On-Line Separation and Determination of Trivalent and Hexavalent Chromium with a New Liquid Membrane Annular Contactor Coupled to Inductively Coupled Plasma Optical Emission Spectrometry
Abstract
1. Introduction
2. Materials and Methods
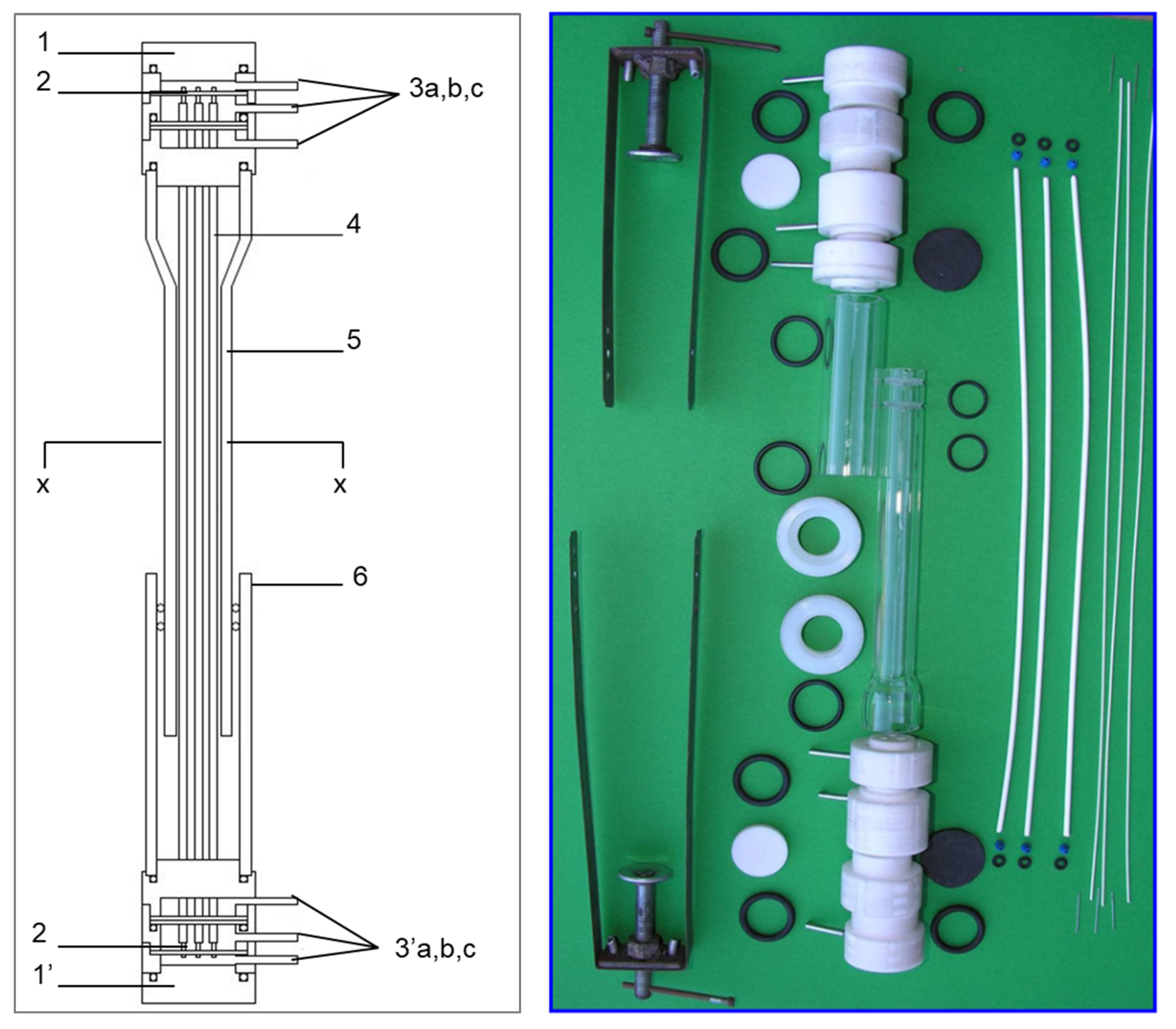
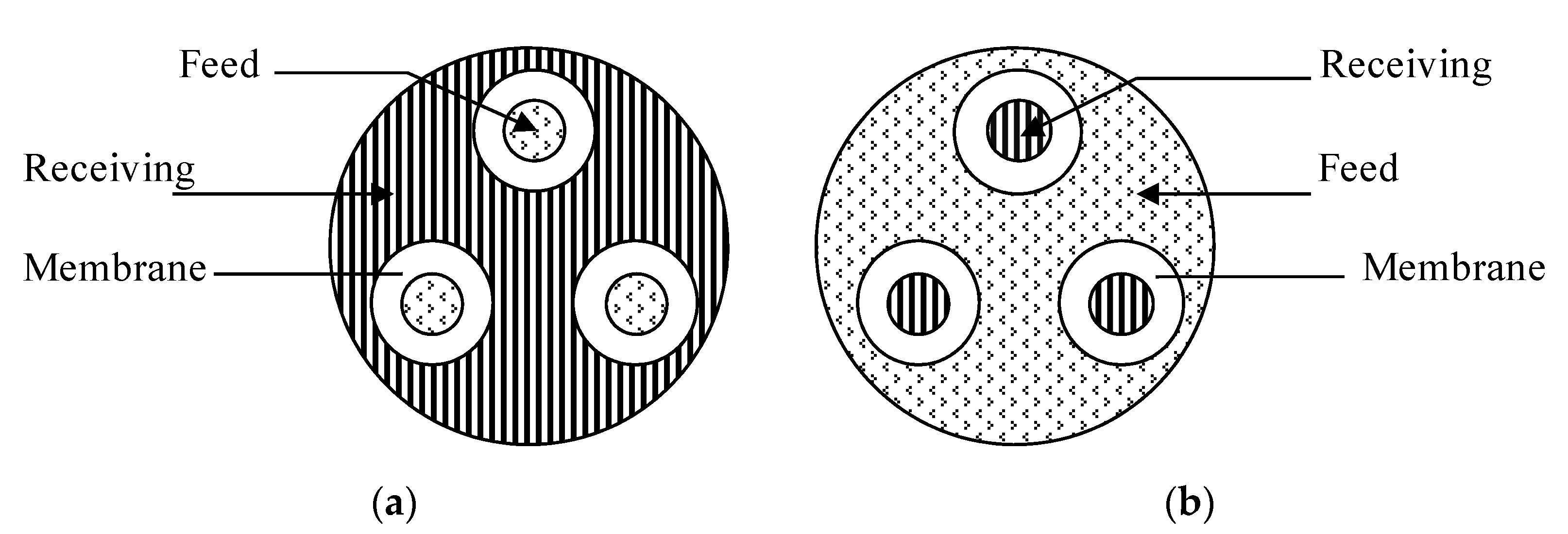
2.1. Design and Development of the TMC
2.2. Analytical Determination
2.3. Selection of Carrier, Membrane Solvent, and Receiving Solution
3. Results and Discussion
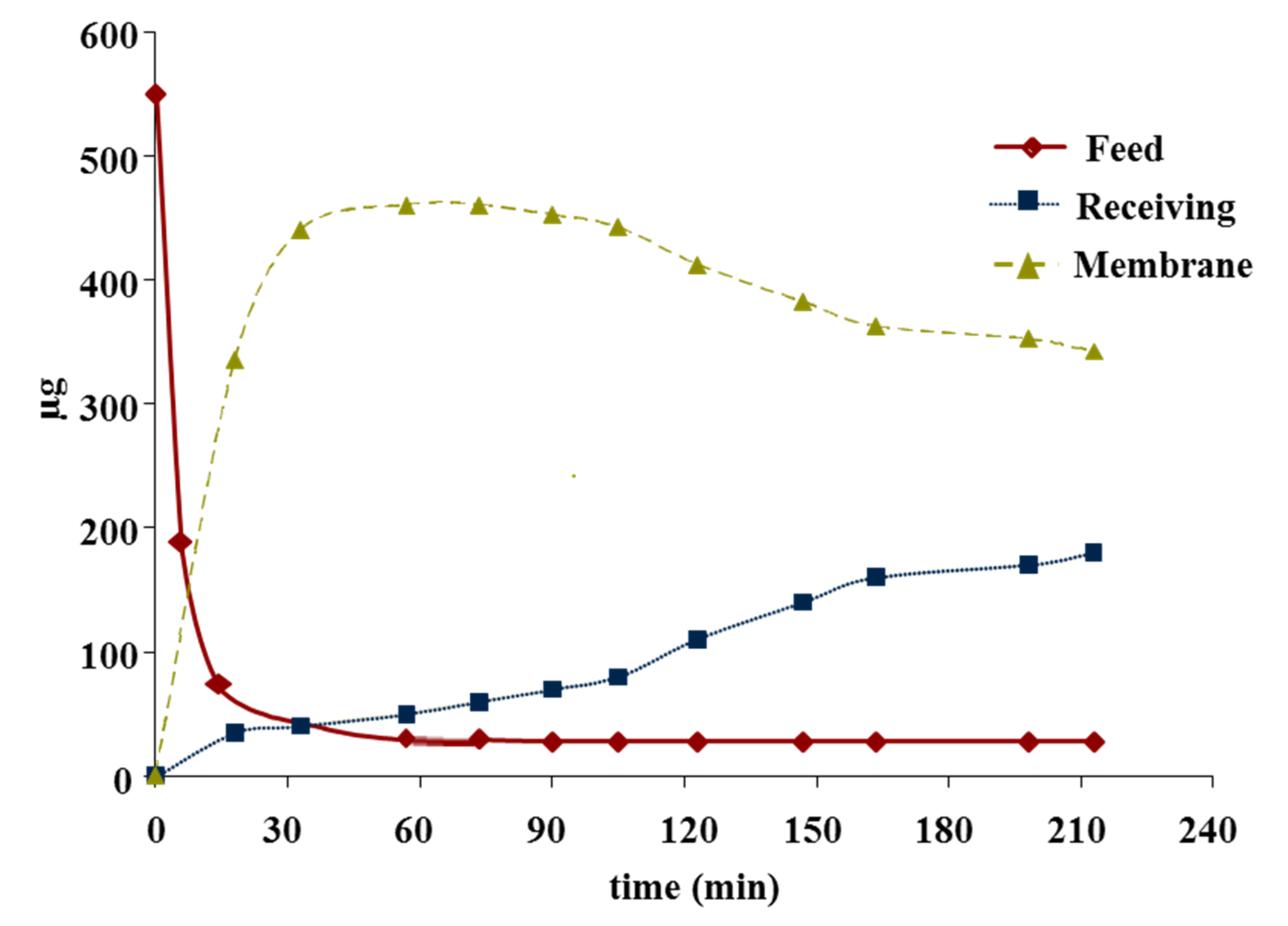
3.1. TMC Performance for Cr(VI) Transport
3.2. Coupling of the TMC with ICP-OES and On-Line Determination of Cr(III) and Cr(VI)
Optimization of the Quantification Procedure
3.3. Study Limitation
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avudainayagam, S.; Megharaj, M.; Owens, G.; Kookana, R.S.; Chittleborough, D.; Naidu, R. Chemistry of chromium in soils with emphasis on tannery waste sites. Rev. Environ. Contam. Toxicol. 2003, 178, 53–91. [Google Scholar] [PubMed]
- Lunk, H.J. Discovery, properties and applications of chromium and its compounds. ChemTexts 2015, 1, 1–17. [Google Scholar] [CrossRef]
- Canepari, S.; Castellano, P.; Astolfi, M.L.; Materazzi, S.; Ferrante, R.; Fiorini, D.; Curini, R. Release of particles, organic compounds, and metals from crumb rubber used in synthetic turf under chemical and physical stress. Environ. Sci. Pollut. Res. 2015, 25, 1448–1459. [Google Scholar] [CrossRef]
- Tajima, H.; Yoshida, T.; Ohnuma, A.; Fukuyama, T.; Hayashi, K.; Yamaguchi, S.; Ohtsuka, R.; Sasaki, J.; Tomita, M.; Kojima, S.; et al. Pulmonary injury and antioxidant response in mice exposed to arsenate and hexavalent chromium and their combination. Toxicology 2010, 267, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, M.L.; Marconi, E.; Lorini, L.; Valentino, F.; Silva, F.; Ferreira, B.S.; Canepari, S.; Majone, M. Elemental concentration and migratability in bioplastics derived from organic waste. Chemosphere 2020, 25, 127472. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Element, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1997. [Google Scholar]
- Lai, A.; Astolfi, M.L.; Bertelli, V.; Gatto Agostinelli, V.; Zeppilli, M.; Majone, M. Chromate fate and effect in bioelectrochemical systems for remediation of chlorinated solvents. New Biotechnol. 2021, 60, 27–35. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Trace-elements in human nutrition. Report of a WHO Expert Committee. Tech. Rep. Ser. 1973, 532, 1–65. [Google Scholar]
- Levina, A.; Lay, P.A. Chemical properties and toxicity of chromium(III) nutritional supplements. Chem. Res. Toxicol. 2008, 21, 563–571. [Google Scholar] [CrossRef]
- Bhattacharya, P.T.; Misra, S.R.; Hussain, M. Nutritional aspects of essential trace elements in oral health and disease: An extensive review. Scientifica 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- World Health Organization (WHO)—International Agency for Research on Cancer (IARC). IARC monographs on the evaluation of carcinogenic risks to humans. Chromium, nickel and welding. Sci. Publ. Ser. 1990, 49, 1–677. [Google Scholar]
- Legislative decree n. 30 of 16 March 2009. Available online: https://www.minambiente.it/sites/default/files/D.Lgs_._16-3-2009_n._30.pdf (accessed on 17 March 2021).
- Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the Protection of Groundwater against Pollution and Deterioration. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:372:0019:0031:EN:PDF (accessed on 17 March 2021).
- Decree 14 November 2016, concerning amendments of the Annex I to Legislative Decree n. 31 of 2 February 2001, on “Implementation of Directive 98/83/ EC on the Quality of Water Intended for Human Consumption”. Council Directive 98/83/EC, Off. J. Eur. Comm. L330/32 (1998). Available online: http://extwprlegs1.fao.org/docs/pdf/ita162901.pdf (accessed on 17 March 2021).
- Rama Jyothi, N.; Mohammad Farook, N.A.; Cho, M.; Shim, J. Analysis and speciation of chromium in environmental matrices by various analytical techniques. Asian J. Chem. 2013, 25, 4125–4136. [Google Scholar] [CrossRef]
- El-Sheikh, A.H.; Abu Hilal, M.M.; Sweileh, J.A. Bio-separation, speciation and determination of chromium in water using partially pyrolyzed olive pomace sorbent. Bioresour. Technol. 2011, 102, 5749–5756. [Google Scholar] [CrossRef] [PubMed]
- Saçmaci, S.; Kartal, S.; Yilmaz, Y.; Saçmaci, M.; Soykan, C. A new chelating resin: Synthesis, characterization and application for speciation of chromium (III)/(VI) species. Chem. Eng. J. 2012, 181–182, 746–753. [Google Scholar] [CrossRef]
- Abdolmohammad-Zadeh, H.; Sadeghi, G.H. A nano-structured material for reliable speciation of chromium and manganese in drinking waters, surface waters and industrial wastewater effluents. Talanta 2012, 94, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Habila, M.; Unsal, Y.E.; Alothman, Z.A.; Shabaka, A.; Tuzen, M.; Soylak, M. Speciation of chromium in natural waters, tea, and soil with membrane filtration flame atomic absorption spectrometry. Anal. Lett. 2015, 48, 2258–2271. [Google Scholar] [CrossRef]
- Liang, P.; Sang, H. Speciation of chromium in water samples with cloud point extraction separation and preconcentration and determination by graphite furnace atomic absorption spectrometry. J. Hazard. Mater. 2008, 154, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Mandiwana, K.L.; Panichev, N.; Panicheva, S. Determination of chromium(VI) in black, green and herbal teas. Food Chem. 2011, 129, 1839–1843. [Google Scholar] [CrossRef]
- Moghadam, M.R.; Dadfarnia, S.; Shabani, A.M.H. Speciation and determination of ultra-trace amounts of chromium by solidified floating organic drop microextraction (SFODME) and graphite furnace atomic absorption spectrometry. J. Hazard. Mater. 2011, 186, 169–174. [Google Scholar] [CrossRef]
- Bermejo-Barrera, P.; Barciela-Alonso, M.C.; Pérez-Fernández, B.; Bermejo-Barrera, A. Direct speciation analysis of Cr(VI) by electrothermal atomic absorption spectrometry, based on the volatilization of Cr(III)–thenoyltrifluoracetonate from the graphite furnace. Spectrochim. Acta B 2003, 58, 167–173. [Google Scholar] [CrossRef]
- Zou, A.M.; Tang, X.Y.; Chen, M.L.; Wang, J.H. Preconcentration and speciation of chromium in a sequential injection system incorporating dual mini-columns coupled with electrothermal atomic absorption spectrometry. Spectrochim. Acta B 2008, 63, 607–611. [Google Scholar] [CrossRef]
- Jorge, E.O.; Rocha, M.M.; Fonseca, I.T.E.; Neto, M.M.M. Studies on the stripping voltammetric determination and speciation of chromium at a rotating-disc bismuth film electrode. Talanta 2010, 81, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Swietlik, R.; Molik, A.; Molenda, M.; Trojanowska, M.; Siwiec, J. Chromium (III/VI) speciation in urban aerosol. Atmos. Environ. 2011, 45, 1364–1368. [Google Scholar] [CrossRef]
- Sumida, T.; Sabarudin, A.; Oshima, M.; Motomizu, S. Speciation of chromium in seawater by ICP-AES with dual mini-columns containing chelating resin. Anal. Sci. 2006, 22, 161–164. [Google Scholar] [CrossRef]
- Li, Y.; Hu, B.; Jiang, Z.; Wu, Y. Speciation of chromium in water samples by cloud point extraction combined with low temperature electrothermal vaporization ICP-OES. Anal. Lett. 2006, 39, 809–822. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Babaei, L.H.; Badiei, A.; Saberian, K. A novel method for fast enrichment and monitoring of hexavalent and trivalent chromium at the ppt level with modified silica MCM-41 and its determination by inductively coupled plasma optical emission spectrometry. Quim. Nova 2006, 29, 440–443. [Google Scholar] [CrossRef]
- Narukawa, T.; Riley, K.W.; French, D.H.; Chiba, K. Speciation of chromium in Australian fly ash. Talanta 2007, 73, 178–184. [Google Scholar] [CrossRef]
- Zhang, N.; Suleiman, J.S.; He, M.; Hu, B. Chromium(III)-imprinted silica gel for speciation analysis of chromium in environmental water samples with ICP-MS detection. Talanta 2008, 75, 536–543. [Google Scholar] [CrossRef]
- Markiewicz, B.; Komorowicz, I.; Sajnóg, A.; Belter, M.; Barałkiewicz, D. Chromium and its speciation in water samples by HPLC/ICP-MS—Technique establishing metrological traceability: A review since 2000. Talanta 2015, 132, 814–828. [Google Scholar] [CrossRef]
- Astolfi, M.L.; Di Filippo, P.; Gentili, A.; Canepari, S. Semiautomatic sequential extraction of polycyclic aromatic hydrocarbons and elemental bio-accessible fraction by accelerated solvent extraction on a single particulate matter sample. Talanta 2017, 174, 838–844. [Google Scholar] [CrossRef]
- Monasterio, R.P.; Altamirano, J.C.; Martínez, L.D.; Wuilloud, R.G. A novel fiber-packed column for on-line preconcentration and speciation analysis of chromium in drinking water with flame atomic absorption spectrometry. Talanta 2009, 77, 1290–1294. [Google Scholar] [CrossRef]
- Şahan, S.; Saçmaci, S.; Kartal, S.; Saçmaci, M.; Şahin, U.; Ülgen, A. Development of a new on-line system for the sequential speciation and determination of chromium species in various samples using a combination of chelating and ion exchange resins. Talanta 2014, 120, 391–397. [Google Scholar] [CrossRef]
- Gil, R.A.; Cerutti, S.; Gásquez, J.A.; Olsina, R.A.; Martinez, L.D. Preconcentration and speciation of chromium in drinking water samples by coupling of on-line sorption on activated carbon to ETAAS determination. Talanta 2006, 68, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Gong, D.; Xu, B.; Chi, Q.; Zhang, X. Development of a novel, fast, sensitive method for chromium speciation in wastewater based on an organic polymer as solid phase extraction material combined with HPLC–ICP-MS. Talanta 2016, 147, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Alguacil, F.J.; Alonso, M.; Sastre, A.M. Facilitated supported liquid membrane transport of gold(I) and gold(III) using Cyanex 921. J. Membr. Sci. 2005, 252, 237–244. [Google Scholar] [CrossRef]
- López-López, J.A.; Mendiguchía, C.; Pinto, J.J.; Moreno, C. Liquid membranes for quantification and speciation of trace metals in natural waters. Trends Anal. Chem. 2010, 29, 645–653. [Google Scholar] [CrossRef]
- Zeng, C.; Yang, F.; Zhou, N. Hollow fiber supported liquid membrane extraction coupled with thermospray flame furnace atomic absorption spectrometry for the speciation of Sb(III) and Sb(V) in environmental and biological samples. Microchem. J. 2011, 98, 307–311. [Google Scholar] [CrossRef]
- Güell, R.; Fontàs, C.; Anticó, E.; Salvadó, V.; Crespo, J.G.; Velizarov, S. Transport and separation of arsenate and arsenite from aqueous media by supported liquid and anion-exchange membranes. Sep. Purif. Technol. 2011, 80, 428–434. [Google Scholar] [CrossRef]
- San Román, M.F.; Bringas, E.; Ibañez, R.; Ortiz, I. Liquid membrane technology: Fundamentals and review of its applications. J. Chem. Technol. Biotechnol. 2010, 85, 2–10. [Google Scholar] [CrossRef]
- Guha, A.K.; Majumdar, S.; Sirkar, K.K. Gas separation modes in a hollow fiber contained liquid membrane permeate. Ind. Eng. Chem. Res. 1992, 31, 593–604. [Google Scholar] [CrossRef]
- Sastre, A.M.; Kumar, A.; Shukla, J.P.; Singh, R.K. Improved techniques in liquid membrane separations: An overview. Sep. Purif. Methods 1998, 27, 213–298. [Google Scholar] [CrossRef]
- Dai, X.P.; Yang, Z.F.; Luo, R.G.; Sirkar, K.K. Lipase-facilitated separation of organic acids in a hollow fiber contained liquid membrane module. J. Membr. Sci. 2000, 171, 183–196. [Google Scholar] [CrossRef]
- Mat, N.C.; Lou, Y.; Lipscomb, G.G. Hollow fiber membrane modules. Curr. Opin. Chem. Eng. 2014, 4, 18–24. [Google Scholar] [CrossRef]
- Kavousi, F.; Syron, E.; Semmens, M.; Casey, E. Hydrodynamic and gas transfer performance of confined hollow fibre membrane modules with the aid of computational fluid dynamics. J. Membr. Sci. 2016, 513, 117–128. [Google Scholar] [CrossRef]
- Trivunac, K.; Stevanovic, S.; Mitrovic, M. Pertraction of phenol in hollow-fiber membrane contactors. Desalination 2004, 162, 93–101. [Google Scholar] [CrossRef]
- Kocherginsky, N.M.; Yang, Q.; Seelam, L. Recent advances in supported liquid membrane technology. Sep. Purif. Technol. 2007, 53, 171–177. [Google Scholar] [CrossRef]
- Pabby, A.K.; Sastre, A.M. State-of-the-art review on hollow fibre contactor technology and membrane-based extraction processes. J. Membr. Sci. 2013, 430, 263–303. [Google Scholar] [CrossRef]
- Galán, B.; Calzada, M.; Ortiz, I. Recycling of Cr(VI) by membrane solvent extraction: Long term performance with mathematical model. Chem. Eng. J. 2006, 124, 71–79. [Google Scholar] [CrossRef]
- Güell, R.; Anticó, E.; Salvadó, V.; Fontàs, C. Efficient hollow fiber supported liquid membrane system for the removal and preconcentration of Cr(VI) at trace levels. Sep. Purif. Technol. 2008, 62, 389–393. [Google Scholar] [CrossRef]
- Bey, S.; Criscuoli, A.; Simone, S.; Figoli, A.; Benamor, M.; Drioli, E. Hydrophilic PEEK-WC hollow fibre membrane contactors for chromium(VI) removal. Desalination 2011, 283, 16–24. [Google Scholar] [CrossRef]
- Coker, V.S.; Garrity, A.; Wennekes, W.B.; Roesink, H.D.; Cutting, R.S.; Lloyd, J.R. Cr(VI) and azo dye removal using a hollow-fibre membrane system functionalized with a biogenic Pd-magnetite catalyst. Environ. Technol. 2014, 35, 1046–1054. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Saf, A.O.; Alpaydin, S.; Coskun, A.; Ersoz, M. Selective transport and removal of Cr(VI) through polymer inclusion membrane containing 5-(4-phenoxyphenil)-6H-1,3,4-thiadiazin-2-amine as carrier. J. Membr. Sci. 2011, 377, 241–248. [Google Scholar] [CrossRef]
- Alpaydin, S.; Saf, A.Ö.; Bozkurt, S.; Sirit, A. Kinetic study on removal of toxic metal Cr(VI) through a bulk liquid membrane containing p-tert-butylcalix[4]arene derivative. Desalination 2011, 275, 166–171. [Google Scholar] [CrossRef]
- Parinejad, M.; Yaftian, M.R. A study on the removal of chromium(VI) oxoanions from acid solutions by using oxonium ion-crown ether complexes as mobile carrier agents. Iran. J. Chem. Chem. Eng. 2007, 26, 19–27. [Google Scholar]
- Usapein, P.; Lothongkum, A.W.; Ramakul, P.; Pancharoen, U. Efficient transport and selective extraction of Cr(VI) from waste pickling solution of the stainless steel-cold rolled plate process using Aliquat 336 via HFSLM. Korean J. Chem. Eng. 2009, 26, 791–798. [Google Scholar] [CrossRef]
- Bucci, R.; Canepari, S.; Cardarelli, E.; Girelli, A.M.; Pietrodangelo, A.; Valiente, M. Carrier-mediated transport of amino acids through bulk liquid membranes. Sep. Sci. Technol. 2004, 39, 3821–3838. [Google Scholar] [CrossRef]
- Chaudry, M.A.; Bukhari, N.; Mazhar, M.; Tazeen, F. Vanadium(V) ions transport through tri-n-octyl amine cyclohexane supported liquid membranes. Sep. Purif. Technol. 2007, 54, 227–233. [Google Scholar] [CrossRef]
- Zaghbani, A.; Tayeb, R.; Dhahbi, M. Studies on the transport of chromium(III) through a supported liquid membrane containing D2EHPA as carrier. Desalin. Water Treat. 2009, 12, 247–255. [Google Scholar] [CrossRef][Green Version]
- Djane, N.K.; Ndung’u, K.; Jhonsson, C.; Sartz, H.; Tornstrom, T.; Mathiasson, L. Chromium speciation in natural waters using serially connected supported liquid membranes. Talanta 1999, 48, 1121–1132. [Google Scholar] [CrossRef]
- Dermatas, D.; Mpouras, T.; Chrysochoou, M.; Panagiotakis, I.; Vatseris, C.; Linardos, N.; Theologou, E.; Boboti, N.; Xenidis, A.; Papassiopi, N.; et al. Origin and concentration profile of chromium in a Greek aquifer. J. Hazard. Mater. 2015, 281, 35–46. [Google Scholar] [CrossRef]
- Diniz, K.M.; Tarley, C.R.T. Speciation analysis of chromium in water samples through sequential combination of dispersive magnetic solid phase extraction using mesoporous amino-functionalized Fe3O4/SiO2 nanoparticles and cloud point extraction. Microchem. J. 2015, 123, 185–195. [Google Scholar] [CrossRef]
- Catalani, S.; Fostinelli, J.; Gilberti, M.E.; Apostoli, P. Application of a metal free high performance liquid chromatography with inductively coupled plasma mass spectrometry (HPLC–ICP-MS) for the determination of chromium species in drinking and tap water. Int. J. Mass Spectrom. 2015, 387, 31–37. [Google Scholar] [CrossRef]
- Bahadir, Z.; Bulut, V.N.; Hidalgo, M.; Soylak, M.; Margui, E. Cr speciation in water samples by dispersive liquid–liquid microextraction combined with total reflection X-ray fluorescence spectrometry. Spectrochim. Acta B 2016, 115, 46–51. [Google Scholar] [CrossRef]
- Hu, L.; Cai, Y.; Jiang, G. Occurrence and speciation of polymeric chromium(III), monomeric chromium(III) and chromium(VI) in environmental samples. Chemosphere 2016, 156, 4–20. [Google Scholar] [CrossRef] [PubMed]





| Parameters | Off-Line Mode | On-Line Mode |
|---|---|---|
| Plasma gas flow (L/min) | 16.5 | 16.5 |
| Auxiliary gas flow (L/min) | 1.50 | 1.50 |
| RF Power (kW) | 1.30 | 1.30 |
| Nebulizer pressure (KPa) | 230 | 230 |
| Rinse time (s) | 15 | 0 |
| Instrument stabilization delay (s) | 45 | 0 |
| Replicate read time (s) | 30 | 20 |
| Number of replicates | 3 | 5 |
| Pump rate (rpm) | 20 | 5 |
| [Cr(III)] (µg/L) | [Cr(VI)] (µg/L) | Flow Rate (mL/min) | E% |
|---|---|---|---|
| E% of Cr(VI) in function of the peristaltic pump flow rate | |||
| 0.0 | 63.5 | 0.20 | 97.1 ± 0.3 |
| 0.0 | 63.5 | 0.25 | 95.8 ± 0.7 |
| 0.0 | 63.5 | 0.30 | 92.3 ± 0.7 |
| 0.0 | 63.5 | 0.40 | 88.1 ± 0.9 |
| 0.0 | 63.5 | 0.50 | 86.3 ± 1.1 |
| 0.0 | 63.5 | 0.70 | 74.9 ± 1.3 |
| E% of Cr(VI) as a function of the concentration in the feed solution entering the contactor | |||
| 0.0 | 3.0 | 0.30 | 85.6 ± 0.9 |
| 0.0 | 5.0 | 0.30 | 90.3 ± 0.7 |
| 0.0 | 10.0 | 0.30 | 95.8 ± 0.2 |
| 0.0 | 15.0 | 0.30 | 96.3 ± 0.4 |
| 0.0 | 50.0 | 0.30 | 97.7 ± 0.5 |
| 0.0 | 100.0 | 0.30 | 96.7 ± 0.2 |
| E% of Cr(VI) as a function of the Cr(VI)/Cr(III) ratio | |||
| 20.0 | 5.0 | 0.30 | 78.2 ± 0.8 |
| 20.0 | 10.0 | 0.30 | 85.9 ± 0.9 |
| 20.0 | 20.0 | 0.30 | 88.3 ± 0.6 |
| 20.0 | 30.0 | 0.30 | 92.5 ± 0.3 |
| 20.0 | 60.0 | 0.30 | 95.1 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astolfi, M.L.; Ginese, D.; Ferrante, R.; Marconi, E.; Girelli, A.M.; Canepari, S. On-Line Separation and Determination of Trivalent and Hexavalent Chromium with a New Liquid Membrane Annular Contactor Coupled to Inductively Coupled Plasma Optical Emission Spectrometry. Processes 2021, 9, 536. https://doi.org/10.3390/pr9030536
Astolfi ML, Ginese D, Ferrante R, Marconi E, Girelli AM, Canepari S. On-Line Separation and Determination of Trivalent and Hexavalent Chromium with a New Liquid Membrane Annular Contactor Coupled to Inductively Coupled Plasma Optical Emission Spectrometry. Processes. 2021; 9(3):536. https://doi.org/10.3390/pr9030536
Chicago/Turabian StyleAstolfi, Maria Luisa, Domenico Ginese, Riccardo Ferrante, Elisabetta Marconi, Anna Maria Girelli, and Silvia Canepari. 2021. "On-Line Separation and Determination of Trivalent and Hexavalent Chromium with a New Liquid Membrane Annular Contactor Coupled to Inductively Coupled Plasma Optical Emission Spectrometry" Processes 9, no. 3: 536. https://doi.org/10.3390/pr9030536
APA StyleAstolfi, M. L., Ginese, D., Ferrante, R., Marconi, E., Girelli, A. M., & Canepari, S. (2021). On-Line Separation and Determination of Trivalent and Hexavalent Chromium with a New Liquid Membrane Annular Contactor Coupled to Inductively Coupled Plasma Optical Emission Spectrometry. Processes, 9(3), 536. https://doi.org/10.3390/pr9030536







