jordan. Jordan J. Agric. Sci. 2008, 4, 265–274. [Google Scholar]





| Nutrient | Unit | Value Per 100 g |
|---|---|---|
| Main components | ||
| Energy | kJ | 100 |
| Total carbohydrates | G | 5.88 |
| Sugars | G | 3.53 |
| Protein | G | 0.98 |
| Fiber | G | 3.00 |
| Lipid | G | 0.18 |
| Water | G | 92.3 |
| Minerals | ||
| Calcium | mg | 9.00 |
| Iron | mg | 0.23 |
| Sodium | mg | 2.00 |
| Potassium | mg | 229.0 |
| Magnesium | mg | 14.0 |
| Phosphorus | mg | 24.0 |
| Zinc | mg | 0.16 |
| Vitamins | ||
| Vitamin C | mg | 2.20 |
| Thiamin | mg | 0.039 |
| Niacin | mg | 0.649 |
| Riboflavin | mg | 0.037 |
| Folate | µg | 22.0 |
| Vitamin B6 | mg | 0.084 |
| Vitamin A, RAE * | µg | 1.00 |
| Vitamin E (α-tocopherol) | mg | 0.30 |
| Vitamin K (phylloquinone) | µg | 3.50 |
| Lipids | ||
| Fatty acids, total saturated | G | 0.034 |
| Fatty acids, total monosaturated | G | 0.016 |
| Fatty acids, total polysaturated | g | 0.076 |
| Reducing Power | DPPH Scavenging (IC50) | |
|---|---|---|
| Total polyphenol content | 0.723 | −0.471 |
| Total flavonoid content | 0.698 | −0.460 |
| Compound Name | UC | LC | MC | HC |
|---|---|---|---|---|
| Tannic acid | 8.67 | 14.64 | 283.29 | 138.39 |
| Resorcinol | 0.09 | 0.15 | ND | ND |
| Chlorogenic acid | 4.79 | 12.13 | 48.93 | 77.04 |
| Caffeic acid | 0.21 | ND | 2.77 | 0.57 |
| Vanillin | 0.08 | ND | 0.54 | 0.15 |
| Acetyl salicylic acid | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamatullah, A.M.; Ahmed, M.A.; Alkaltham, M.S.; Hayat, K.; Aloumi, N.S.; Al-Dossari, A.M.; Al-Harbi, L.N.; Arzoo, S. Effect of Air-Frying on the Bioactive Properties of Eggplant (Solanum melongena L.). Processes 2021, 9, 435. https://doi.org/10.3390/pr9030435
Salamatullah AM, Ahmed MA, Alkaltham MS, Hayat K, Aloumi NS, Al-Dossari AM, Al-Harbi LN, Arzoo S. Effect of Air-Frying on the Bioactive Properties of Eggplant (Solanum melongena L.). Processes. 2021; 9(3):435. https://doi.org/10.3390/pr9030435
Chicago/Turabian StyleSalamatullah, Ahmad Mohammad, Mohammed Asif Ahmed, Mohammed Saeed Alkaltham, Khizar Hayat, Najla Sulaiman Aloumi, Alhanouf Mohammed Al-Dossari, Laila Naif Al-Harbi, and Shaista Arzoo. 2021. "Effect of Air-Frying on the Bioactive Properties of Eggplant (Solanum melongena L.)" Processes 9, no. 3: 435. https://doi.org/10.3390/pr9030435
APA StyleSalamatullah, A. M., Ahmed, M. A., Alkaltham, M. S., Hayat, K., Aloumi, N. S., Al-Dossari, A. M., Al-Harbi, L. N., & Arzoo, S. (2021). Effect of Air-Frying on the Bioactive Properties of Eggplant (Solanum melongena L.). Processes, 9(3), 435. https://doi.org/10.3390/pr9030435







