Mead Production Using Immobilized Cells of Saccharomyces cerevisiae: Reuse of Sodium Alginate Beads
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Sample
2.2. Honeys Quality
2.3. Pollen Analysis
2.4. Mead Production
2.4.1. Preparation of Honey Must
2.4.2. Yeast Hydration and Immobilization
2.4.3. Conditions and Monitoring of Fermentation
2.5. Physical–Chemical Characterization of Honey Must and Mead
2.6. Characterization of Alginate Beads at the End of Fermentation
2.7. Statistic Treatment
3. Results and Discussion
3.1. Pollen Analysis of Honey
3.2. Physical–Chemical Characterization of the Honey
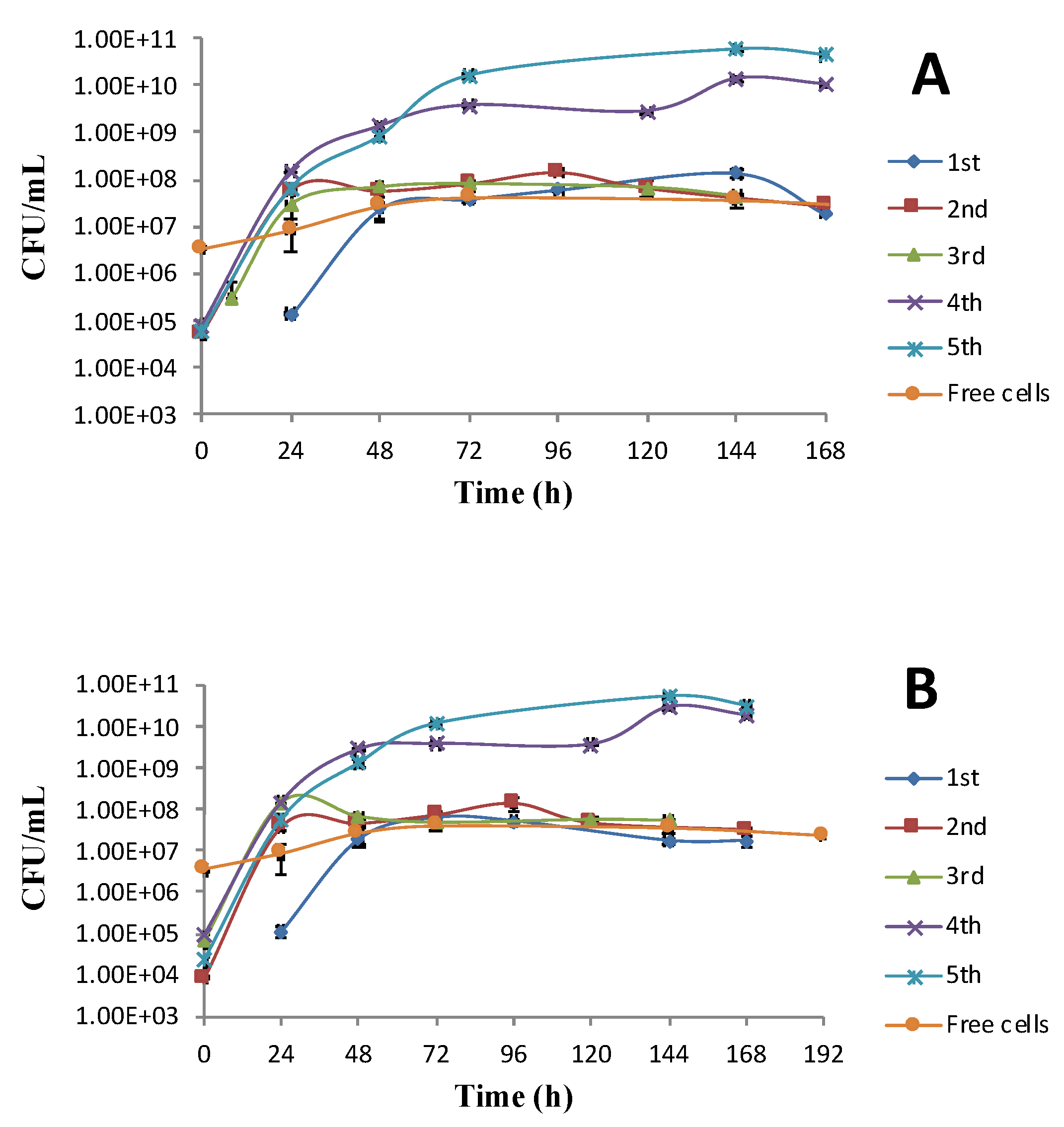
3.3. Mead Production Using Immobilized Cells in Alginate Beads
3.4. Fermentation Performance during Reuse of Immobilized Cells
3.5. Physical–Chemical Characterization of the Final Product
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sroka, P.; Tuszyński, T. Changes in organic acid contents during mead wort fermentation. Food Chem. 2007, 104, 1250–1257. [Google Scholar] [CrossRef]
- Navrátil, M.; Sturdík, E.; Gemeiner, P. Batch and continuous mead production with pectate immobilised, ethanol-tolerant yeast. Biotechnol. Lett. 2001, 23, 977–982. [Google Scholar] [CrossRef]
- Pereira, A.; Mendes-Ferreira, A.; Oliveira, J.; Estevinho, L.; Mendes-Faia, A. High-cell-density fermentation of Saccharomyces cerevisiae for the optimisation of mead production. Food Microbiol. 2013, 33, 114–123. [Google Scholar] [CrossRef] [PubMed]
- McConnell, D.S.; Schramm, K.D. Mead success: Ingredients, processes and techniques. Zymurgy Spring 1995, 4, 33–39. [Google Scholar]
- Pereira, A.P.; Dias, T.; Andrade, J.; Ramalhosa, E.; Estevinho, L.M. Mead production: Selection and characterization assays of Saccharomyces cerevisiae strains. Food Chem. Toxicol. 2009, 47, 2057–2063. [Google Scholar] [CrossRef]
- Mendes-Ferreira, A.; Cosme, F.; Barbosa, C.; Falco, V.; Inês, A.; Mendes-Faia, A. Optimization of honey-must preparation ad alcoholic fermentation by Saccharomyces cerevisiae for mead production. Int. J. Food Microbiol. 2010, 144, 193–198. [Google Scholar] [CrossRef]
- Roldán, A.; Van Muiswinkel, G.; Lasanta, C.; Palacios, V.; Caro, I. Influence of pollen addition on mead elaboration: Physicochemical and sensory characteristics. Food Chem. 2011, 126, 574–582. [Google Scholar] [CrossRef]
- Ukpabi, U.J. Quality evaluation of meads produced with cassava (Manihot esculenta) floral honey under farm conditions in Nigeria. Trop. Subtrop. Agroecosyst. 2006, 6, 37–41. [Google Scholar]
- Wintersteen, C.L.; Andrae, L.M.; Engeseth, N.J. Effect of Heat Treatment on Antioxidant Capacity and Flavor Volatiles of Mead. J. Food Sci. 2005, 70, C119–C126. [Google Scholar] [CrossRef]
- Ivorra, C.; Pérez-Ortín, J.E.; Del Olmo, M. An inverse correlation between stress resistance and stuck fermentations in wine yeasts. A molecular study. Biotechnol. Bioeng. 1999, 64, 698–708. [Google Scholar] [CrossRef]
- Casellas, G.B. Effect of Low Temperature Fermentation and Nitrogen Content on Wine Yeast Metabolism. Ph.D. Thesis, Universitat Rovira i Virgili, Spain, France, 2005. [Google Scholar]
- Champagne, C.P.; Gaudy, C.; Poncelet, D.; Neufeld, R.J. Lactococcus lactis release from calcium alginate beads. Appl. Environ. Microbiol. 1992, 58, 1429–1434. [Google Scholar] [CrossRef]
- Genisheva, Z.; Macedo, S.; Mussatto, S.I.; Teixeira, J.A.; Oliveira, J.M. Production of White Wine by Sacchomyces cerevisiae immobilized on grape pomace. J. Inst. Brew. 2012, 118, 163–173. [Google Scholar] [CrossRef]
- Coelho, T.C. Avaliação das Condições de Imobilização de Células de Candida Guilliermondii FTI 20037 em Bucha Vegetal (Luffa Cylindrica) Visando a Produção de Xilitol. Master’s Thesis, Universidade de São Paulo, São Paulo, Brazil, 2007. [Google Scholar]
- Covizzi, L.G.; Giese, E.C.; Gomes, E.; Dekker, R.F.H.; Da Silva, R. Imobilização de células microbianas e suas aplicações biotecnológicas. Semin. Ciências Exatas Tecnológicas 2007, 28, 143. [Google Scholar] [CrossRef]
- Inal, M.; Yiğitoğlu, M. Production of bioethanol by immobilized Saccharomyces Cerevisiae onto modified sodium alginate gel. J. Chem. Technol. Biotechnol. 2011, 86, 1548–1554. [Google Scholar] [CrossRef]
- Hernández, R.M.; Orive, G.; Murua, A.; Pedraz, J.L. Microcapsules and microcarriers for in situ cell delivery. Adv. Drug Deliv. Rev. 2010, 62, 711–730. [Google Scholar] [CrossRef]
- Najafpour, G.; Younesi, H.; Ismail, K.S.K. Ethanol fermentation in an immobilized cell reactor using Saccharomyces cerevisiae. Bioresour. Technol. 2004, 92, 251–260. [Google Scholar] [CrossRef]
- Liouni, M.; Drichoutis, P.; Nerantzis, E.T. Studies of the mechanical properties and the fermentation behavior of double layer alginate–chitosan beads, using Saccharomyces cerevisiae entrapped cells. World J. Microbiol. Biotechnol. 2007, 24, 281–288. [Google Scholar] [CrossRef]
- Carvalho, W.; Canilha, L.; Silva, S.S. Uso de Biocatalizadores Imobilizados: Uma alternativa para a condução de bioprocessos. Rev. Anal. 2006, 23, 60–70. [Google Scholar]
- Oliveira, M.A. Produção de Cerveja de Baixo teor Alcoólico Utilizando Leveduras Imobilizadas em Biopolímero. Master’s Thesis, Universidade Tiradentes, Aracaju, Brazil, 2011. [Google Scholar]
- Divies, C.; Cachon, R. Wine Production by immobilized cell systems. In Applications of Cell Immobilisation Biotechnology, Focus on Biotechnology, 1st ed.; Nedović, V., Willaert, R., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 8B, pp. 285–293. [Google Scholar]
- Wang, S.; Song, C.; Chen, G.; Guo, T.; Liu, J.; Zhang, B.; Takeuchi, S. Characteristics and biodegradation properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/organophilic montmorillonite (PHBV/OMMT) nanocomposite. Polym. Degrad. Stab. 2005, 87, 69–76. [Google Scholar] [CrossRef]
- Nikolić, S.; Mojović, L.; Rakin, M.; Pejn, D.; Nedović, V. Effect of different fermentation parameters on bioethanol production from corn meal hydrolyzates by free and immobilized cells of Saccharomyces cerevisiae var. ellipsoideus. J. Chem. Technol. Biotechnol. 2008, 84, 497–503. [Google Scholar] [CrossRef]
- Vilela, A.; Schuller, D.; Mendes-Faia, A.; Côrte-Real, M. Reduction of volatile acidity of acidic wines by immobilized Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 2013, 97, 4991–5000. [Google Scholar] [CrossRef]
- Ciani, M.; Ferraro, L. Enhanced Glycerol Content in Wines Made with Immobilized Candida stellata Cells. Appl. Environ. Microbiol. 1996, 62, 128–132. [Google Scholar] [CrossRef]
- Kostov, G.; Angelov, M.; Mihaylov, I.; Poncelet, D. Mechanical properties of Ca-alginate beads for ethanol fermentation with immobilized yeast. Rev. Genie Ind. 2010, 5, 25–35. [Google Scholar]
- Drichoutis, P.; Nerantzes, E.T.; Liouni, M. Continuous production of wine in a tower fermentor using entrapped yeast cells in double layer alginate-chitosan beads. e-J. Sci. Technol. 2007, 6, 51–60. [Google Scholar]
- Harmonized Methods of the International Honey Commission, International Honey Commission, IHC Methods. 2009. Available online: https://www.ihc-platform.net/ihcmethods2009.pdf (accessed on 1 November 2020).
- Sancho, M.T.; Muniategui, S.; Sánchez, P.; Huidobro, J.F.; Simal, J. Mieles del Pais Vasco, XI: Evaluación de los distintos tipos de cenizas. An. Bromatol. 1991, 4, 311–324. [Google Scholar]
- Bogdanov, S.; Martin, P.; Lüllmann, C. Harmonised methods of the European Honey Commision. Apidologie 1997, 28, 1–59. [Google Scholar]
- Anonymous. Métodos oficiales de análisis para la miel. Orden de 12 de Junio de 1986 de la Presidência del Gobierno, “Boletín Oficial del Estado” no 145 de 18 de Junio de XXIII—Edulcorantes naturales y derivados. 1986. Available online: https://www.boe.es/eli/es/o/1986/06/12/(3) (accessed on 26 February 2020).
- Bianchi, E.M. Control de Calidad de la Miel y la Cera; Organización de las Naciones Unidas para la Agriculture y Alimentación (FAO): Rome, Italy, 1990. [Google Scholar]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. Compendium of International Methods of Wine and Must Analysis, Volume 1; OIV: Paris, France, 2021. [Google Scholar]
- Aerny, J. Composés azotes des moûts et des vins. Revue Suisse de Viticulture, Arboriculture. Horticulture 1996, 28, 161–165. [Google Scholar]
- International Organisation of Vine and Wine. Compendium of International Methods of Wine and Must Analysis, Volume 2; OIV: Paris, France, 2021. [Google Scholar]
- Curvelo-Garcia, A.S. Controlo de Qualidade Dos vinhos. Química Enológica e Métodos Analíticos; Instituto da Vinha e do Vinho: Lisboa, Portugal, 1998. [Google Scholar]
- Göksungur, Y.; Zorlu, N. Production of Ethanol from Beet Molasses by Ca- Alginate Immobilized Yeast Cells in a Packed-Bed Bioreactor. Turk. J. Biol. 2001, 25, 265–275. [Google Scholar]
- Maia, M.; Russo-Almeida, P.; Pereira, J.O. Caracterização do Espectro Polínico dos Méis do Alentejo (Portugal). Silva Lusit. 2005, 13, 95–103. [Google Scholar]
- Bogdanov, S.; Ruoh, K.; Oddo, L.P. Physico-chemical methods for the characterization of unifloral honeys: A review. Apidologie 2004, 35, S4–S17. [Google Scholar] [CrossRef]
- De Rodríguez, G.O.; De Ferrer, B.S.; Ferrer, A.; Rodríguez, B. Characterization of honey produced in Venezuela. Food Chem. 2004, 84, 499–502. [Google Scholar] [CrossRef]
- Vargas, T. Avaliação da Qualidade do mel Produzido na Região dos Campos Gerais do Paraná. Master’s Thesis, Universidade Estadual de Ponta Grossa, Ponta Grossa, Brazil, 2006. [Google Scholar]
- Al, M.L.; Daniel, D.; Moise, A.R.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Acquarone, C.; Buera, P.; Elizalde, B. Pattern of pH and electrical conductivity upon honey dilution as a complementary tool for discriminating geographical origin of honeys. Food Chem. 2007, 101, 695–703. [Google Scholar] [CrossRef]
- Küçük, M.; Kolaylı, S.; Karaoğlu, Ş.; Ulusoy, E.; Baltacı, C.; Candan, F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Evangelista-Rodrigues, A.; Silva, E.M.S.d.; Beserra, E.M.F.; Rodrigues, M.L. Análise físico-química dos méis das abelhas Apis mellifera e Melipona scutellaris produzidos em regiões distintas no Estado da Paraíba. Ciência Rural 2005, 35, 1166–1171. [Google Scholar] [CrossRef][Green Version]
- Abu-Tarboush, H.M.; Al-Kahtani, H.A.; El-Sarrage, M.S. Foral-type identication and quality evaluation of some honey types. Food Chem. 1993, 26, 17–33. [Google Scholar]
- Iurlina, M.O.; Fritz, R. Characterization of microorganisms in Argentinean honeys from different sources. Int. J. Food Microbiol. 2005, 105, 297–304. [Google Scholar] [CrossRef]
- Souza, D.C.; Bazlen, K. Análises Preliminares de Características Físico-Químicas de Méis de Tiúba (Melipona compressipes) do Piauí; XII Congresso Brasileiro de Apicultura: Salvador, BA, USA, 1998. [Google Scholar]
- Aroucha, E.M.M.; Oliveira, A.J.F.; Nunes, G.H.S.; Maracajá, P.B. Qualidade do mel de abelha produzidos pelos Incubados da iagram e comercializado no Município de Mossoró/RN. Caatinga Mossoró 2004, 21, 211–217. [Google Scholar]
- Valbuena, A.O. Contribución a la Denominación de Origen de la Miel de la Alcarria. Ph.D. Thesis, Facultad de Ciencias Biológicas de la Universidad Complutense de Madrid, Spain, France, 1992. [Google Scholar]
- Fallico, B.; Arena, E.; Verzera, A.; Zappalà, M. The European Food Legislation and its impact on honey sector. Accredit. Qual. Assur. 2006, 11, 49–54. [Google Scholar] [CrossRef]
- Carillo Magana, F.A. Meliponicultura: El Mundo de las Abejas Nativas de Yucatán; Blackwell Publishing: Mérida, México, 1998. [Google Scholar]
- Azeredo, L.C.; Azeredo, M.; De Souza, S.; Dutra, V. Protein contents and physicochemical properties in honey samples of Apis mellifera of different floral origins. Food Chem. 2003, 80, 249–254. [Google Scholar] [CrossRef]
- Smidsrød, O.; Skjåk-Bræk, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Mendes-Faia, A.; Côrte-Real, M. Redução da acidez volátil de vinhos por células de Saccharomyces cerevisiae imobilizadas em esferas de alginato-quitosano. Enologia 2012, 38–42. [Google Scholar]
- Duran, P.M.; Baley, J.E. Effects of immmobilization on growth, fermentations properties and macromolecular composition of S. cerevisiae attached to gelatin. Biotecnol. Bioeng. 1986, 28, 73–87. [Google Scholar] [CrossRef]
- Bezbradica, D.; Obradovic, B.; Leskosek-Cukalovic, I.; Bugarski, B.; Nedovic, V. Immobilization of yeast cells in PVA particles for beer fermentation. Process. Biochem. 2007, 42, 1348–1351. [Google Scholar] [CrossRef]
- Nikolaou, E.; Soufleros, E.H.; Bouloumpasi, E.; Tzanetakis, N. Selection of indigenous Saccharomyces cerevisiae strains according to their oenological characteristics and vinification results. Food Microbiol. 2006, 23, 205–211. [Google Scholar] [CrossRef]
- Gomes, T. Produção de Hidromel: Efeitos das Condições de Fermentação. Master’s Thesis, Instituto Politécnico de Bragança, Bragança, Portugal, 2010. [Google Scholar]
- Filipe-Ribeiro, L.; Mendes-Faia, A. Validation and comparison of analytical methods used to evaluate the nitrogen status of grape juice. Food Chem. 2007, 100, 1272–1277. [Google Scholar] [CrossRef]
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology. Handb. Enol. 2005, 1. [Google Scholar] [CrossRef]


| Parameter | Honey |
|---|---|
| Humidity (%) | 17.2 ± 0.2 |
| Acidity (meq. Ac/Kg) | 16.0 ± 0.7 |
| Electrical Conductivity (mS/cm) | 0.80 ± 0.01 |
| Total Ash (%) | 0.56 ± 0.02 |
| pH | 4.47 ± 0.06 |
| Diastatic Index (Schade scale) | 13.3 ± 0.5 |
| HMF (mg/Kg) | 8.3 ± 1.0 |
| Reducing sugars (%) | 62.4 ± 0.6 |
| Apparent Sucrose (%) | 8.9 ± 0.3 |
| Fermentation | pH | °Brix | Total Acidity | Assimilable Nitrogen | Reducing Sugars | |
|---|---|---|---|---|---|---|
| Type | N° | (g/L Tartaric Acid) | (mg/L) | (g/L) | ||
| Free cells | 3.71 ± 0.06 | 23.0 ± 0.1 | 1.33 ± 0.09 | 254 ± 7 | 221 ± 17 | |
| Immobilized cells | 1st | 3.83 ± 0.06 | 24.5 ± 0.1 | 1.58 ± 0.01 | 212 ± 11 | 274 ± 23 |
| 2nd | 3.82 ± 0.09 | 24.3 ± 0.1 | 1.55 ± 0.04 | 238 ± 7 | 246 ± 9 | |
| 3rd | 3.83 ± 0.04 | 23.5 ± 0.1 | 1.25 ± 0.04 | 243 ± 4 | 252 ± 22 | |
| 4th | 3.83 ± 0.06 | 24.4 ± 0.2 | 1.4 ± 0.1 | 259 ± 1 | 230 ± 8 | |
| 5th | 3.83 ± 0.04 | 24.5 ± 0.1 | 1.45 ± 0.04 | 289 ± 18 | 245 ± 10 | |
| Fermentation | µc | BF | Yethanol/sugars | |
|---|---|---|---|---|
| Type | N° | (h−1) | (CFU/mL) | (%) |
| Free cells | Control | 0.15 ± 0.006 c | 2.4 × 107 ± 5 × 106 a | 58 ± 4 a |
| Immobilized cells with 2% alginate | 1st | 0.11 ± 0.04 b | 1.8 × 107 ± 2 × 106 a | 48 ± 5 a |
| 2nd | 0.18 ± 0.01 def | 2.70 × 107 ± 1 × 106 a | 54.56 ± 0.05 a | |
| 3rd | 0.19 ± 0.0004 fg | 4.45 × 107 ± 7 × 105 a | 55 ± 1 a | |
| 4th | 0.15 ± 0.0002 cd | 1.04 × 1010 ± 3 × 108 b | 58 ± 2 a | |
| 5th | 0.16 ± 0.006 cde | 4.4 × 1010 ± 2 × 109 e | 52.4 ± 0.3 a | |
| Immobilized cells with 4% alginate | 1st | 0.080 ± 0.006 a | 1.8 × 107 ± 6 × 106 a | 48 ± 4 a |
| 2nd | 0.19 ± 0.004 fg | 3.2 × 107 ± 4 × 106 a | 54 ± 3 a | |
| 3rd | 0.21 ± 0.02 g | 5.4 × 107 ± 3 × 106 a | 53 ± 5 a | |
| 4th | 0.17 ± 0.0007 cde | 1.8 × 1010 ± 1 × 109 c | 58 ± 2 a | |
| 5th | 0.15 ± 0.001 cd | 3.1 × 1010 ± 3 × 109 d | 56 ± 2 a | |
| Fermentation | pH | Volatile Acidity | Total Acidity | Assimilable Nitrogen | Total SO2 | Alcohol Content | Reducing Sugars | |
|---|---|---|---|---|---|---|---|---|
| Type | N° | (g/L Acetic Acid) | (g/L Tartaric Acid) | (mg/L) | (mg/L) | (%) | (g/L) | |
| Free cells | Control | 3.35 ± 0.13 a | 0.78 ± 0.03 a | 4.25 ± 0.16 ab | 32.67 ± 4.04 ab | 39.25 ± 1.48 c | 11.33 ± 0.12 a | 24.02 ± 0.61 ab |
| Immobilized cells with 2% alginate | 1st | 3.63 ± 0.10 c | 0.82 ± 0.03 ab | 4.58 ± 0.08 b | 21.00 ± 0.01 a | 28.16 ± 0.01 b | 12.03 ± 0.12 bc | 21.08 ± 3.22 a |
| 2nd | 3.44 ± 0.02 abc | 1.08 ± 0.01 f | 5.36 ± 0.27 c | 42.00 ± 0.01 bc | 30.72 ± 0.01 b | 11.80 ± 0.14 abc | 24.97 ± 0.55 ab | |
| 3rd | 3.51 ± 0.16 abc | 0.96 ± 0.01 de | 4.58 ± 0.01 b | 38.50 ± 4.95 bc | 30.72 ± 0.01 b | 11.80 ± 0.14 abc | 24.77 ± 3.36 ab | |
| 4th | 3.51 ± 0.02 abc | 0.84 ± 0.08 abc | 4.05 ± 0.32 a | 42.00 ± 0.01 bc | 30.72 ± 3.62 b | 11.50 ± 0.28 ab | 28.94 ± 0.60 b | |
| 5th | 3.47 ± 0.01 abc | 0.93 ± 0.04 bcde | 4.39 ± 0.05 ab | 73.50 ± 4.95 d | 16.64 ± 1.81 a | 11.55 ± 0.50 ab | 29.34 ± 1.43 b | |
| Immobilized cells with 4% alginate | 1st | 3.61 ± 0.01 bc | 0.86 ± 0.03 abcd | 4.58 ± 0.08 b | 23.33 ± 4.04 a | 28.16 ± 0.01 b | 12.10 ± 0.10 c | 19.46 ± 2.40 a |
| 2nd | 3.37 ± 0.10 ab | 1.02 ± 0.01 ef | 5.50 ± 0.04 c | 46.67 ± 4.04 bc | 30.72 ± 2.56 b | 11.97 ± 0.12 bc | 24.58 ± 1.65 ab | |
| 3rd | 3.46 ± 0.04 abc | 0.94± 0.03 cde | 4.10 ± 0.04 a | 42.00 ± 0.01 bc | 30.72 ± 0.01 b | 11.90 ± 0.01 bc | 25.34 ± 0.71 ab | |
| 4th | 3.48 ± 0.06 abc | 0.92 ± 0.03 bcde | 4.48 ± 0.11 ab | 49.00 ± 7.00 c | 31.57 ± 1.48 b | 11.70 ± 0.01 abc | 28.08 ± 2.33 b | |
| 5th | 3.47 ± 0.04 abc | 0.94 ± 0.03 cde | 4.20 ± 0.15 ab | 77.00 ± 7.00 d | 16.21 ± 1.48 a | 11.97 ± 0.12 bc | 29.48 ± 0.40 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa-Dias, M.L.; Paula, V.B.; Dias, L.G.; Estevinho, L.M. Mead Production Using Immobilized Cells of Saccharomyces cerevisiae: Reuse of Sodium Alginate Beads. Processes 2021, 9, 724. https://doi.org/10.3390/pr9040724
Sousa-Dias ML, Paula VB, Dias LG, Estevinho LM. Mead Production Using Immobilized Cells of Saccharomyces cerevisiae: Reuse of Sodium Alginate Beads. Processes. 2021; 9(4):724. https://doi.org/10.3390/pr9040724
Chicago/Turabian StyleSousa-Dias, Miguel L., Vanessa Branco Paula, Luís G. Dias, and Letícia M. Estevinho. 2021. "Mead Production Using Immobilized Cells of Saccharomyces cerevisiae: Reuse of Sodium Alginate Beads" Processes 9, no. 4: 724. https://doi.org/10.3390/pr9040724
APA StyleSousa-Dias, M. L., Paula, V. B., Dias, L. G., & Estevinho, L. M. (2021). Mead Production Using Immobilized Cells of Saccharomyces cerevisiae: Reuse of Sodium Alginate Beads. Processes, 9(4), 724. https://doi.org/10.3390/pr9040724








