Fractionation of Tilapia By-Product Protein Hydrolysate Using Multilayer Configuration of Ultrafiltration Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Tilapia By-Product Mince
2.2. Preparation of Tilapia Protein Hydrolysate Using Alcalase
2.3. Membrane Type and Module
2.4. Preparation of Membranes and Procedure for Cleaning
2.5. Experiments of Membrane Filtration
2.5.1. Permeate Flux
2.5.2. Peptide Transmission
2.6. Peptides Content Measurement
2.7. Determination of Peptides Distribution
2.8. Statistical Analysis
3. Results and Discussion
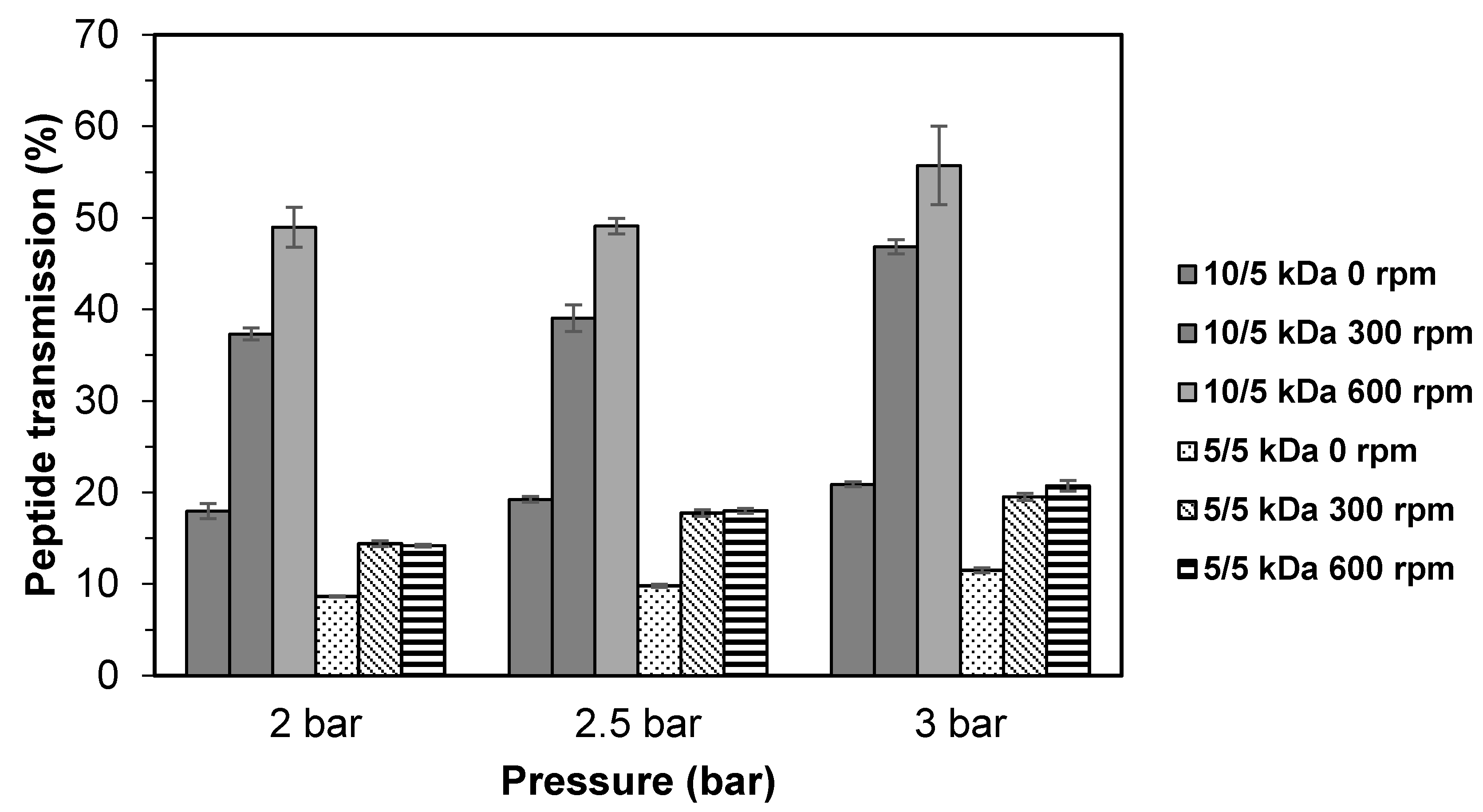
3.1. Effects of Stirring Speed on Fractionation of TB Protein Hydrolysate Using Multilayer Membranes
3.2. Effects of Solution pH on the Fractionation of TB Protein Hydrolysate Using Multilayer Membranes
3.3. Effects of Salt Concentration (NaCl) on the Fractionation of TB Protein Hydrolysate Using Multilayer Membranes
3.4. Peptides Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zamorano-Apodaca, J.C.; García-Sifuentes, C.O.; Carvajal-Millán, E.; Vallejo-Galland, B.; Scheuren-Acevedo, S.M.; Elena, L.S.M. Biological and functional properties of peptide fractions obtained from collagen hydrolysate derived from mixed by-products of different fish species. Food Chem. 2020, in press. [Google Scholar] [CrossRef]
- Saidi, S.; Deratani, A.; Belleville, M.P.; Amar, R.B. Production and fractionation of tuna by-product protein hydrolysate by ultrafiltration and nanofiltration: Impact on interesting peptides fractions and nutritional properties. Food Res. Int. 2014, 65, 453–461. [Google Scholar] [CrossRef]
- Bougatef, A.; Nedjar-Arroume, N.; Ravallec-Plé, R.; Leroy, Y.; Guillochon, D.; Barkia, A.; Nasri, M. Angiotensin I-converting enzyme (ACE) inhibitory activities of sardinelle (Sardinella aurita) by-products protein hydrolysates obtained by treatment with microbial and visceral fish serine proteases. Food Chem. 2008, 111, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Nasir, S.N.A.M.; Sarbon, N.M. Angiotensin converting enzyme (ACE), antioxidant activity and functional properties of shortfin scad (Decapterus macrosoma) muscle protein hydrolysate at different molecular weight variations. Biocatal. Agric. Biotechnol. 2019, 20, 101254. [Google Scholar] [CrossRef]
- Robert, M.; Zatylny-Gaudina, C.; Fournierd, V.; Corree, E.; Le Corguillée, G.; Bernayc, B.; Henry, J. Molecular characterization of peptide fractions of a Tilapia (Oreochromis niloticus) by-product hydrolysate and in vitro evaluation of antibacterial activity. Process Biochem. 2015, 50, 487–492. [Google Scholar] [CrossRef]
- Wang, K.; Siddanakoppalub, P.N.; Ahmed, I.; Pavase, T.R.; Lin, H.; Li, Z. Purification and identification of anti-allergic peptide from Atlantic Salmon (Salmo salar) byproduct enzymatic hydrolysates. J. Funct. Foods. 2020, 72, 104084. [Google Scholar] [CrossRef]
- Khairkar, S.R.; Amol, V.; Pansare, A.V.; Shedge, A.A.; Chhatre, S.Y.; Suresh, A.K.; Chakrabarti, S.; Patil, V.R.; Nagarkar, A.A. Hydrophobic interpenetrating polyamide-PDMS membranes for desalination, pesticides removal and enhanced chlorine tolerance. Chemosphere 2020, 258, 127179. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Kristinsson, H.G. ACE-inhibitory activity of tilapia protein hydrolysates. Food Chem. 2009, 117, 582–588. [Google Scholar] [CrossRef]
- Kim, S.; Je, J.; Kim, S. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ranathunga, S.; Rajapakse, N.; Kim, S.K. Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster). Eur. Food Res. Technol. 2006, 222, 310–315. [Google Scholar] [CrossRef]
- Cui, Z.F.; Jiang, Y.; Field, R.W. Chapter 1—Fundamentals of Pressure-Driven membrane separation processes. In Membrane Technology; Cui, Z.F., Muralidhara, H.S., Eds.; Elsevier: Burlington, MA, USA, 2010; pp. 1–18. [Google Scholar]
- Atkinson, A.J.; Wang, J.; Grzebyk, K.; Zhang, Z.; Jung, D.; Zeng, D.; Pollard, A.; Gold, A.; Coronell, O. Scalable fabrication of anti-biofouling membranes through 2-aminoimidazole incorporation during polyamide casting. J. Membr. Sci. 2019, 579, 151–161. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Seyedpour, S.F.; Aktij, S.A.; Giagnorio, M.; Bazrafshan, N.; Mollahosseini, A.; Samadi, F.; Ahmadalipour, S.; Firouzjaei, F.D.; Esfahani, M.R.; et al. Recent advances in functionalized polymer membranes for biofouling control and mitigation in forward osmosis. J. Membr. Sci. 2020, 596, 117604. [Google Scholar] [CrossRef]
- Das, R.; Bhattacherjee, C.; Ghosh, S. Effects of operating parameters and nature of fouling behavior in ultrafiltration of sesame protein hydrolysate. Desalination 2009, 237, 268–276. [Google Scholar] [CrossRef]
- Saidi, S.; Deratani, A.; Amar, R.B.; Belleville, M.P. Fractionation of a tuna dark muscle hydrolysate by a two-step membrane process. Sep. Purif. Technol. 2013, 108, 28–36. [Google Scholar] [CrossRef]
- Prata-Vidal, M.; Bouhallab, S.; Henry, G.; Aimar, P. An experimental study of caseinomacropeptide hydrolysis by trypsin in a continuous membrane reactor. Biochem. Eng. J. 2001, 8, 195–202. [Google Scholar] [CrossRef]
- Md. Yunos, K.F.; Field, R.W. Rejection amplification in the ultrafiltration of binary protein mixtures using sandwich configurations. Chem. Eng. Process. 2008, 47, 1053–1060. [Google Scholar] [CrossRef]
- Feins, M.; Sirkar, K. Highly selective membranes in protein ultrafiltration. Biotechnol. Bioeng. 2004, 86, 603–611. [Google Scholar] [CrossRef]
- Feins, M.; Sirkar, K. Novel internally staged ultrafiltration for protein purification. J. Membr. Sci. 2005, 248, 137–148. [Google Scholar] [CrossRef]
- Md Yunos, K.F.; Field, R.W. Effect of sandwich configuration of ultrafiltration membranes on protein fractionation. Desalination 2006, 199, 222–224. [Google Scholar] [CrossRef]
- Field, R.W.; Md Yunos, K.F.; Cui, Z. Separation of proteins using sandwich membranes. Desalination 2009, 245, 597–605. [Google Scholar] [CrossRef]
- El-Sayed, A.M. Tilapia Culture; CABI Publishing: Oxfordshire, UK, 2006. [Google Scholar]
- Roslan, J.; Mustapa Kamal, S.M.; Yunos, K.F.; Abdullah, N. Optimization of enzymatic hydrolysis of tilapia (Oreochromis niloticus) by-product using response surface methodology. Int. Food Res. J. 2015, 22, 1117–1123. [Google Scholar]
- Roslan, J.; Mustapa Kamal, S.M.; Yunos, K.F.; Abdullah, N. Characterization of Fish Protein Hydrolysate from Tilapia (Oreochromis niloticus) by-Product. Agric. Agric. Sci. Procedia 2014, 2, 312–319. [Google Scholar] [CrossRef]
- Ghosh, R. Protein Bioseparation using Ultrafiltration; Imperial College Press: London, UK, 2003; p. 166. [Google Scholar]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT User’s Guide, Version 6, 4th ed.; SAS Institute Inc.: Cary, NC, USA, 1989; Volume 2. [Google Scholar]
- Roslan, J.; Mustapa Kamal, S.M.; Yunos, K.F.; Abdullah, N. Assessment on multilayer ultrafiltration membrane for fractionation of tilapia by-product protein hydrolysate with angiotensin I-converting enzyme (ACE) inhibitory activity. Sep. Purif. Technol. 2017, 173, 250–257. [Google Scholar] [CrossRef]
- Roslan, J.; Mustapa Kamal, S.M.; Yunos, K.F.; Abdullah, N. Evaluation on performance of dead-end ultrafiltration membrane in fractionating tilapia by-product protein hydrolysate. Sep. Purif. Technol. 2018, 195, 21–29. [Google Scholar] [CrossRef]
- Sarkar, D.; Bhattacharya, A.; Bhattacharjee, C. Modelling the performances of a standard single stirred ultrafiltration cell using variable velocity back transport flux. Desalination 2010, 261, 89–98. [Google Scholar] [CrossRef]
- Wan, Y.; Ghosh, R.; Cui, Z. Fractionation of Proteins Using Ultrafiltration: Developments and Challenges. Dev. Chem. Eng. Mineral. Process. 2005, 13, 121–136. [Google Scholar] [CrossRef]
- Datta, D.; Bhattacharjee, S.; Nath, A.; Das, R.; Bhattacharjee, C.; Datta, S. Separation of ovalbumin from chicken egg white using two-stage ultrafiltration technique. Sep. Purif. Technol. 2009, 66, 353–361. [Google Scholar] [CrossRef]
- Sarkar, P.; Ghosh, S.; Dutta, S.; Sen, D.; Bhattrjee, C. Effect of different operating parameters on the recovery of proteins from casein whey using a rotating disc membrane ultrafiltration cell. Desalination 2009, 249, 5–11. [Google Scholar] [CrossRef]
- Aravind, U.K.; Mathew, J.; Aravindakumar, C.T. Transport studies of BSA, lysozyme and ovalbumin through chitosan/polystyrene sulfonate multilayer membrane. J. Membr. Sci. 2007, 299, 146–155. [Google Scholar] [CrossRef]
- Ghosh, R.; Cui, Z.F. Fractionation of BSA and lysozyme using ultrafiltration: Effect of pH and membrane pretreatment. J. Membr. Sci. 1998, 139, 17–28. [Google Scholar] [CrossRef]
- Wang, Y.N.; Tang, C.Y. Protein fouling of nanofiltration, reverse osmosis, and ultrafiltration membranes-The role of hydrodynamic conditions, solution chemistry, and membrane properties. J. Membr. Sci. 2011, 376, 275–282. [Google Scholar] [CrossRef]
- Wan, Y.; Lu, J.; Cui, Z. Separation of lysozyme from chicken egg white using ultrafiltration. Sep. Purif. Technol. 2006, 48, 133–142. [Google Scholar] [CrossRef]
- Groleau, P.E.; Morin, P.; Gauthier, S.F.; Pouliot, Y. Effect of physicochemical conditions on peptide-peptide interactions in a tryptic hydrolysate of β-lactoglobulin and identification of aggregating peptides. J. Agric. Food Chem. 2003, 51, 4370–4375. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Riera, F.A. Influence of ionic strength on peptide membrane fractionation. Sep. Purif. Technol. 2013, 119, 129–135. [Google Scholar] [CrossRef]
- Lin, S.H.; Hung, C.L.; Juang, R.S. Effect of operating parameters on the separations of proteins in aqueous solutions by dead-end ultrafiltration. Desalination 2008, 234, 116–125. [Google Scholar] [CrossRef]
- She, Q.; Tang, C.Y.; Wang, Y.N.; Zhang, Z. The role of hydrodynamic conditions and solution chemistry on fouling during ultrafiltration. Desalination 2009, 249, 1079–1087. [Google Scholar] [CrossRef]
- Pouliot, Y.; Wijers, M.C.; Gauthier, S.F.; Nadeau, L. Fractionation of whey protein hydrolysates using charged UF/NF membranes. J. Membr. Sci. 1999, 158, 105–114. [Google Scholar] [CrossRef]
- Nau, F.; Kerhervé, F.L.; Leonil, J.; Daufin, G. Selective separation of tryptic beta-casein peptides through ultrafiltration membranes: Influence of ionic interactions. Biotechnol. Bioeng. 1995, 46, 246–253. [Google Scholar] [CrossRef]






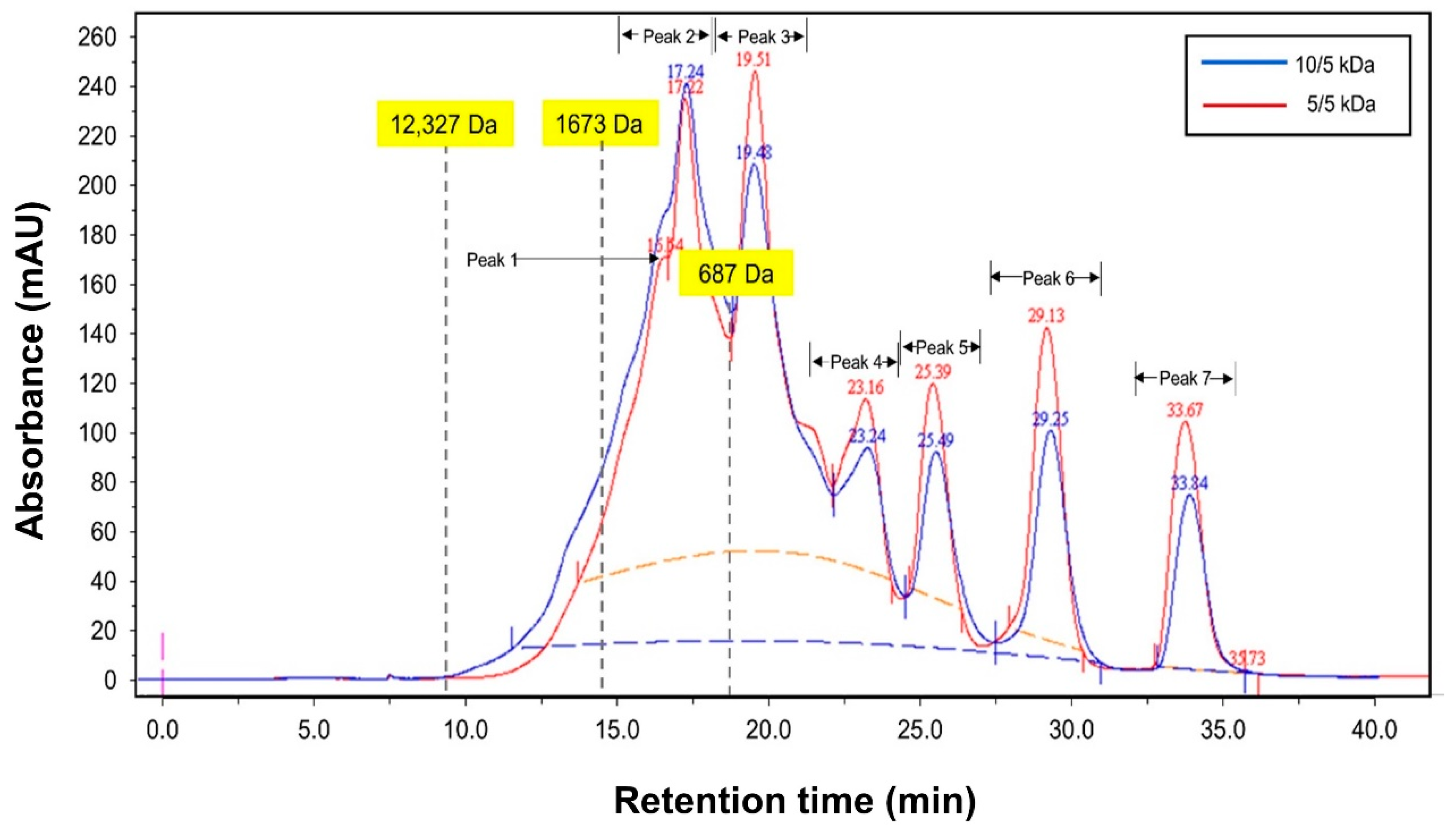
| Membrane Configuration (kDa) | Peptide Concentration (g/L) | |
|---|---|---|
| 10/5 | 5/5 | |
| Peptide fraction (<1500 Da) | ||
| Peak 1 (1088 Da) | ND | 0.14 ± 0.04 |
| Peak 2 (895 Da) | 0.56 ± 0.06 | 0.22 ± 0.08 |
| Total Concentration (<1500 Da) | 0.56 | 0.36 |
| Peptide fraction (<500 Da) | ||
| Peak 3 | 0.33 ± 0.03 | 0.28 ± 0.08 |
| Peak 4 | 0.01 ± 0.02 | 0.08 ± 0.03 |
| Peak 5 | 0.08 ± 0.01 | 0.07 ± 0.01 |
| Peak 6 | 0.09 ± 0.01 | 0.12 ± 0.03 |
| Peak 7 | 0.07 ± 0.01 | 0.10 ± 0.02 |
| Total concentration (<500 Da) | 0.58 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roslan, J.; Mustapa Kamal, S.M.; Md. Yunos, K.F.; Abdullah, N. Fractionation of Tilapia By-Product Protein Hydrolysate Using Multilayer Configuration of Ultrafiltration Membrane. Processes 2021, 9, 446. https://doi.org/10.3390/pr9030446
Roslan J, Mustapa Kamal SM, Md. Yunos KF, Abdullah N. Fractionation of Tilapia By-Product Protein Hydrolysate Using Multilayer Configuration of Ultrafiltration Membrane. Processes. 2021; 9(3):446. https://doi.org/10.3390/pr9030446
Chicago/Turabian StyleRoslan, Jumardi, Siti Mazlina Mustapa Kamal, Khairul Faezah Md. Yunos, and Norhafizah Abdullah. 2021. "Fractionation of Tilapia By-Product Protein Hydrolysate Using Multilayer Configuration of Ultrafiltration Membrane" Processes 9, no. 3: 446. https://doi.org/10.3390/pr9030446
APA StyleRoslan, J., Mustapa Kamal, S. M., Md. Yunos, K. F., & Abdullah, N. (2021). Fractionation of Tilapia By-Product Protein Hydrolysate Using Multilayer Configuration of Ultrafiltration Membrane. Processes, 9(3), 446. https://doi.org/10.3390/pr9030446






