Metal Accumulation Profile of Catharanthus roseus (L.) G.Don and Celosia argentea L. with EDTA Co-Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth of Seedlings
2.2. Selection and Acclimatization of Uniform Seedlings
2.3. Treatment Plan and Experimental Conditions
2.4. Plant Harvesting
2.5. Plant Analysis
2.5.1. Plant Physiological Parameters
2.5.2. Measurement of Metal Content in Different Plant Parts
2.6. Translocation Factor
2.7. Statistical Analysis
3. Results
3.1. Physiological Parameters of C. roseus and C. argentea
3.2. Plant Biomass
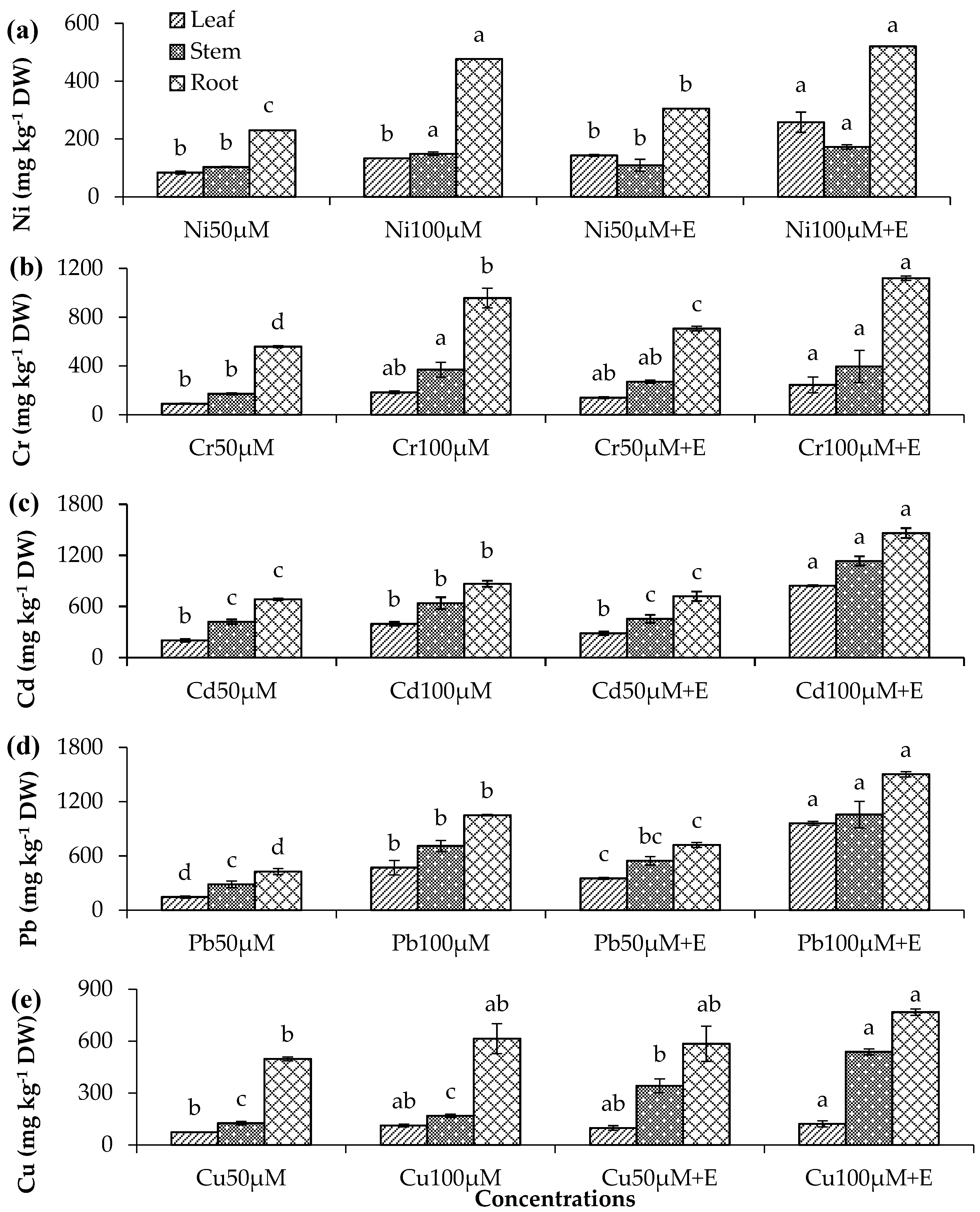
3.3. Metal Uptake in C. roseus and C. argentea
3.4. Translocation Factor, Bioaccumulation Coefficient, and Bioconcentration Factor for Different MCs in C. roseus and C. argentea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cheema, A.I.; Liu, G.; Yousaf, B.; Abbas, Q.; Zhou, H. A comprehensive review of biogeochemical distribution and fractionation of lead isotopes for source tracing in distinct interactive environmental compartments. Sci. Total Environ. 2020, 719, 135658. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Mushtaq, M.U.; Iqbal, A.; Nawaz, I.; Mirza, C.R.; Yousaf, S.; Farooq, G.; Ali, M.A.; Khan, A.H.A.; Iqbal, M. Enhanced uptake of Cd, Cr, and Cu in Catharanthus roseus (L.) G.Don by Bacillus cereus: Application of moss and compost to reduce metal availability. Environ. Sci. Pollut. R. 2020, 27, 39807–39818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, Z.; Cai, G.; Mi, W.; Xu, W. Bioaccumulation and changes of trace metals over the last two decades in marine organisms from Guangdong coastal regions, South China. J. Environ. Sci. 2020, 98, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, A.; Cui, C. Remediation of heavy metal-contaminated soils by electrokinetic technology: Mechanisms and applicability. Chemosphere 2021, 265, 129071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, X.; Yang, L.; Chu, Y. Research on progress in combined remediation technologies of heavy metal polluted sediment. Int. J. Environ. Res. Public Health 2019, 16, 5098. [Google Scholar] [CrossRef]
- Aftab, N.; Saleem, K.; Khan, A.H.A.; Butt, T.A.; Mirza, C.R.; Hussain, J.; Farooq, G.; Tahir, A.; Yousaf, S.; Zafar, M.I.; et al. Cosmos sulphureus Cav. is more tolerant to lead than copper and chromium in hydroponics system. Int. J. Environ. Sci. Technol. 2020, 1–10. [Google Scholar] [CrossRef]
- Song, Y.; Kirkwood, N.; Maksimović, Č.; Zheng, X.; O’Connor, D.; Jin, Y.; Hou, D. Nature based solutions for contaminated land remediation and brownfield redevelopment in cities: A review. Sci. Total Environ. 2019, 663, 568–579. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Nawaz, I.; Qu, Z.; Butt, T.A.; Yousaf, S.; Iqbal, M. Reduced growth response of ornamental plant Nicotiana alata L. upon selected heavy metals uptake, with co-application of ethylenediaminetetraacetic acid. Chemosphere 2020, 241, 125006. [Google Scholar] [CrossRef]
- Patra, D.K.; Pradhan, C.; Patra, H.K. Toxic metal decontamination by phytoremediation approach: Concept, challenges, opportunities and future perspectives. Environ. Technol. Innov. 2020, 18, 100672. [Google Scholar] [CrossRef]
- Raza, A.; Khan, A.H.A.; Nawaz, I.; Qu, Z.; Yousaf, S.; Ali, M.A.; Sayal, A.U.; Iqbal, M. Evaluation of arsenic-induced stress in Dahlia pinnata Cav: Morphological and physiological response. Soil Sediment Contam. 2019, 28, 716–728. [Google Scholar] [CrossRef]
- Iqbal, A.; Mushtaq, M.U.; Khan, A.H.; Nawaz, I.; Yousaf, S.; Iqbal, M. Influence of Pseudomonas japonica and organic amendments on the growth and metal tolerance of Celosia argentea L. Environ. Sci. Pollut. R. 2020, 27, 24671–24685. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Kiyani, A.; Mirza, C.R.; Butt, T.A.; Barros, R.; Ali, B.; Iqbal, M.; Yousaf, S. Ornamental plants for the phytoremediation of heavy metals: Present knowledge and future perspectives. Environ. Res. 2021, 195, 110780. [Google Scholar] [CrossRef]
- Al-Mefleh, N.K.; Othman, Y.A.; Tadros, M.J.; Al-Assaf, A.; Talozi, S. An assessment of treated greywater reuse in irrigation on growth and protein content of Prosopis and Albizia. Horticulturae 2021, 7, 38. [Google Scholar] [CrossRef]
- Kanwal, U.; Ali, S.; Shakoor, M.B.; Farid, M.; Hussain, S.; Yasmeen, T.; Adrees, M.; Bharwana, S.A.; Abbas, F. EDTA ameliorates phytoextraction of lead and plant growth by reducing morphological and biochemical injuries in Brassica napus L. under lead stress. Environ. Sci. Pollut. R. 2014, 21, 9899–9910. [Google Scholar] [CrossRef] [PubMed]
- Menhas, S.; Hayat, K.; Niazi, N.K.; Zhou, P.; Amna Bundschuh, J.; Naeem, M.; Munis, M.F.; Yang, X.; Chaudhary, H.J. Microbe-EDTA mediated approach in the phytoremediation of lead-contaminated soils using maize (Zea mays L.) plants. Int. J. Phytoremediat. 2020, 1–12. [Google Scholar] [CrossRef]
- Ehsan, M.; Viveros, F.M.L.; Hernaandez, V.E.; Barakat, M.A.; Ortega, A.R.; Maza, A.V.; Monter, J.V. Zinc and cadmium accumulation by Lupinus uncinatus Schldl. grown in nutrient solution. Int. J. Environ. Sci. Technol. 2015, 12, 307–316. [Google Scholar] [CrossRef][Green Version]
- January, M.C.; Cutright, T.J.; Keulen, H.V.; Wei, R. Hydroponic phytoremediation of Cd, Cr, Ni, As, and Fe: Can Helianthus annuus hyperaccumulate multiple heavy metals? Chemosphere 2008, 70, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Ali, S.; Farid, M.; Shakoor, M.B.; Rizwan, M.; Ibrahim, M.; Hayat, T.; Ali, B. EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ. Sci. Pollut. R. 2015, 22, 1534–1544. [Google Scholar] [CrossRef]
- Arshad, M.; Khan, A.H.A.; Hussain, I.; Zaman, B.; Anees, M.; Iqbal, M.; Soja, G.; Lindef, C.; Yousaf, S. The reduction of chromium (VI) phytotoxicity and phytoavailability to wheat (Triticum aestivum L.) using biochar and bacteria. Appl. Soil Ecol. 2017, 114, 90–98. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Kiyani, A.; Cheema, A.S.; Tareen, U.; Nawaz, I.; Iqbal, M.; Yousaf, S. Integrative application of soil conditioners and bio-augmentation for enhanced heavy metal stabilization from wastewater and improved growth of Nicotiana alata L. and Petunia hydrida L. J. Plant Growth Regul. 2021, 40, 240–253. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region; International Center for Agricultural Research in the Dry Areas (ICARDA): Beirut, Lebanon, 2013. [Google Scholar]
- Khalid, N.; Hussain, M.; Young, H.S.; Ashraf, M.; Hameed, M.; Ahmad, R. Lead concentrations in soils and some wild plant species along two busy roads in Pakistan. Bull. Environ. Contam. Tox. 2018, 100, 250–258. [Google Scholar] [CrossRef]
- González-De Zayas, R.; Yera, A.B.; Artiles, M.M.; González, A.L.; Sandoval, F.S.C.; Merino-Ibarra, M. Trace metals in sediments of seven coastal lagoons of the Sabana-Camagüey Archipelago, Cuba. Soil Sediment Contam. 2020, 331–349. [Google Scholar]
- Nematollahi, M.J.; Keshavarzi, B.; Zaremoaiedi, F.; Rajabzadeh, M.A.; Moore, F. Ecological-health risk assessment and bioavailability of potentially toxic elements (PTEs) in soil and plant around a copper smelter. Environ. Monit. Assess. 2020, 192, 639. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokberdoran, F.; Ahmad, Z.; Xie, Y. Determination of heavy metal tolerance threshold in a bamboo species (Arundinaria pygmaea) as treated with silicon dioxide nanoparticles. Glob. Ecol. Conserv. 2020, 24, e01306. [Google Scholar] [CrossRef]
- Iqbal, M.; Nawaz, I.; Hassan, Z.; Hakvoort, H.W.J.; Bliek, M.; Aarts, M.G.M.; Schat, H. Expression of HMA4 cDNAs of the zinc hyperaccumulator Noccaea caerulescens from endogenous NcHMA4 promoters does not complement the zinc-deficiency phenotype of the Arabidopsis thaliana hma2hma4 double mutant. Front. Plant Sci. 2013, 4, 404. [Google Scholar] [CrossRef] [PubMed]
- Saifullah; Ghafoor, A.; Zia, M.H.; Murtaza, G.; Waraich, E.A.; Bibi, S.; Srivastava, P. Comparison of organic and inorganic amendments for enhancing soil lead phytoextraction by wheat (Triticum aestivum L.). Int. J. Phytoremediat. 2010, 12, 633–649. [Google Scholar] [CrossRef]
- Yang, M.; Xiao, X.-Y.; Miao, X.-F.; Guo, Z.-H.; Wang, F.-Y. Effect of amendments on growth and metal uptake of giant reed (Arundo donax L.) grown on soil contaminated by arsenic, cadmium and lead. Trans. Nonferr. Metal. Soc. 2012, 22, 1462–1469. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Butt, T.A.; Mirza, C.R.; Yousaf, S.; Nawaz, I.; Iqbal, M. Combined application of selected heavy metals and EDTA reduced the growth of Petunia hybrida L. Sci. Rep. 2019, 9, 4138. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Rizvi, A.H. Excess lead alters growth, metabolism and translocation of certain nutrients in radish. Chemosphere 2008, 70, 1539–1544. [Google Scholar] [CrossRef]
- Rossato, L.V.; Nicoloso, F.T.; Farias, J.G.; Cargnelluti, D.; Tabaldi, L.A.; Antes, F.G.; Dressler, V.L.; Morsch, V.M.; Schetinger, M.R.C. Effects of lead on the growth, lead accumulation and physiological responses of Pluchea sagittalis. Ecotoxicology 2012, 21, 111–123. [Google Scholar] [CrossRef]
- Székely, Á.; Poór, P.; Bagi, I.; Csiszár, J.; Gémes, K.; Horváth, F.; Tari, I. Effect of EDTA on the growth and copper accumulation of sweet sorghum and sudangrass seedlings. Acta Biol. Szeged. 2011, 55, 159–164. [Google Scholar]
- Peralta, J.R.; Gardea-Torresdey, J.L.; Tiemann, K.J.; Gomez, E.; Arteaga, S.; Rascon, E.; Parsons, J.G. Uptake and effects of five heavy metals on seed germination and plant growth in Alfalfa (Medicago sativa L.). Bull. Environ. Contam. Tox. 2001, 66, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, S.M.; Bhatti, H.N.; Khalid, M.; Haq, M.A.; Shahzad, S.M. Potential of sunflower (Helianthus annuus L.) for phytoremediation of nickle (Ni) and lead (Pb) contaminated water. Pak. J. Bot. 2010, 42, 4017–4026. [Google Scholar]
- Sekhar, K.C.; Kamala, C.T.; Chary, N.S.; Balaram, V.; Garcia, G. Potential of Hemidesmus indicus for phytoextraction of lead from industrially contaminated soils. Chemosphere 2005, 58, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-E.; Peng, H.-Y.; Jiang, L.-Y.; He, Z.-L. Phytoextraction of copper from contaminated soil by Elsholtzia splendens as affected by EDTA, citric acid, and compost. Int. J. Phytoremediat. 2005, 7, 69–83. [Google Scholar] [CrossRef]
- Liang, S.-X.; Jin, Y.; Liu, W.; Li, X.; Shen, S.-G.; Ding, L. Feasibility of Pb phytoextraction using nano-materials assisted ryegrass: Results of a one-year field-scale experiment. J. Environ. Manag. 2017, 190, 170–175. [Google Scholar] [CrossRef]
- Mirzaei, M.; Verrelst, J.; Bakhtiari, A.R.; Marofi, S. Potential use of grapevine cv Askari for heavy metal phytoremediation purposes at greenhouse scale. Environ. Sci. Pollut. Res. 2021, 28, 12447–12458. [Google Scholar] [CrossRef]
- Usman, K.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci. Rep. 2019, 9, 5658. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef]
- Chen, H.; Cutright, T.J. The interactive effects of chelator, fertiliser, and rhizobacteria for enhancing phytoremediation of heavy metal contaminated soil. J. Soil. Sediment 2002, 2, 203–210. [Google Scholar] [CrossRef]

| Catharanthus roseus | Celosia argentea | |||||||
|---|---|---|---|---|---|---|---|---|
| Root Length (cm) | Shoot Length (cm) | Total Leaves Plant−1 | Leaf Area (cm2) | Root Length (cm) | Shoot Length (cm) | Total Leaves Plant−1 | Leaf Area (cm2) | |
| Control 1 | 5.2 ± 1.2 b12, bc3, c4, b5 | 7.6 ± 1.6 b1, a1345 | 11.7 ± 1.2 c1, ab1, b3, a4 | 1.92 ± 0.32 b124, a3, ab5 | 45.7 ± 1.7 a12345 | 15.0 ± 0.6 a12345 | 19.7 ± 0.7 a12345 | 20.14 ± 0.48 bc1, b23, a4 |
| Control + EDTA 1 | 4.9 ± 0.4 b125, c34 | 6.7 ± 0.3 bc1, b1345 | 8.0 ± 0.0 d1, b25, c34 | 1.44 ± 0.02 c1, b25, bc34 | 7.7 ± 0.6 d124, b3, c5 | 8.1 ± 0.8 c123, bc4, b5 | 13.0 ± 0.0 b12345 | 3.08 ± 0.48 d15, c234 |
| Ni 50 | 16.8 ± 1.4 a | 8.8 ± 0.5 a | 20.0 ± 0.0 a | 2.71 ± 0.23 a | 35.5 ± 1.9 b | 12.4 ± 0.9 a | 14.3 ± 1.3 b | 68.47 ± 3.20 a |
| Ni 100 | 15.9 ± 2.1 a | 8.1 ± 0.5 a | 16.7 ± 0.3 b | 2.30 ± 0.17 ab | 50.1 ± 1.4 a | 11.3 ± 1.9 b | 13.3 ± 2.0 b | 36.66 ± 1.70 b |
| Ni 50 + EDTA | 4.9 ± 0.2 b | 4.5 ± 0.7 c | 9.3 ± 0.7 c | 1.04 ± 0.11 d | 6.9 ± 0.3 c | 6.9 ± 1.8 c | 9.7 ± 0.9 c | 1.43 ± 0.24 d |
| Ni 100 + EDTA | 4.9 ± 0.2 b | 5.4 ± 0.1 c | 10.7 ± 0.9 c | 1.28 ± 0.17 c | 6.1 ± 0.2 c | 4.9 ± 1.4 d | 9.0 ± 0.0 c | 1.29 ± 0.18 d |
| Cr 50 | 14.6 ± 1.8 a | 7.4 ± 1.1 a | 10.7 ± 0.3 b | 2.30 ± 0.33 a | 43.1 ± 1.6 a | 13.1 ± 0.5 a | 17.7 ± 1.4 a | 50.00 ± 3.41 a |
| Cr 100 | 11.7 ± 0.7 a | 6.1 ± 0.5 b | 11.0 ± 0.0 ab | 0.70 ± 0.03 d | 35.1 ± 2.3 b | 10.8 ± 0.8 ab | 15.0 ± 1.1 ab | 8.23 ± 1.22 bc |
| Cr 50 + EDTA | 3.2 ± 0.5 b | 3.9 ± 0.8 d | 9.7 ± 0.9 b | 0.55 ± 0.02 d | 6.9 ± 0.7 c | 6.0 ± 0.6 c | 11.0 ± 0.6 b | 7.59 ± 0.20 b |
| Cr 100 + EDTA | 4.5 ± 0.1 b | 5.2 ± 0.1 c | 13.0 ± 0.6 a | 0.81 ± 0.07 c | 6.4 ± 0.4 c | 5.3 ± 0.7 d | 11.7 ± 0.7 b | 2.06 ± 0.43 c |
| Cd 50 | 8.4 ± 1.2 a | 7.1 ± 0.7 a | 9.3 ± 0.3 bc | 1.63 ± 0.10 b | 6.0 ± 0.6 b | 9.2 ± 0.3 b | 12.3 ± 0.3 b | 27.25 ± 1.08 a |
| Cd 100 | 6.4 ± 0.6 b | 6.7 ± 0.1 b | 12.3 ± 0.9 a | 1.62 ± 0.08 b | 5.4 ± 0.4 c | 6.8 ± 0.3 c | 13.0 ± 1.528 b | 0.95 ± 0.45 d |
| Cd 50 + EDTA | 4.9 ± 0.4 c | 5.5 ± 1.0 c | 13.0 ± 1.1 a | 1.30 ± 0.15 c | 7.0 ± 0.4 b | 6.6 ± 0.7 c | 12.0 ± 1.1 b | 4.27 ± 0.26 c |
| Cd 100 + EDTA | 3.2 ± 0.5 c | 4.5 ± 0.4 c | 10.0 ± 0.6 b | 0.89 ± 0.10 d | 6.1 ± 0.1 b | 5.5 ± 0.3 c | 10.3 ± 0.7 b | 4.30 ± 0.98 c |
| Pb 50 | 15.3 ± 1.8 a | 8.1 ± 0.8 a | 12.0 ± 1.1 a | 2.83 ± 0.44 a | 40.5 ± 2.6 a | 9.2 ± 0.4 b | 12.3 ± 1.2 b | 11.20 ± 1.00 b |
| Pb 100 | 7.7 ± 0.2 b | 6.5 ± 0.5 b | 12.3 ± 0.9 a | 1.88 ± 0.31 b | 37.3 ± 1.5 b | 11.8 ± 1.1 b | 15.0 ± 1.1 b | 9.29 ± 1.19 b |
| Pb 50 + EDTA | 5.4 ± 0.3 c | 5.3 ± 0.4 c | 9.0 ± 0.6 b | 1.01 ± 0.07 c | 15.0 ± 0.5 c | 7.2 ± 0.6 bc | 12.7 ± 0.9 b | 5.13 ± 0.16 bc |
| Pb 100 + EDTA | 4.8 ± 0.1 c | 5.3 ± 0.4 c | 7.7 ± 0.9 c | 1.34 ± 0.42 bc | 10.7 ± 0.3 c | 5.4 ± 0.6 c | 10.3 ± 0.9 b | 2.60 ± 0.06 c |
| Cu 50 | 8.4 ± 1.0 a | 5.6 ± 0.6 c | 9.0 ± 0.6 b | 1.08 ± 0.06 c | 6.1 ± 0.5 c | 8.1 ± 1.0 b | 11.3 ± 0.9 b | 2.16 ± 0.17 b |
| Cu 100 | 4.2 ± 0.4 b | 4.1 ± 0.1 d | 10.0 ± 0.6 a | 2.47 ± 0.18 a | 7.1 ± 0.6 c | 6.4 ± 0.7 c | 9.0 ± 0.6 c | 1.92 ± 0.05 d |
| Cu 50 + EDTA | 4.3 ± 0.6 b | 4.5 ± 0.4 d | 10.0 ± 1.5 a | 1.73 ± 0.12 b | 15.9 ± 2.0 b | 6.8 ± 1.3 c | 14.3 ± 0.3 ab | 5.54 ± 0.42 c |
| Cu 100 + EDTA | 2.6 ± 0.4 c | 4.7 ± 0.1 d | 9.3 ± 1.3 b | 0.85 ± 0.09 c | 17.1 ± 0.1 b | 7.3 ± 0.2 bc | 11.0 ± 0.6 b | 3.87 ± 0.63 cd |
| Root Fresh Weight (g) | Root Dried Weight (g) | Root Fresh to Dried Weight Ratio | Stem Fresh Weight (g) | Stem Dried Weight (g) | Stem Fresh To Dried Weight Ratio | Leaf Fresh Weight (g) | Leaf Dried Weight (g) | Leaf Fresh to Dried Weight Ratio | |
|---|---|---|---|---|---|---|---|---|---|
| Control 1 | 0.041 ± 0.001 a12345 | 0.022 ± 0.001 a12345 | 1.86 ± 0.01 b1234, d5 | 0.048 ± 0.005 b1, a2354 | 0.027 ± 0.001 a12345 | 1.78 ± 0.12 b1235, d3 | 0.289 ± 0.004 a12345 | 0.038 ± 0.006 b1, a2345 | 7.60 ± 0.31 a12345 |
| Control + EDTA 1 | 0.035 ± 0.003 ab1245, b3 | 0.018 ± 0.003 a12, b345 | 2.00 ± 0.30 a1234, c5 | 0.032 ± 0.006 c12, b345 | 0.018 ± 0.001 b12345 | 1.78 ± 0.13 b1235, d3 | 0.190 ± 0.008 b12345 | 0.025 ± 0.004 c1, b2345 | 7.60 ± 0.21 a12345 |
| Ni 50 | 0.041 ± 0.008 a | 0.020 ± 0.004 a | 2.05 ± 0.06 a | 0.041 ± 0.005 b | 0.020 ± 0.004 b | 2.05 ± 0.04 a | 0.207 ± 0.031 ab | 0.050 ± 0.004 a | 4.14 ± 0.16 b |
| Ni 100 | 0.030 ± 0.002 b | 0.020 ± 0.001 a | 1.47 ± 0.05 c | 0.051 ± 0.006 a | 0.026 ± 0.002 a | 1.96 ± 0.13 a | 0.158 ± 0.024 c | 0.033 ± 0.001 b | 4.78 ± 0.12 b |
| Ni 50 + EDTA | 0.032 ± 0.005 b | 0.016 ± 0.002 ab | 1.99 ± 0.29 a | 0.044 ± 0.001 b | 0.024 ± 0.003 ab | 1.83 ± 0.18 a | 0.049 ± 0.010 d | 0.019 ± 0.000 d | 2.57 ± 0.18 c |
| Ni 100 + EDTA | 0.030 ± 0.006 b | 0.014 ± 0.001 b | 2.18 ± 0.36 a | 0.056 ± 0.031 a | 0.027 ± 0.001 a | 2.07 ± 0.06 a | 0.046 ± 0.018 cd | 0.018 ± 0.006 c | 2.50 ± 0.11 d |
| Cr 50 | 0.025 ± 0.005 b | 0.019 ± 0.006 a | 1.31 ± 0.21 b | 0.035 ± 0.009 bc | 0.018 ± 0.005 b | 1.94 ± 0.05 a | 0.230 ± 0.012 b | 0.044 ± 0.014 a | 5.22 ± 0.39 b |
| Cr 100 | 0.019 ± 0.005 c | 0.014 ± 0.005 b | 1.35 ± 0.16 b | 0.031 ± 0.008 c | 0.018 ± 0.003 b | 1.73 ± 0.05 b | 0.141 ± 0.028 bc | 0.025 ± 0.006 b | 5.64 ± 0.20 b |
| Cr 50 + EDTA | 0.038 ± 0.015 ab | 0.020 ± 0.005 a | 1.89 ± 0.06 b | 0.035 ± 0.004 bc | 0.026 ± 0.004 a | 1.34 ± 0.05 c | 0.060 ± 0.010 c | 0.027 ± 0.001 b | 2.22 ± 0.16 c |
| Cr 100 + EDTA | 0.036 ± 0.019 ab | 0.018 ± 0.006 ab | 2.01 ± 0.32 a | 0.039 ± 0.011 b | 0.026 ± 0.010 a | 1.50 ± 0.19 b | 0.058 ± 0.009 c | 0.026 ± 0.003 b | 2.23 ± 0.33 c |
| Cd 50 | 0.037 ± 0.009 ab | 0.020 ± 0.008 a | 1.86 ± 0.30 b | 0.038 ± 0.010 b | 0.015 ± 0.008 bc | 2.53 ± 0.11 a | 0.100 ± 0.014 c | 0.036 ± 0.014 a | 2.77 ± 1.14 b |
| Cd 100 | 0.025 ± 0.006 c | 0.019 ± 0.006 ab | 1.31 ± 0.02 c | 0.037 ± 0.005 b | 0.014 ± 0.001 c | 2.64 ± 0.10 b | 0.098 ± 0.013 c | 0.035 ± 0.003 a | 2.80 ± 0.21 b |
| Cd 50 + EDTA | 0.023 ± 0.004 c | 0.018 ± 0.006 ab | 1.21 ± 0.05 c | 0.028 ± 0.007 c | 0.013 ± 0.001 c | 2.15 ± 0.13 c | 0.060 ± 0.008 d | 0.028 ± 0.011 b | 2.14 ± 0.11 b |
| Cd 100 + EDTA | 0.018 ± 0.002 d | 0.011 ± 0.004 c | 1.63 ± 0.01 bc | 0.025 ± 0.007 c | 0.016 ± 0.005 bc | 1.56 ± 0.09 d | 0.035 ± 0.000 e | 0.017 ± 0.002 c | 2.06 ± 0.41 b |
| Pb 50 | 0.030 ± 0.003 b | 0.023 ± 0.002 a | 1.30 ± 0.02 c | 0.034 ± 0.003 b | 0.026 ± 0.001 a | 1.30 ± 0.13 a | 0.130 ± 0.012 c | 0.039 ± 0.001 a | 3.33 ± 0.05 b |
| Pb 100 | 0.027 ± 0.002 c | 0.019 ± 0.002 a | 1.46 ± 0.05 c | 0.028 ± 0.016 c | 0.020 ± 0.001 b | 1.40 ± 0.07 c | 0.104 ± 0.020 d | 0.034 ± 0.005 a | 3.06 ± 0.12 bc |
| Pb 50 + EDTA | 0.021 ± 0.001 c | 0.016 ± 0.002 ab | 1.32 ± 0.12 c | 0.030 ± 0.004 bc | 0.019 ± 0.002 b | 1.56 ± 0.10 c | 0.074 ± 0.013 de | 0.025 ± 0.019 b | 2.96 ± 0.18 c |
| Pb 100 + EDTA | 0.020 ± 0.003 c | 0.013 ± 0.001 c | 1.54 ± 0.03 bc | 0.020 ± 0.083 d | 0.013 ± 0.003 c | 1.53 ± 0.03 c | 0.056 ± 0.016 e | 0.022 ± 0.002 b | 2.54 ± 0.01 c |
| Cu 50 | 0.035 ± 0.029 b | 0.014 ± 0.011 b | 2.50 ± 0.29 b | 0.026 ± 0.004 c | 0.015 ± 0.003 b | 1.73 ± 0.17 b | 0.052 ± 0.005 c | 0.022 ± 0.004 b | 2.36 ± 0.04 b |
| Cu 100 | 0.025 ± 0.007 c | 0.010 ± 0.006 bc | 2.50 ± 0.28 b | 0.026 ± 0.001 c | 0.014 ± 0.001 b | 1.85 ± 0.10 b | 0.042 ± 0.004 c | 0.019 ± 0.005 c | 2.21 ± 0.02 b |
| Cu 50 + EDTA | 0.028 ± 0.006 c | 0.008 ± 0.005 c | 3.50 ± 0.52 a | 0.014 ± 0.006 d | 0.005 ± 0.002 c | 2.80 ± 0.20 a | 0.034 ± 0.004 d | 0.014 ± 0.001 c | 2.42 ± 0.03 b |
| Cu 100 + EDTA | 0.023 ± 0.020 c | 0.007 ± 0.020 c | 3.28 ± 0.12 a | 0.013 ± 0.005 d | 0.006 ± 0.003 c | 2.16 ± 0.22 a | 0.019 ± 0.002 e | 0.010 ± 0.002 d | 1.90 ± 0.05 c |
| Root Fresh Weight (g) | Root Dried Weight (g) | Root Fresh to Dried Weight Ratio | Stem Fresh Weight (g) | Stem Dried Weight (g) | Stem Fresh to Dried Weight Ratio | Leaf Fresh Weight (g) | Leaf Dried Weight (g) | Leaf Fresh to Dried Weight Ratio | |
|---|---|---|---|---|---|---|---|---|---|
| Control 1 | 2.634 ± 0.500 a12345 | 0.713 ± 0.024 a12345 | 3.65 ± 0.58 c13, b2, a3, d5 | 0.820 ± 0.113 b12, a35, bc4 | 0.043 ± 0.004 b125, a3, c4 | 19.32 ± 2.67 b12, a35, ab4 | 1.042 ± 0.002 c14, b2, a35 | 0.071 ± 0.000 bc1, b25, a3, d4 | 14.69 ± 0.02 b12, a345 |
| Control + EDTA 1 | 0.224 ± 0.073 c123, d4, b5 | 0.076 ± 0.04 a12, b345 | 3.86 ± 1.06 c13, b2, a3, d5 | 0.688 ± 0.073 b125, a3, c4 | 0.038 ± 0.005 c14, b25, a3 | 18.40 ± 0.74 b12, a35, ab4 | 1.127 ± 0.024 c14, b2, a35 | 0.070 ± 0.001 bc1, b25, a3, d4 | 16.07 ± 0.24 b12, a34, a5 |
| Ni 50 | 1.290 ± 0.046 b | 0.075 ± 0.005 a | 17.31 ± 0.51 a | 3.150 ± 0.191 a | 0.150 ± 0.026 a | 22.01 ± 2.72 a | 3.293 ± 0.014 a | 0.117 ± 0.013 b | 28.37 ± 2.60 a |
| Ni 100 | 0.331 ± 0.020 c | 0.059 ± 0.008 b | 5.72 ± 0.41 bc | 0.425 ± 0.026 b | 0.022 ± 0.002 c | 19.35 ± 0.45 b | 1.185 ± 0.179 c | 0.117 ± 0.041 b | 11.82 ± 4.19 c |
| Ni 50 + EDTA | 0.152 ± 0.007 c | 0.021 ± 0.001 c | 7.40 ± 0.30 b | 0.189 ± 0.013 c | 0.058 ± 0.003 b | 3.26 ± 0.04 c | 2.210 ± 0.248 b | 0.156 ± 0.010 a | 14.00 ± 0.73 c |
| Ni 100 + EDTA | 0.154 ± 0.026 c | 0.024 ± 0.004 c | 6.38 ± 0.02 b | 0.212 ± 0.042 c | 0.065 ± 0.015 b | 3.34 ± 0.16 c | 0.131 ± 0.007 d | 0.052 ± 0.007 c | 2.60 ± 0.25 d |
| Cr 50 | 0.598 ± 0.138 b | 0.082 ± 0.003 a | 7.22 ± 1.45 a | 2.458 ± 0.161 a | 0.133 ± 0.018 a | 18.80 ± 1.30 b | 0.105 ± 0.021 c | 0.031 ± 0.007 c | 3.42 ± 0.26 c |
| Cr 100 | 0.302 ± 0.097 bc | 0.040 ± 0.019 b | 8.67 ± 1.26 s | 0.549 ± 0.012 c | 0.036 ± 0.001 b | 15.11 ± 0.10 c | 0.275 ± 0.056 c | 0.065 ± 0.013 b | 4.24 ± 0.08 c |
| Cr 50 + EDTA | 0.145 ± 0.009 cd | 0.037 ± 0.007 b | 4.09 ± 0.59 b | 0.479 ± 0.125 c | 0.019 ± 0.003 c | 24.41 ± 3.40 a | 3.193 ± 0.170 a | 0.154 ± 0.010 a | 20.70 ± 0.17 a |
| Cr 100 + EDTA | 0.083 ± 0.040 d | 0.031 ± 0.011 b | 2.95 ± 1.54 c | 0.417 ± 0.035 c | 0.038 ± 0.009 c | 11.79 ± 1.76 c | 0.925 ± 0.214 b | 0.054 ± 0.012 c | 17.24 ± 0.49 b |
| Cd 50 | 0.128 ± 0.007 b | 0.046 ± 0.009 c | 2.97 ± 0.54 b | 0.194 ± 0.016 b | 0.018 ± 0.002 c | 10.94 ± 0.27 b | 0.216 ± 0.049 c | 0.017 ± 0.002 c | 12.24 ± 1.39 b |
| Cd 100 | 0.085 ± 0.009 c | 0.073 ± 0.008 b | 1.16 ± 0.02 c | 0.197 ± 0.007 b | 0.023 ± 0.004 c | 9.15 ± 1.12 b | 0.251 ± 0.024 c | 0.068 ± 0.010 a | 3.71 ± 0.16 c |
| Cd 50 + EDTA | 0.115 ± 0.032 b | 0.042 ± 0.009 c | 2.63 ± 0.23 b | 0.065 ± 0.003 c | 0.015 ± 0.001 c | 4.44 ± 0.19 c | 0.077 ± 0.003 b | 0.057 ± 0.009 b | 1.36 ± 0.29 c |
| Cd 100 + EDTA | 0.065 ± 0.003 c | 0.037 ± 0.003 c | 1.78 ± 0.08 c | 0.190 ± 0.017 b | 0.012 ± 0.002 d | 16.95 ± 1.75 ab | 0.075 ± 0.002 b | 0.063 ± 0.001 a | 1.18 ± 0.01 c |
| Pb 50 | 1.247 ± 0.009 b | 0.061 ± 0.001 c | 20.54 ± 0.28 a | 1.450 ± 0.308 b | 0.061 ± 0.013 b | 24.03 ± 0.15 a | 2.089 ± 0.030 b | 0.129 ± 0.000 b | 16.24 ± 0.22 a |
| Pb 100 | 0.532 ± 0.017 c | 0.029 ± 0.002 d | 18.69 ± 1.03 b | 2.013 ± 0.342 a | 0.097 ± 0.012 a | 20.56 ± 0.91 a | 3.380 ± 0.456 a | 0.221 ± 0.029 a | 15.29 ± 0.09 a |
| Pb 50 + EDTA | 0.498 ± 0.059 c | 0.025 ± 0.004 d | 20.45 ± 0.97 a | 0.685 ± 0.005 c | 0.041 ± 0.000 c | 16.73 ± 0.19 b | 1.228 ± 0.022 c | 0.095 ± 0.002 c | 12.94 ± 0.06 ab |
| Pb 100 + EDTA | 0.181 ± 0.026 d | 0.012 ± 0.001 d | 15.20 ± 0.56 b | 0.307 ± 0.006 d | 0.016 ± 0.003 d | 20.27 ± 3.64 a | 0.428 ± 0.011 d | 0.037 ± 0.001 d | 11.63 ± 0.34 b |
| Cu 50 | 0.024 ± 0.009 c | 0.004 ± 0.001 d | 5.88 ± 0.28 c | 0.431 ± 0.031 c | 0.052 ± 0.001 a | 8.23 ± 0.41 b | 0.308 ± 0.005 c | 0.041 ± 0.003 c | 7.61 ± 0.37 d |
| Cu 100 | 0.018 ± 0.003 c | 0.005 ± 0.001 d | 3.51 ± 0.14 d | 0.136 ± 0.007 d | 0.016 ± 0.002 c | 8.58 ± 0.73 b | 0.299 ± 0.020 c | 0.045 ± 0.004 c | 6.73 ± 0.24 d |
| Cu 50 + EDTA | 0.658 ± 0.056 b | 0.034 ± 0.004 c | 19.29 ± 0.63 a | 0.867 ± 0.076 a | 0.050 ± 0.004 a | 17.16 ± 0.26 a | 1.341 ± 0.147 a | 0.104 ± 0.009 a | 12.86 ± 0.40 b |
| Cu 100 + EDTA | 0.233 ± 0.076 b | 0.015 ± 0.004 d | 15.09 ± 1.18 b | 0.521 ± 0.004 bc | 0.031 ± 0.001 bc | 16.88 ± 0.22 a | 0.524 ± 0.044 b | 0.055 ± 0.005 c | 9.51 ± 0.144 c |
| C. roseus | C. argentea | |||||
|---|---|---|---|---|---|---|
| Leaves | Stem | Roots | Leaves | Stem | Roots | |
| Ni 50 | 4.194 ± 0.335 | 2.067 ± 0.413 | 4.602 ± 0.748 | 8.817 ± 0.990 * | 12.338 ± 2.138 * | 14.856 ± 0.990 * |
| Ni 100 | 4.386 ± 0.133 * | 3.862 ± 0.297 * | 9.535 ± 0.281 * | 8.734 ± 1.972 * | 4.753 ± 0.432 | 14.551 ± 1.972 * |
| Ni 50 + EDTA | 2.720 ± 0.010 | 2.632 ± 0.328 | 4.878 ± 0.505 | 5.084 ± 0.218 | 6.427 ± 0.332 | 4.593 ± 0.218 |
| Ni 100 + EDTA | 4.639 ± 0.546 * | 4.650 ± 0.172 * | 9.287 ± 0.429 * | 8.435 ± 1.217 | 11.707 ± 2.701 * | 7.307 ± 1.217 |
| Cr 50 | 3.942 ± 1.254 | 3.075 ± 0.854 | 10.607 ± 1.562 | 3.004 ± 1.158 | 19.721 ± 2.669 * | 31.654 ± 1.158 * |
| Cr 100 | 4.576 ± 1.098 | 6.638 ± 1.106 | 13.392 ± 2.759 | 7.091 ± 0.467 | 9.591 ± 0.266 | 17.826 ± 5.467 |
| Cr 50 + EDTA | 3.753 ± 0.139 | 7.024 ± 1.080 | 14.125 ± 2.045 | 29.203 ± 3.620 * | 4.500 ± 0.710 | 19.136 ± 3.620 |
| Cr 100 + EDTA | 6.356 ± 1.177 * | 10.280 ± 2.95 * | 20.156 ± 3.839 * | 16.121 ± 2.977 | 14.812 ± 3.508 | 22.481 ± 3.977 |
| Cd 50 | 5.024 ± 1.953 | 2.024 ± 1.079 | 13.875 ± 2.196 | 3.422 ± 0.147 | 7.559 ± 0.839 | 31.419 ± 6.147 |
| Cd 100 | 11.315 ± 0.969 * | 4.722 ± 0.337 * | 19.298 ± 3.963 * | 26.893 ± 3.935 | 14.682 ± 2.553 * | 63.286 ± 6.935 * |
| Cd 50 + EDTA | 4.001 ± 0.572 | 0.502 ± 0.093 | 4.444 ± 1.089 | 16.222 ± 3.471 | 6.812 ± 0.454 | 30.200 ± 6.471 |
| Cd 100 + EDTA | 3.346 ± 0.394 | 1.959 ± 0.212 | 7.598 ± 1.277 | 53.164 ± 4.384 * | 13.611 ± 2.268 * | 54.073 ± 7.384 * |
| Pb 50 | 5.647 ± 1.448 | 7.415 ± 0.285 | 9.807 ± 0.859 | 18.072 ± 0.739 | 24.340 ± 5.187 | 45.128 ± 2.739 * |
| Pb 100 | 15.984 ± 2.351 | 14.196 ± 0.709 * | 19.943 ± 2.359 * | 55.179 ± 3.043 * | 54.171 ± 6.701 * | 44.136 ± 3.043 * |
| Pb 50 + EDTA | 8.780 ± 0.702 | 10.365 ± 1.091 | 11.543 ± 1.793 | 16.877 ± 3.321 | 29.632 ± 3.200 | 20.754 ± 3.320 |
| Pb 100 + EDTA | 21.142 ± 5.529 * | 13.734 ± 1.694 * | 19.543 ± 2.017 * | 21.092 ± 2.067 | 17.519 ± 3.284 | 24.804 ± 2.067 |
| Cu 50 | 1.612 ± 0.525 | 1.887 ± 0.377 | 6.961 ± 1.194 * | 1.193 ± 0.099 | 3.624 ± 0.069 | 0.399 ± 0.099 |
| Cu 100 | 2.130 ± 0.681 * | 2.951 ± 0.167 * | 6.940 ± 0.280 * | 4.779 ± 0.424 | 3.085 ± 0.385 | 2.124 ± 0.424 |
| Cu 50 + EDTA | 1.366 ± 0.021 | 1.705 ± 0.682 | 4.676 ± 0.193 | 7.343 ± 0.612 | 6.660 ± 0.533 | 5.209 ± 0.612 |
| Cu 100 + EDTA | 1.213 ± 0.006 | 3.126 ± 0.613 * | 5.373 ± 1.179 | 12.163 ± 2.458 * | 11.477 ± 1.370 * | 9.218 ± 2.458 * |
| C. roseus | C. argentea | |||||
|---|---|---|---|---|---|---|
| Translocation Factor | Bioaccumulation Coefficient | Bioconcentration Factor | Translocation Factor | Bioaccumulation Coefficient | Bioconcentration Factor | |
| Ni 50 | 0.814 ± 0.314 | 0.064 ± 0.003 | 0.078 ± 0.008 | 0.796 ± 0.104 | 0.054 ± 0.003 | 0.067 ± 0.010 |
| Ni 100 | 0.590 ± 0.207 | 0.048 ± 0.001 | 0.081 ± 0.006 | 1.629 ± 0.131 * | 0.068 ± 0.005 * | 0.042 ± 0.002 |
| Ni 50 + EDTA | 0.829 ± 0.187 * | 0.086 ± 0.008 * | 0.104 ± 0.005 * | 0.656 ± 0.128 | 0.049 ± 0.002 | 0.075 ± 0.002 * |
| Ni 100 + EDTA | 0.826 ± 0.166 * | 0.073 ± 0.007 | 0.089 ± 0.000 | 1.124 ± 0.196 | 0.058 ± 0.001 | 0.052 ± 0.011 |
| Cr 50 | 0.466 ± 0.060 | 0.100 ± 0.005 | 0.215 ± 0.003 | 0.635 ± 0.160 | 0.094 ± 0.005 | 0.148 ± 0.003 |
| Cr 100 | 0.577 ± 0.204 * | 0.106 ± 0.014 | 0.184 ± 0.015 | 0.843 ± 0.111 | 0.072 ± 0.003 | 0.086 ± 0.004 |
| Cr 50 + EDTA | 0.579 ± 0.019 * | 0.157 ± 0.009 * | 0.272 ± 0.007 * | 0.825 ± 0.069 | 0.164 ± 0.010 * | 0.199 ± 0.005 * |
| Cr 100 + EDTA | 0.571 ± 0.071 * | 0.123 ± 0.038 | 0.215 ± 0.004 | 0.949 ± 0.081 * | 0.132 ± 0.019 | 0.139 ± 0.002 |
| Cd 50 | 0.396 ± 0.174 | 0.049 ± 0.004 | 0.123 ± 0.015 * | 0.910 ± 0.298 | 0.111 ± 0.008 | 0.122 ± 0.001 |
| Cd 100 | 0.650 ± 0.261 | 0.059 ± 0.003 * | 0.090 ± 0.009 | 1.193 ± 0.524 | 0.092 ± 0.008 | 0.077 ± 0.003 |
| Cd 50 + EDTA | 0.735 ± 0.099 * | 0.032 ± 0.004 | 0.044 ± 0.003 | 1.027 ± 0.222 | 0.131 ± 0.012 | 0.128 ± 0.010 * |
| Cd 100 + EDTA | 0.462 ± 0.094 | 0.028 ± 0.003 | 0.061 ± 0.000 | 1.354 ± 0.142 * | 0.176 ± 0.005 * | 0.130 ± 0.005 * |
| Pb 50 | 1.008 ± 0.487 | 0.042 ± 0.005 | 0.041 ± 0.003 | 0.729 ± 0.271 | 0.052 ± 0.003 | 0.071 ± 0.001 |
| Pb 100 | 1.124 ± 0.090 | 0.057 ± 0.007 | 0.051 ± 0.000 | 0.531 ± 0.253 | 0.039 ± 0.001 | 0.073 ± 0.001 |
| Pb 50 + EDTA | 1.243 ± 0.209 | 0.087 ± 0.006 | 0.070 ± 0.003 | 1.085 ± 0.580 * | 0.087 ± 0.003 * | 0.080 ± 0.006 |
| Pb 100 + EDTA | 1.342 ± 0.198 * | 0.097 ± 0.008 * | 0.073 ± 0.001 * | 0.806 ± 0.277 | 0.089 ± 0.006 * | 0.100 ± 0.003 * |
| Cu 50 | 0.400 ± 0.058 | 0.063 ± 0.004 | 0.156 ± 0.004 | 0.991 ± 0.153 | 0.031 ± 0.004 | 0.031 ± 0.003 |
| Cu 100 | 0.456 ± 0.200 | 0.044 ± 0.003 | 0.097 ± 0.014 | 0.704 ± 0.345 | 0.047 ± 0.002 | 0.067 ± 0.005 |
| Cu 50 + EDTA | 0.750 ± 0.227 * | 0.138 ± 0.017 * | 0.184 ± 0.032 * | 1.330 ± 0.114 * | 0.064 ± 0.003 | 0.048 ± 0.003 |
| Cu 100 + EDTA | 0.859 ± 0.077 * | 0.104 ± 0.006 | 0.121 ± 0.003 | 0.962 ± 0.247 | 0.093 ± 0.001 * | 0.097 ± 0.003 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qurban, M.; Mirza, C.R.; Khan, A.H.A.; Khalifa, W.; Boukendakdji, M.; Achour, B.; Yousaf, S.; Nawaz, I.; Butt, T.A.; Iqbal, M. Metal Accumulation Profile of Catharanthus roseus (L.) G.Don and Celosia argentea L. with EDTA Co-Application. Processes 2021, 9, 598. https://doi.org/10.3390/pr9040598
Qurban M, Mirza CR, Khan AHA, Khalifa W, Boukendakdji M, Achour B, Yousaf S, Nawaz I, Butt TA, Iqbal M. Metal Accumulation Profile of Catharanthus roseus (L.) G.Don and Celosia argentea L. with EDTA Co-Application. Processes. 2021; 9(4):598. https://doi.org/10.3390/pr9040598
Chicago/Turabian StyleQurban, Muneeba, Cyrus Raza Mirza, Aqib Hassan Ali Khan, Walid Khalifa, Mustapha Boukendakdji, Belkacem Achour, Sohail Yousaf, Ismat Nawaz, Tayyab Ashfaq Butt, and Mazhar Iqbal. 2021. "Metal Accumulation Profile of Catharanthus roseus (L.) G.Don and Celosia argentea L. with EDTA Co-Application" Processes 9, no. 4: 598. https://doi.org/10.3390/pr9040598
APA StyleQurban, M., Mirza, C. R., Khan, A. H. A., Khalifa, W., Boukendakdji, M., Achour, B., Yousaf, S., Nawaz, I., Butt, T. A., & Iqbal, M. (2021). Metal Accumulation Profile of Catharanthus roseus (L.) G.Don and Celosia argentea L. with EDTA Co-Application. Processes, 9(4), 598. https://doi.org/10.3390/pr9040598









