Pressure-Driven Membrane Process: A Review of Advanced Technique for Heavy Metals Remediation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ultrafiltration in Heavy Metal Removal
2.2. Nanofiltration for the Removal of Heavy Metals from Wastewater
2.3. Reverse Osmosis in Heavy Metal Removal from Wastewater
| Characteristics of Membrane | Heavy Metal Targeted | Initial Metal Concentration (mg/L) | pH | Pressure (bar) | Removal Efficiency (%) | Ref |
|---|---|---|---|---|---|---|
| Flat organic membranes MWCO = 1000 Da Permeability = 1.6 × 10−3 L m−2 s−1 bar−1 | Ni(II) Cu(II) | 69 | 1.2 | 14 | 76.1 72.6 | [106] |
| Polyamide membrane supported by diaminobenzenesulfonic acid (DABSA) MWCO = 500 Da Permeability = 11.8 L m−2 h−1 bar−1 Negative charged | Cr(VI) | 460 | 9 | 4 | >99 | [107] |
| Chitosan/polyvinyl alcohol/montmorillonite clay membrane Active area = 0.00385 m2 | Cr(VI) | 50 | 7 | 1 | (84–88.34) | [108] |
| PAN/Sulfonated Polyarylene ether benzonitrile MWCO = 300 Da Permeability = 7.62 LMH.bar−1 | Pb(II) Cd(II) | 1000 | (2–5) | 6 | 94.6 95.1 | [109] |
| Spiral wound Polymeric membrane (NF270–2540) Active area = 2.6 m2 Negatively charged MWCO = (200–400) Da | Co(II) Ni(II) | 20 | (3.4–5.6) 3.4 | 6 10 | 100 91.94 | [110] |
| Polyamide membrane (NF270) Active area = 0.00076 m2. The surface of the membrane is positively charged for pH less than 4 and negatively charged for a higher value. | Cd(II) Mn(II) Pb(II) | 1000 | 1.5 | 4 | 99 89 74 | [111] |
| Polyamide membrane Active area = 0.47 m2 Isoelectric point is at pH 3.3–4 | Pb(II) Ni(II) | 1 | 5.5 | 6–8 | 86 93 | [112] |
| Polyethersulfone Membrane Area = 0.00001256 m2 Membrane fabricated with 0.5 wt% magnetic graphene based nanocomposites | Cu(II) | 20 | 5 | 4 | 92 | [113] |
| Thin-film composite. Area = 0.024 m2 The isoelectric point is 3.6. Membrane surface is negatively charged | Pb(II) | 150 | 5.8 | 25 | 99 | [114] |
| Polyamide thin film composite membrane MWCO = 400 Zeta potential = −36.8 mV | Cr(VI) As(V) | 0.1 | 8 | 8.18 | (25–95) | [115] |
| NF 300 Active area = 2.5 m2 | As(V) | 0.015 to 0.02 | 5 | 50 | 99.8 | [116] |
| Aromatic polyamide membrane MWCO = (200–300) Water permeability = 2.4 × 10−8 m3/(s.m2.kPa) | As(V) | 0.2 | 7.5 | 10 | >94 | [74] |
| Thin film composite membrane (NF 2) Active area = 0.01 m2 | As(V) | 0.150–0.252 | 7 | 11.76 | (97–100) | [117] |
| NF Hollow fibre membrane MWCO = 520 Da Isoelectric point = 6.6 Pure water flux = 47.5 L/(m2.h) | Ni(II) Cr(VI) Cu(II) | 142.23 121.23 56.55 | 2.31 | 4 | 94.99 95.76 95.33 | [118] |
| Polyamide composite membrane MWCO ranges between 150 and 300 Effective area = 0.00572 m2 | Cu(II) | 230 | 4.5 | 6.89 | 98.1 | [119] |
| Thin-film Composite membrane Area = 0.0036 m2 | Pb(II) | 400 | 3 | 30 | 97.5 | [120] |
| Thin-film composite membrane (AFC 40) Effective area = 0.024 m2 | Co(II) | 100 | 3 | 25 | 97 | [64] |
| NF 300 membrane Effective area = 0.015 m2 | Cd(II) Ni(II) | 5 | 5 | 20 | 97.26 98.90 | [121] |
| Polyamide membrane (NF270) Membrane surface area = 0.0012 m2 Isoelectric point = 3.3 | Cu(II) | 25,000 | (3–10) | 30 | 99.5 | [122] |
| Negatively charged microporous NF, Nanomax50 | Cu(II) | 200 | < 4.5 | 3 | 66 | [123] |
| NF spiral-wound membrane | Cu(II) | 50 | 5 | 3.8 | >95 | [124] |
| Polyamide membrane (NTR 729HF) MWCO = 700 pH 6.5 Effective membrane area = 0.006 m2 | As(V) As(III) | 0.5 | (5–9) | 0.1–5 | 81 57 | [125] |
| Polyamide thin film composite (NF90- 2540) Active surface area = 2.6 m2 MWCO = 200 Da | As(V) | 0.1 | 8 | 6 | >90 | [76] |
| Composite polyamide spiral wound membrane(NFI) Membrane area = 0.75 m2 Pure water permeability = 3.20 L/hm2 bar | Cr(VI) | 1000 | 5–8 | 99 | [73] |
| Characteristics of Membrane | Heavy Metal Targeted | Initial Metal Concentration (mg/L) | Operating Conditions | Pressure (bar) | Removal Efficiency | Ref |
|---|---|---|---|---|---|---|
| TFC spiral wound membrane Active area = 1.95 m2 Allowable operating pH range = 4–11 Max operating temperature = 45 °C Max feed turbidity, NTU = 1 Max feed SDI = 5 | Cu(II) Ni(II) | 500 | Na2EDTA was added as a chelating agent at pH 5 | 5.06 | 99.5 | [126] |
| Disk membranes Polyamide selective layer is supported on the polysulfone layer | Cu(II) | 20–100 | Addition of Sodium dodecyl sulphate increased the removal efficiency | Low pressure | 70–95 | [127] |
| Polyamide thin film composite membrane MWCO = N.A Pure water permeability = 0.75 L m−2 d−1 kPa−1 pH range = 3–10 Zeta potential = −4.5 mV | Cr(VI) As(V) | 0.1 | 8 | 5.13 | >90 | [106] |
| Polyamide membrane Active area = 0.014 m2 | As(V) | 0.1 | 10–40 | 99.75 | [128] | |
| Brackish water membrane Active surface area = 0.014 m2 | As(III) | pH 9.6 | 40 | 99 | [129] | |
| SWHR membrane (Filmtec) | As(V) As(III) | 0.2 | pH 4 pH 9.1 | 10–35 | 96.8 92.5 | [130] |
| Polyamide spiral wound membrane Membrane surface area = 2.5 m2 pH range = (4–11) | Cu(II) Cd(II) | 500 | 13 | 98 99 | [131] | |
| SE and MPF44 NF membranes Active membrane surface area = 0.0028 m2 | Cu(II) | 2 M | 35 | >95 | [67] |
2.4. Fouling of Membranes
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Ihsanullah; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Taseidifar, M.; Ziaee, M.; Pashley, R.M.; Ninham, B.W. Ion flotation removal of a range of contaminant ions from drinking water. J. Environ. Chem. Eng. 2019, 7, 103263. [Google Scholar] [CrossRef]
- Jaafari, J.; Yaghmaeian, K. Optimization of heavy metal biosorption onto freshwater algae (Chlorella coloniales) using response surface methodology (RSM). Chemosphere 2019, 217, 447–455. [Google Scholar] [CrossRef]
- Ahmed, M.J.K.; Ahmaruzzaman, M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Process Eng. 2016, 10, 39–47. [Google Scholar] [CrossRef]
- Patil, D.S.; Chavan, S.M.; Oubagaranadin, J.U.K. A review of technologies for manganese removal from wastewaters. J. Environ. Chem. Eng. 2016, 4, 468–487. [Google Scholar] [CrossRef]
- Aslam, A.; Thomas-Hall, S.R.; Mughal, T.; Zaman, Q.U.; Ehsan, N.; Javied, S.; Schenk, P.M. Heavy metal bioremediation of coal-fired flue gas using microalgae under different CO2 concentrations. J. Environ. Manag. 2019, 241, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Kirkelund, G.M.; Jensen, P.E.; Ottosen, L.M.; Pedersen, K.B. Comparison of two- and three-compartment cells for electrodialytic removal of heavy metals from contaminated material suspensions. J. Hazard. Mater. 2019, 367, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendergast, M.T.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.S.; Baek, K.; Yang, J.W. Crossflow ultrafiltration of surfactant solutions. Desalination 2005, 184, 385–394. [Google Scholar] [CrossRef]
- Mungray, A.A.; Kulkarni, S.V.; Mungray, A.K. Removal of heavy metals from wastewater using micellar enhanced ultrafiltration technique: A review. Cent. Eur. J. Chem. 2012, 10, 27–46. [Google Scholar] [CrossRef]
- Chhatre, A.J.; Marathe, K.V. Dynamic analysis and optimization of surfactant dosage in micellar enhanced ultrafiltration of nickel from aqueous streams. Sep. Sci. Technol. 2006, 41, 2755–2770. [Google Scholar] [CrossRef]
- Nura, C.S.; Chattree, A.; Singh, R.P.; Nath, S. Removal of hexavalent chromium by mixed micelles of cetyl trimethyl ammonium bromide using micellar enhanced ultrafiltration. IJCS 2017, 5, 627–631. [Google Scholar]
- Landaburu-Aguirre, J.; García, V.; Pongrácz, E.; Keiski, R.L. The removal of zinc from synthetic wastewaters by micellar-enhanced ultrafiltration: Statistical design of experiments. Desalination 2009, 240, 262–269. [Google Scholar] [CrossRef]
- Samper, E.; Rodríguez, M.; De la Rubia, M.A.; Prats, D. Removal of metal ions at low concentration by micellar-enhanced ultrafiltration (MEUF) using sodium dodecyl sulfate (SDS) and linear alkylbenzene sulfonate (LAS). Sep. Purif. Technol. 2009, 65, 337–342. [Google Scholar] [CrossRef]
- Ghosh, G.; Bhattacharya, P.K. Hexavalent chromium ion removal through micellar enhanced ultrafiltration. Chem. Eng. J. 2006, 119, 45–53. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Chang, W.S.; Nguyen, N.C.; Chen, S.S.; Chang, H.M. Influence of micelle properties on micellar-Enhanced ultrafiltration for chromium recovery. Water Sci. Technol. 2015, 72, 2045–2051. [Google Scholar] [CrossRef]
- Rafique, R.F.; Lee, S. Micellar Enhanced Ultrafiltration (MEUF) and activated carbon fiber (ACF) hybrid processes for the removal of cadmium from an aqueous solution. Korean Chem. Eng. Res. 2014, 52, 775–780. [Google Scholar] [CrossRef] [Green Version]
- Tortora, F.; Innocenzi, V.; Prisciandaro, M.; Mazziotti di Celso, G.; Vegliò, F. Analysis of membrane performance in Ni and Co removal from liquid wastes by means of micellar-enhanced ultrafiltration. Desalin. Water Treat. 2016, 57, 22860–22867. [Google Scholar] [CrossRef]
- Bahmani, P.; Maleki, A.; Rezaee, R.; Khamforosh, M.; Yetilmezsoy, K.; Dehestani Athar, S.; Gharibi, F. Simultaneous removal of arsenate and nitrate from aqueous solutions using micellar-enhanced ultrafiltration process. J. Water Process Eng. 2019, 27, 24–31. [Google Scholar] [CrossRef]
- Huang, J.H.; Zeng, G.M.; Zhou, C.F.; Li, X.; Shi, L.J.; He, S.B. Adsorption of surfactant micelles and Cd2+/Zn2+ in micellar-enhanced ultrafiltration. J. Hazard. Mater. 2010, 183, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Landaburu-Aguirre, J.; Pongrácz, E.; Perämäki, P.; Keiski, R.L. Micellar-enhanced ultrafiltration for the removal of cadmium and zinc: Use of response surface methodology to improve understanding of process performance and optimisation. J. Hazard. Mater. 2010, 180, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Danis, U.; Aydiner, C. Investigation of process performance and fouling mechanisms in micellar-enhanced ultrafiltration of nickel-contaminated waters. J. Hazard. Mater. 2009, 162, 577–587. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, F.; Zeng, G.; Li, X.; Gu, Y.; Shi, L.; Liu, W.; Shi, Y. Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere 2017, 173, 199–206. [Google Scholar] [CrossRef]
- Verma, S.P.; Sarkar, B. Simultaneous removal of Cd (II) and p-cresol from wastewater by micellar-enhanced ultrafiltration using rhamnolipid: Flux decline, adsorption kinetics and isotherm studies. J. Environ. Manag. 2018, 213, 217–235. [Google Scholar] [CrossRef]
- Innocenzi, V.; Prisciandaro, M.; Tortora, F.; Mazziotti di Celso, G.; Vegliò, F. Treatment of WEEE industrial wastewaters: Removal of yttrium and zinc by means of micellar enhanced ultra filtration. Waste Manag. 2018, 74, 393–403. [Google Scholar] [CrossRef]
- Lee, S.H.; Shrestha, S. Application of micellar enhanced ultrafiltration (MEUF) process for zinc (II) removal in synthetic wastewater: Kinetics and two-parameter isotherm models. Int. Biodeterior. Biodegrad. 2014, 95, 241–250. [Google Scholar] [CrossRef]
- Häyrynen, P.; Landaburu-Aguirre, J.; Pongrácz, E.; Keiski, R.L. Study of permeate flux in micellar-enhanced ultrafiltration on a semi-pilot scale: Simultaneous removal of heavy metals from phosphorous rich real wastewaters. Sep. Purif. Technol. 2012, 93, 59–66. [Google Scholar] [CrossRef]
- Abbasi-Garravand, E.; Mulligan, C.N. Using micellar enhanced ultrafiltration and reduction techniques for removal of Cr(VI) and Cr(III) from water. Sep. Purif. Technol. 2014, 132, 505–512. [Google Scholar] [CrossRef]
- Schwarze, M.; Groß, M.; Moritz, M.; Buchner, G.; Kapitzki, L.; Chiappisi, L.; Gradzielski, M. Micellar enhanced ultrafiltration (MEUF) of metal cations with oleylethoxycarboxylate. J. Membr. Sci. 2015, 478, 140–147. [Google Scholar] [CrossRef]
- Tanhaei, B.; Pourafshari Chenar, M.; Saghatoleslami, N.; Hesampour, M.; Laakso, T.; Kallioinen, M.; Sillanpää, M.; Mänttäri, M. Simultaneous removal of aniline and nickel from water by micellar-enhanced ultrafiltration with different molecular weight cut-off membranes. Sep. Purif. Technol. 2014, 124, 26–35. [Google Scholar] [CrossRef]
- Channarong, B.; Lee, S.H.; Bade, R.; Shipin, O.V. Simultaneous removal of nickel and zinc from aqueous solution by micellar-enhanced ultrafiltration and activated carbon fiber hybrid process. Desalination 2010, 262, 221–227. [Google Scholar] [CrossRef]
- Tanhaei, B.; Pourafshari Chenar, M.; Saghatoleslami, N.; Hesampour, M.; Kallioinen, M.; Sillanpää, M.; Mänttäri, M. Removal of nickel ions from aqueous solution by micellar-enhanced ultrafiltration, using mixed anionic-non-ionic surfactants. Sep. Purif. Technol. 2014, 138, 169–176. [Google Scholar] [CrossRef]
- Li, X.; Zeng, G.M.; Huang, J.H.; Zhang, D.M.; Shi, L.J.; He, S.B.; Ruan, M. Simultaneous removal of cadmium ions and phenol with MEUF using SDS and mixed surfactants. Desalination 2011, 276, 136–141. [Google Scholar] [CrossRef]
- Huang, J.; Shi, L.; Zeng, G.; Li, H.; Huang, H.; Gu, Y.; Shi, Y.; Yi, K.; Li, X. Removal of Cd(Ⅱ) by micellar enhanced ultrafiltration: Role of SDS behaviors on membrane with low concentration. J. Clean. Prod. 2019, 209, 53–61. [Google Scholar] [CrossRef]
- Huang, J.; Li, H.; Zeng, G.; Shi, L.; Gu, Y.; Shi, Y.; Tang, B.; Li, X. Removal of Cd(II) by MEUF-FF with anionic-nonionic mixture at low concentration. Sep. Purif. Technol. 2018, 207, 199–205. [Google Scholar] [CrossRef]
- Li, C.W.; Liu, C.K.; Yen, W.S. Micellar-enhanced ultrafiltration (MEUF) with mixed surfactants for removing Cu(II) ions. Chemosphere 2006, 63, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Ennigrou, D.J.; Sik Ali, M.B.; Dhahbi, M.; Ferid, M. Removal of heavy metals from aqueous solution by polyacrylic acid enhanced ultrafiltration. Desalin. Water Treat. 2015, 56, 2682–2688. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Wang, X.; Huang, W.; Lawless, D.; Feng, X. Removal of heavy metals from water using polyvinylamine by polymer-enhanced ultrafiltration and flocculation. Sep. Purif. Technol. 2016, 158, 124–136. [Google Scholar] [CrossRef]
- Aroua, M.K.; Zuki, F.M.; Sulaiman, N.M. Removal of chromium ions from aqueous solutions by polymer-enhanced ultrafiltration. J. Hazard. Mater. 2007, 147, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.R.; Mao, L.J. Removal of heavy metal ions from aqueous solution by ultrafiltration assisted with copolymer of maleic acid and acrylic acid. Desalination 2013, 329, 78–85. [Google Scholar] [CrossRef]
- Kim, H.J.; Baek, K.; Kim, B.K.; Yang, J.W. Humic substance-enhanced ultrafiltration for removal of cobalt. J. Hazard. Mater. 2005, 122, 31–36. [Google Scholar] [CrossRef]
- Muthumareeswaran, M.R.; Alhoshan, M.; Agarwal, G.P. Ultrafiltration membrane for effective removal of chromium ions from potable water. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Molinari, R.; Poerio, T.; Argurio, P. Selective separation of copper(II) and nickel(II) from aqueous media using the complexation-ultrafiltration process. Chemosphere 2008, 70, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, J.; Qiu, Y.R. Removal of Ni (II) and Cr (III) by complexation-ultrafiltration using rotating disk membrane and the selective separation by shear induced dissociation. Chem. Eng. Process. Process Intensif. 2019, 135, 236–244. [Google Scholar] [CrossRef]
- Sánchez, J.; Espinosa, C.; Pooch, F.; Tenhu, H.; Pizarro, G.D.C.; Oyarzún, D.P. Poly(N,N-dimethylaminoethyl methacrylate) for removing chromium (VI) through polymer-enhanced ultrafiltration technique. React. Funct. Polym. 2018, 127, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Camarillo, R.; Pérez, Á.; Cañizares, P.; de Lucas, A. Removal of heavy metal ions by polymer enhanced ultrafiltration. Batch process modeling and thermodynamics of complexation reactions. Desalination 2012, 286, 193–199. [Google Scholar] [CrossRef]
- Labanda, J.; Khaidar, M.S.; Llorens, J. Feasibility study on the recovery of chromium (III) by polymer enhanced ultrafiltration. Desalination 2009, 249, 577–581. [Google Scholar] [CrossRef]
- Camarillo, R.; Llanos, J.; García-Fernández, L.; Pérez, Á.; Cañizares, P. Treatment of copper (II)-loaded aqueous nitrate solutions by polymer enhanced ultrafiltration and electrodeposition. Sep. Purif. Technol. 2010, 70, 320–328. [Google Scholar] [CrossRef]
- Llanos, J.; Camarillo, R.; Pérez, Á.; Cañizares, P. Polymer supported ultrafiltration as a technique for selective heavy metal separation and complex formation constants prediction. Sep. Purif. Technol. 2010, 73, 126–134. [Google Scholar] [CrossRef]
- Jellouli Ennigrou, D.; Gzara, L.; Ramzi Ben Romdhane, M.; Dhahbi, M. Cadmium removal from aqueous solutions by polyelectrolyte enhanced ultrafiltration. Desalination 2009, 246, 363–369. [Google Scholar] [CrossRef]
- Ennigrou, D.J.; Ben Sik Ali, M.; Dhahbi, M. Copper and Zinc removal from aqueous solutions by polyacrylic acid assisted-ultrafiltration. Desalination 2014, 343, 82–87. [Google Scholar] [CrossRef]
- Lam, B.; Déon, S.; Morin-Crini, N.; Crini, G.; Fievet, P. Polymer-enhanced ultrafiltration for heavy metal removal: Influence of chitosan and carboxymethyl cellulose on filtration performances. J. Clean. Prod. 2018, 171, 927–933. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.; Zhang, Y.; Lawless, D.; Feng, X. Batch process of polymer-enhanced ultrafiltration to recover mercury (II) from wastewater. J. Membr. Sci. 2016, 514, 229–240. [Google Scholar] [CrossRef]
- Chou, Y.H.; Choo, K.H.; Chen, S.S.; Yu, J.H.; Peng, C.Y.; Li, C.W. Copper recovery via polyelectrolyte enhanced ultrafiltration followed by dithionite based chemical reduction: Effects of solution pH and polyelectrolyte type. Sep. Purif. Technol. 2018, 198, 113–120. [Google Scholar] [CrossRef]
- Ennigrou, D.J.; Gzara, L.; Romdhane, M.R.B.; Dhahbi, M. Retention of cadmium ions from aqueous solutions by poly(ammonium acrylate) enhanced ultrafiltration. Chem. Eng. J. 2009, 155, 138–143. [Google Scholar] [CrossRef]
- Cañizares, P.; Pérez, Á.; Llanos, J.; Rubio, G. Preliminary design and optimisation of a PEUF process for Cr(VI) removal. Desalination 2008, 223, 229–237. [Google Scholar] [CrossRef]
- Barakat, M.A.; Schmidt, E. Polymer-enhanced ultrafiltration process for heavy metals removal from industrial wastewater. Desalination 2010, 256, 90–93. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.R.; Zhang, Y.; Lawless, D.; Feng, X. Removal of mercury (II) from wastewater by polyvinylamine-enhanced ultrafiltration. Sep. Purif. Technol. 2015, 154, 1–10. [Google Scholar] [CrossRef]
- Llanos, J.; Pérez, Á.; Cañizares, P. Copper recovery by polymer enhanced ultrafiltration (PEUF) and electrochemical regeneration. J. Membr. Sci. 2008, 323, 28–36. [Google Scholar] [CrossRef]
- Cañizares, P.; Pérez, Á.; Camarillo, R.; Llanos, J.; López, M.L. Selective separation of Pb from hard water by a semi-continuous polymer-enhanced ultrafiltration process (PEUF). Desalination 2007, 206, 602–613. [Google Scholar] [CrossRef]
- Tajuddin, M.H.; Yusof, N.; Wan Azelee, I.; Wan Salleh, W.N.; Ismail, A.F.; Jaafar, J.; Aziz, F.; Nagai, K.; Razali, N.F. Development of copper-aluminum layered double hydroxide in thin film nanocomposite nanofiltration membrane for water purification process. Front. Chem. 2019, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ye, C.C.; An, Q.F.; Wu, J.K.; Zhao, F.Y.; Zheng, P.Y.; Wang, N.X. Nanofiltration membranes consisting of quaternized polyelectrolyte complex nanoparticles for heavy metal removal. Chem. Eng. J. 2019, 359, 994–1005. [Google Scholar] [CrossRef]
- Yaroshchuk, A.E. Non-steric mechanisms of nanofiltration: Superposition of Donnan and dielectric exclusion. Sep. Purif. Technol. 2001, 22–23, 143–158. [Google Scholar] [CrossRef]
- Yaroshchuk, A.E. Dielectric exclusion of ions from membranes. Adv. Colloid Interface Sci. 2000, 85, 193–230. [Google Scholar] [CrossRef]
- Cséfalvay, E.; Pauer, V.; Mizsey, P. Recovery of copper from process waters by nanofiltration and reverse osmosis. Desalination 2009, 240, 132–142. [Google Scholar] [CrossRef]
- Gherasim, C.V.; Hancková, K.; Palarčík, J.; Mikulášek, P. Investigation of cobalt(II) retention from aqueous solutions by a polyamide nanofiltration membrane. J. Membr. Sci. 2015, 490, 46–56. [Google Scholar] [CrossRef]
- Murthy, Z.V.P.; Chaudhari, L.B. Application of nanofiltration for the rejection of nickel ions from aqueous solutions and estimation of membrane transport parameters. J. Hazard. Mater. 2008, 160, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Boricha, A.G.; Murthy, Z.V.P. Preparation, characterization and performance of nanofiltration membranes for the treatment of electroplating industry effluent. Sep. Purif. Technol. 2009, 65, 282–289. [Google Scholar] [CrossRef]
- Gherasim, C.V.; Cuhorka, J.; Mikulášek, P. Analysis of lead(II) retention from single salt and binary aqueous solutions by a polyamide nanofiltration membrane: Experimental results and modelling. J. Membr. Sci. 2013, 436, 132–144. [Google Scholar] [CrossRef]
- Gao, J.; Sun, S.P.; Zhu, W.P.; Chung, T.S. Green modification of outer selective P84 nanofiltration (NF) hollow fiber membranes for cadmium removal. J. Membr. Sci. 2016, 499, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Muthukrishnan, M.; Guha, B.K. Effect of pH on rejection of hexavalent chromium by nanofiltration. Desalination 2008, 219, 171–178. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, C.; Wang, Y.; Fan, W.; Luan, Z. Effects of ion concentration and natural organic matter on arsenic(V) removal by nanofiltration under different transmembrane pressures. J. Environ. Sci. 2013, 25, 302–307. [Google Scholar] [CrossRef]
- Urgun-Demirtas, M.; Benda, P.L.; Gillenwater, P.S.; Negri, M.C.; Xiong, H.; Snyder, S.W. Achieving very low mercury levels in refinery wastewater by membrane filtration. J. Hazard. Mater. 2012, 215–216, 98–107. [Google Scholar] [CrossRef]
- Figoli, A.; Cassano, A.; Criscuoli, A.; Mozumder, M.S.I.; Uddin, M.T.; Islam, M.A.; Drioli, E. Influence of operating parameters on the arsenic removal by nanofiltration. Water Res. 2010, 44, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Murthy, Z.V.P.; Chaudhari, L.B. Separation of binary heavy metals from aqueous solutions by nanofiltration and characterization of the membrane using Spiegler-Kedem model. Chem. Eng. J. 2009, 150, 181–187. [Google Scholar] [CrossRef]
- Koseoglu, H.; Kitis, M. The recovery of silver from mining wastewaters using hybrid cyanidation and high-pressure membrane process. Miner. Eng. 2009, 22, 440–444. [Google Scholar] [CrossRef]
- Zeng, G.; He, Y.; Zhan, Y.; Zhang, L.; Pan, Y.; Zhang, C.; Yu, Z. Novel polyvinylidene fluoride nanofiltration membrane blended with functionalized halloysite nanotubes for dye and heavy metal ions removal. J. Hazard. Mater. 2016, 317, 60–72. [Google Scholar] [CrossRef]
- Zhu, W.P.; Gao, J.; Sun, S.P.; Zhang, S.; Chung, T.S. Poly(amidoamine) dendrimer (PAMAM) grafted on thin film composite (TFC) nanofiltration (NF) hollow fiber membranes for heavy metal removal. J. Membr. Sci. 2015, 487, 117–126. [Google Scholar] [CrossRef]
- Gao, J.; Sun, S.P.; Zhu, W.P.; Chung, T.S. Chelating polymer modified P84 nanofiltration (NF) hollow fiber membranes for high efficient heavy metal removal. Water Res. 2014, 63, 252–261. [Google Scholar] [CrossRef]
- Wang, K.Y.; Chung, T.S. Fabrication of polybenzimidazole (PBI) nanofiltration hollow fiber membranes for removal of chromate. J. Membr. Sci. 2006, 281, 307–315. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, C.; Du, S.; Wang, T.; Luan, Z.; Wang, J.; Hou, D. Fabrication of asymmetric poly (m-phenylene isophthalamide) nanofiltration membrane for chromium(VI) removal. J. Environ. Sci. 2010, 22, 1335–1341. [Google Scholar] [CrossRef]
- Nedzarek, A.; Drost, A.; Harasimiuk, F.B.; Tórz, A. The influence of pH and BSA on the retention of selected heavy metals in the nanofiltration process using ceramic membrane. Desalination 2015, 369, 62–67. [Google Scholar] [CrossRef]
- Sayed, S.; Tarek, S.; Dijkstra, I.; Moerman, C. Optimum operation conditions of direct capillary nanofiltration for wastewater treatment. Desalination 2007, 214, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Zolfaghari, G.; Kargar, M. Nanofiltration and microfiltration for the removal of chromium, total dissolved solids, and sulfate from water. MethodsX 2019, 6, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.P.; Sun, S.P.; Gao, J.; Fu, F.J.; Chung, T.S. Dual-layer polybenzimidazole/polyethersulfone (PBI/PES) nanofiltration (NF) hollow fiber membranes for heavy metals removal from wastewater. J. Membr. Sci. 2014, 456, 117–127. [Google Scholar] [CrossRef]
- Lv, J.; Wang, K.Y.; Chung, T.S. Investigation of amphoteric polybenzimidazole (PBI) nanofiltration hollow fiber membrane for both cation and anions removal. J. Membr. Sci. 2008, 310, 557–566. [Google Scholar] [CrossRef]
- Gao, J.; Sun, S.P.; Zhu, W.P.; Chung, T.S. Polyethyleneimine (PEI) cross-linked P84 nanofiltration (NF) hollow fiber membranes for Pb2+ removal. J. Membr. Sci. 2014, 452, 300–310. [Google Scholar] [CrossRef]
- Qi, Y.; Zhu, L.; Shen, X.; Sotto, A.; Gao, C.; Shen, J. Polythyleneimine-modified original positive charged nanofiltration membrane: Removal of heavy metal ions and dyes. Sep. Purif. Technol. 2019, 222, 117–124. [Google Scholar] [CrossRef]
- Zareei, F.; Hosseini, S.M. A new type of polyethersulfone based composite nanofiltration membrane decorated by cobalt ferrite-copper oxide nanoparticles with enhanced performance and antifouling property. Sep. Purif. Technol. 2019, 226, 48–58. [Google Scholar] [CrossRef]
- Rafieian, F.; Jonoobi, M.; Yu, Q. A novel nanocomposite membrane containing modified cellulose nanocrystals for copper ion removal and dye adsorption from water. Cellulose 2019, 26, 3359–3373. [Google Scholar] [CrossRef]
- Gomes, S.; Cavaco, S.A.; Quina, M.J.; Gando-Ferreira, L.M. Nanofiltration process for separating Cr(III) from acid solutions: Experimental and modelling analysis. Desalination. 2010, 254, 80–89. [Google Scholar] [CrossRef]
- López, J.; Reig, M.; Gibert, O.; Cortina, J.L. Increasing sustainability on the metallurgical industry by integration of membrane nanofiltration processes: Acid recovery. Sep. Purif. Technol. 2019, 226, 267–277. [Google Scholar] [CrossRef]
- López, J.; Gibert, O.; Cortina, J.L. Evaluation of an extreme acid-resistant sulphonamide based nanofiltration membrane for the valorisation of copper acidic effluents. Chem. Eng. J. 2020, 127015. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; González-Melgoza, L.L.; García-Depraect, O. Ongoing progress on novel nanocomposite membranes for the separation of heavy metals from contaminated water. Chemosphere 2021, 270, 129421. [Google Scholar] [CrossRef]
- Shahalamqb, A.M.; Al-harthyb, A.; Al-zawhryb, A. Feed water pretreatment in RO systems in the Middle East. Desalination 2015, 37, 16–24. [Google Scholar]
- Rodrigues Pires da Silva, J.; Merçon, F.; Guimarães Costa, C.M.; Radoman Benjo, D. Application of reverse osmosis process associated with EDTA complexation for nickel and copper removal from wastewater. Desalin. Water Treat. 2016, 57, 19466–19474. [Google Scholar] [CrossRef]
- Petrinic, I.; Korenak, J.; Povodnik, D.; Hélix-Nielsen, C. A feasibility study of ultrafiltration/reverse osmosis (UF/RO)-based wastewater treatment and reuse in the metal finishing industry. J. Clean. Prod. 2015, 101, 292–300. [Google Scholar] [CrossRef]
- Mnif, A.; Bejaoui, I.; Mouelhi, M.; Hamrouni, B. Hexavalent Chromium Removal from Model Water and Car Shock Absorber Factory Effluent by Nanofiltration and Reverse Osmosis Membrane. Int. J. Anal. Chem. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Çimen, A. Removal of chromium from wastewater by reverse osmosis. Russ. J. Phys. Chem. A 2015, 89, 1238–1243. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Zhong, C.-M.; Xu, Z.-L.; Fang, X.-H.; Cheng, L. Treatment of Acid Mine Drainage (AMD) by Ultra-Low-Pressure Reverse Osmosis and Nanofiltration. Environ. Eng. Sci. 2007, 24, 1297–1306. [Google Scholar] [CrossRef]
- Ricci, B.C.; Ferreira, C.D.; Aguiar, A.O.; Amaral, M.C.S. Integration of nanofiltration and reverse osmosis for metal separation and sulfuric acid recovery from gold mining effluent. Sep. Purif. Technol. 2015, 154, 11–21. [Google Scholar] [CrossRef]
- Choi, J.; Im, S.J.; Jang, A. Application of volume retarded osmosis—Low pressure membrane hybrid process for recovery of heavy metals in acid mine drainage. Chemosphere 2019, 232, 264–272. [Google Scholar] [CrossRef]
- Balanyà, T.; Labanda, J.; Llorens, J.; Sabaté, J. Influence of chemical speciation on the separation of metal ions from chelating agents by nanofiltration membranes. Sep. Sci. Technol. 2019, 54, 143–152. [Google Scholar] [CrossRef]
- Wei, X.Z.; Gan, Z.Q.; Shen, Y.J.; Qiu, Z.L.; Fang, L.F.; Zhu, B.K. Negatively-charged nanofiltration membrane and its hexavalent chromium removal performance. J. Colloid Interface Sci. 2019, 553, 475–483. [Google Scholar] [CrossRef]
- Sangeetha, K.; Sudha, P.N.; Faleh, A.A.; Sukumaran, A. Novel chitosan based thin sheet nanofiltration membrane for rejection of heavy metal chromium. Int. J. Biol. Macromol. 2019, 132, 939–953. [Google Scholar] [CrossRef]
- Jia, T.Z.; Lu, J.P.; Cheng, X.Y.; Xia, Q.C.; Cao, X.L.; Wang, Y.; Xing, W.; Sun, S.P. Surface enriched sulfonated polyarylene ether benzonitrile (SPEB) that enhances heavy metal removal from polyacrylonitrile (PAN) thin-film composite nanofiltration membranes. J. Membr. Sci. 2019, 580, 214–223. [Google Scholar] [CrossRef]
- Belkhouche, N.-E.; Merad, N.S.; Mesli, M.; Sefrou, Z. Separation of cobalt and nickel by nanofiltration using a FilmTec membrane. Euro-Mediterr. J. Environ. Integr. 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Al-Rashdi, B.A.M.; Johnson, D.J.; Hilal, N. Removal of heavy metal ions by nanofiltration. Desalination 2013, 315, 2–17. [Google Scholar] [CrossRef]
- Maher, A.; Sadeghi, M.; Moheb, A. Heavy metal elimination from drinking water using nanofiltration membrane technology and process optimization using response surface methodology. Desalination 2014, 352, 166–173. [Google Scholar] [CrossRef]
- Abdi, G.; Alizadeh, A.; Zinadini, S.; Moradi, G. Removal of dye and heavy metal ion using a novel synthetic polyethersulfone nanofiltration membrane modified by magnetic graphene oxide/metformin hybrid. J. Membr. Sci. 2018, 552, 326–335. [Google Scholar] [CrossRef]
- Gherasim, C.V.; Mikulášek, P. Influence of operating variables on the removal of heavy metal ions from aqueous solutions by nanofiltration. Desalination 2014, 343, 67–74. [Google Scholar] [CrossRef]
- Yoon, J.; Amy, G.; Chung, J.; Sohn, J.; Yoon, Y. Removal of toxic ions (chromate, arsenate, and perchlorate) using reverse osmosis, nanofiltration, and ultrafiltration membranes. Chemosphere 2009, 77, 228–235. [Google Scholar] [CrossRef]
- Harisha, R.S.; Hosamani, K.M.; Keri, R.S.; Nataraj, S.K.; Aminabhavi, T.M. Arsenic removal from drinking water using thin film composite nanofiltration membrane. Desalination 2010, 252, 75–80. [Google Scholar] [CrossRef]
- Sen, M.; Manna, A.; Pal, P. Removal of arsenic from contaminated groundwater by membrane-integrated hybrid treatment system. J. Membr. Sci. 2010, 354, 108–113. [Google Scholar] [CrossRef]
- Wei, X.; Kong, X.; Wang, S.; Xiang, H.; Wang, J.; Chen, J. Removal of heavy metals from electroplating wastewater by thin-film composite nanofiltration hollow-fiber membranes. Ind. Eng. Chem. Res. 2013, 52, 17583–17590. [Google Scholar] [CrossRef]
- Ku, Y.; Chen, S.W.; Wang, W.Y. Effect of solution composition on the removal of copper ions by nanofiltration. Sep. Purif. Technol. 2005, 43, 135–142. [Google Scholar] [CrossRef]
- Mehdipour, S.; Vatanpour, V.; Kariminia, H.R. Influence of ion interaction on lead removal by a polyamide nanofiltration membrane. Desalination 2015, 362, 84–92. [Google Scholar] [CrossRef]
- Chaudhari, L.B.; Murthy, Z.V.P. Separation of Cd and Ni from multicomponent aqueous solutions by nanofiltration and characterization of membrane using IT model. J. Hazard. Mater. 2010, 180, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Tanninen, J.; Platt, S.; Weis, A.; Nyström, M. Long-term acid resistance and selectivity of NF membranes in very acidic conditions. J. Membr. Sci. 2004, 240, 11–18. [Google Scholar] [CrossRef]
- Chaabane, T.; Taha, S.; Taleb Ahmed, M.; Maachi, R.; Dorange, G. Removal of copper from industrial effluent using a spiral wound module—Film theory and hydrodynamic approach. Desalination 2006, 200, 403–405. [Google Scholar] [CrossRef]
- Sudilovskiy, P.S.; Kagramanov, G.G.; Kolesnikov, V.A. Use of RO and NF for treatment of copper containing wastewaters in combination with flotation. Desalination 2008, 221, 192–201. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Vigneswaran, S.; Ngo, H.H.; Shon, H.K.; Kandasamy, J. Arsenic removal by a membrane hybrid filtration system. Desalination 2009, 236, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Mohsen-Nia, M.; Montazeri, P.; Modarress, H. Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 2007, 217, 276–281. [Google Scholar] [CrossRef]
- ZHANG, L.; WU, Y.; QU, X.; LI, Z.; NI, J. Mechanism of combination membrane and electro-winning process on treatment and remediation of Cu2+ polluted water body. J. Environ. Sci. 2009, 21, 764–769. [Google Scholar] [CrossRef]
- Abejón, A.; Garea, A.; Irabien, A. Arsenic removal from drinking water by reverse osmosis: Minimization of costs and energy consumption. Sep. Purif. Technol. 2015, 144, 46–53. [Google Scholar] [CrossRef]
- Teychene, B.; Collet, G.; Gallard, H.; Croue, J.P. A comparative study of boron and arsenic (III) rejection from brackish water by reverse osmosis membranes. Desalination 2013, 310, 109–114. [Google Scholar] [CrossRef]
- Akin, I.; Arslan, G.; Tor, A.; Cengeloglu, Y.; Ersoz, M. Removal of arsenate [As(V)] and arsenite [As(III)] from water by SWHR and BW-30 reverse osmosis. Desalination 2011, 281, 88–92. [Google Scholar] [CrossRef]
- Qdais, H.A.; Moussa, H. Removal of heavy metals from wastewater by membrane processes: A comparative study. Desalination 2004, 164, 105–110. [Google Scholar] [CrossRef]
- Shirazi, S.; Lin, C.J.; Chen, D. Inorganic fouling of pressure-driven membrane processes—A critical review. Desalination 2010, 250, 236–248. [Google Scholar] [CrossRef]
- Tu, K.L.; Chivas, A.R.; Nghiem, L.D. Effects of membrane fouling and scaling on boron rejection by nano fi ltration and reverse osmosis membranes. Desalination 2011, 279, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Jafari, M.; Vanoppen, M.; van Agtmaal, J.M.C.; Cornelissen, E.R.; Vrouwenvelder, J.S.; Verliefde, A.; van Loosdrecht, M.C.M.; Picioreanu, C. Cost of fouling in full-scale reverse osmosis and nanofiltration installations in the Netherlands. Desalination. 2021, 500, 114865. [Google Scholar] [CrossRef]
- Lyu, Z.; Ng, T.C.A.; Duc, T.T.; Lim, G.J.H.; Gu, Q.; Zhang, L.; Zhang, Z.; Ding, J.; Thien, N.P.; Wang, J.; et al. 3D-printed surface-patterned ceramic membrane with enhanced performance in crossflow filtration. J. Membr. Sci. 2020, 606, 118138. [Google Scholar] [CrossRef]
- Ng, T.C.A.; Lyu, Z.; Gu, Q.; Zhang, L.; Poh, W.J.; Zhang, Z.; Wang, J.; Ng, H.W. Effect of gradient profile in ceramic membranes on filtration characteristics: Implications for membrane development. J. Membr. Sci. 2020, 595, 117576. [Google Scholar] [CrossRef]
- Zhang, L.; Ng, T.C.A.; Liu, X.; Gu, Q.; Pang, Y.; Zhang, Z.; Lyu, Z.; He, Z.; Ng, H.Y.; Wang, J. Hydrogenated TiO2 membrane with photocatalytically enhanced anti-fouling for ultrafiltration of surface water. Appl. Catal. 2020, 264, 118528. [Google Scholar] [CrossRef]
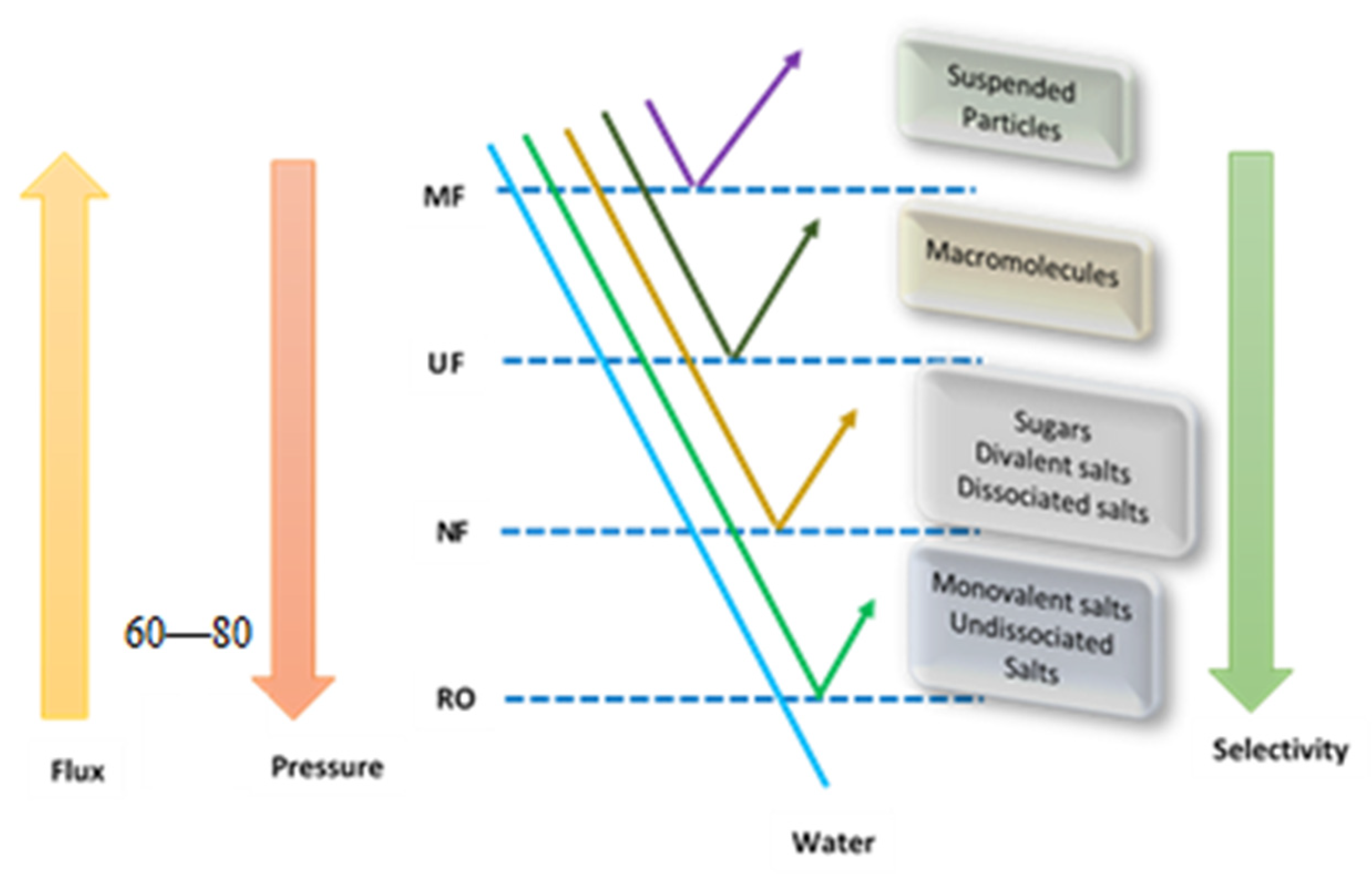
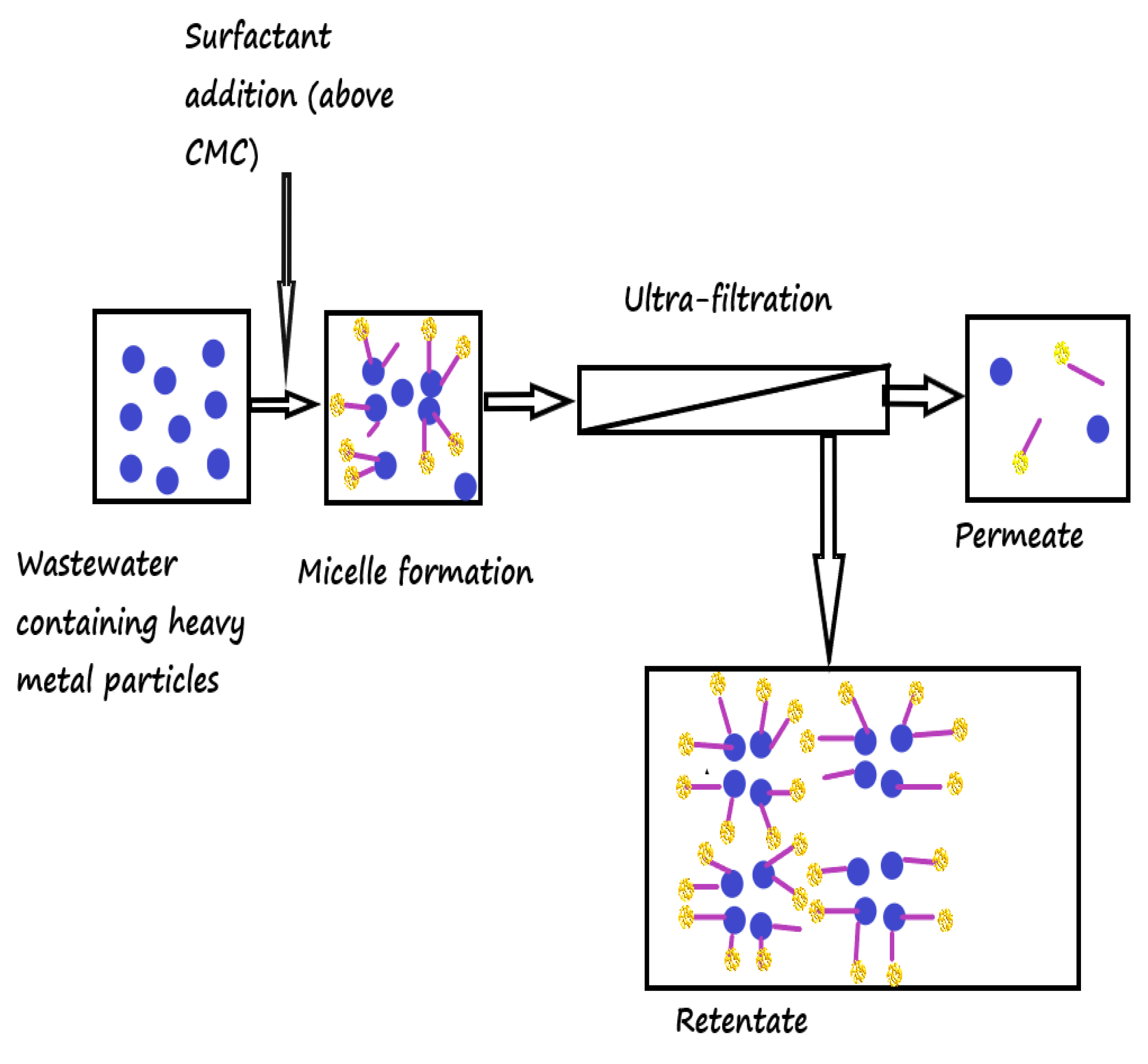
| Membrane Material | Characteristic of Membrane | Heavy Metal Targeted | Surfactant/Complexing Agent Used | Optimum Pressure (Bar) | Surfactant Concentration (mM) | Initial Concentration (mg/L) | pH | % Removal | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Ceramic | MWCO = 210 kDa | Ni(II) Co(II) | Sodium dodecyl sulphate | 2.8 | 0.025 | 10 | 7 | 53 51 | [20] |
| PAN Membrane | Area = 0.00124 m2 | As(V) | Cetyl Pyridinium Chloride (CPC) | 1 | 5 | 1 | 7–8 | 96.9 | [21] |
| Polyether sulphone | MWCO = 6000 g mol−1 Area = 0.3 m2 TMP <= 0.15 MPa | Cd(II) Zn(II) | Sodium dodecyl sulphate | 0.7 | 2.15 | 50 | 92–98 | [22] | |
| Amicon regenerated cellulose | MWCO = 10 kDa | Cd(II) Zn(II) | Sodium dodecyl sulphate | 3 | 13.9 14.2 | 23 | 99 | [23] | |
| Polycarbonate | TMP = 250 kPa | Ni(II) | Sodium lauryl ether sulphate | 2 | 9.2 | 98.6 | [24] | ||
| Polyether sulphone | MWCO = 10 kDa Area = 9.6 cm2 Permeate flux = 150 L.m2/h at 0.35 MPa | Cu(II) Cd(II) Zn(II) Pb(II) | Sodium dodecyl sulphate | 8 | (50–300) | >3 >3 3–10 3–10 | 99 | [25] | |
| Polyether sulphone | MCO = 10 kDa Area = 32.15 × 10−4 m2 | Cd(II) | Rhamnolipid | 2.76 | 8.04 | 60 | 7.8 | 92 | [26] |
| Ceramic | MWCO = 1 kDa | Zn(II) | Sodium dodecyl sulphate | 0.8 | 10 | 2 | 99 | [27] | |
| Polyacrylonitrile (PAN) | MWCO = 300,000 Area = 4.8 m2 | Zn(II) | Sodium dodecyl sulphate | 2 | 0.21 | 19.32 | 7 | 84.67 | [28] |
| Polyether sulphone | MWCO = 10 kDa Area = 1.6 m2 | Cd(II) Cu(II) | Sodium dodecyl sulphate | 3 | 60 | 0.37 0.41 | 85 81 | [29] | |
| Polysulphone | MWCO =10 k Da Area = 0.014 m2 | Cr(VI) Cr(III) | Rhamnolipid | 0.7 | 0.02 | 10 | 6 | 98.7 96.2 | [30] |
| Regenerated Cellulose | MWCO = 10 kDa Are a= 0.0013 m2 | Cu(II) Cd(II) Zn(II) Ni(II) Mg(II) | nonaoxyethylene oleylether carboxylic acid (RO90) | 3 | 30.43 | 920 | 6.5 | >95% | [31] |
| Polysulphone | MWCO = 1 kDa Area = 0.004 m2 | Ni(II) | Sodium dodecyl sulphate | 2.5 | 16 | 10 | 7 | 97% | [32] |
| Polyacrylonitrile (PAN) | MWCO = 100 kDa Area = 0.07 m2 | Ni(II) Zn(II) | Sodium dodecyl sulphate | 1 | 12.75 | 23 | 7 | 96.3 96.7 | [33] |
| Polysulphone | MWCO = 10 kDa Area = 0.004 m2 | Ni(II) | Sodium dodecyl sulphate | 1 | 8 | 10 | 11 | 99 | [34] |
| Polysulphone | MWCO = 10 kDa Area = 0.3 m2 | Cd(II) | Sodium dodecyl sulphate | 0.3 | 8 | 0.45 | 97 | [35] | |
| Polyether sulphone | MWCO = 5 kDa, 10 kDa, 30 kDa Area = 0.00096 m2 | Cd(II) | Sodium dodecyl sulphate | 1 | 4 | 10 | 90 | [36] | |
| Polyether sulphone | MWCO = 8 kDa Area = −0.005 m2 | Cd(II) | Sodium dodecyl sulphate | 7.33 | 50 | 98.4 | [37] | ||
| Hydrophilic | MWCO = 10 kDa | Cu(II) | polyoxyethylene Octyl phenyl ether (Triton-X) plus Sodium dodecyl sulphate | 2.096 | 1.29 5.67 | 9.2 | 5 | 92 | [38] |
| Polyether sulfone | MWCO = 10 kDa Area = 0.003019 m2 | Cd(II) Cu(II) Pb(II) Zn(II) | Sodium dodecyl sulphate | 1 | 9 | 10 | >90 | [16] |
| Membrane | Characteristic of Membrane (MWCO) | Heavy Metal | Surfactant/Complexing Agent Used | Optimum Pressure (bar) | Surfactant Concentration | Initial Concentration (mg/L) | pH | % Removal | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Ceramic | 15 kDa | Cu(II) | Poly (acrylic acid) sodium | 3 | 1 wt% | 160 | 4–5 | 99.5 | [48] |
| Ceramic | 15,000 g/mol | Cr(III) | Polyvinyl alcohol (PVA) | 1 wt% | 92 | 5 | >90% | [49] | |
| Polyether sulphone | 10 kDa | Pb(II) Cu(II) Fe(III) | Polyvinylamine | 2 | 0.1 wt% | 25 | 7 | 99 97 99 | [40] |
| Ceramic | 10 kDa | Cu(II) | Poly (acrylic acid) | 0.4 wt% | 160 | 5.5 | 99.5 | [50] | |
| Ceramic | 10 kDa | Cu(II) Zn(II) | Partially ethoxylated polyethyleneimine (PEPEI) | 3 | 0.06 wt% | 90.62 | 6 | Selectivity ratio Cu(II)/Zn(II) = 12.31 | [51] |
| Polyether sulphone | 10 kDa | Cd(II) | Poly (ammonium acrylate) | 2 | 3.71 × 10−4 mol/L | 46 | 6.32 | 99 | [52] |
| Cellulose | 10 kDa | Cu(II) Zn(II) | Poly (acrylic acid) | 3 | 2 × 10−3 mol/L | 46 | 5 | 97 75 | [53] |
| Thin Film Composite | 3.5 kDa | Ni(II) | Chitosan | 28 | 2 × 10−2 mol/L | 0.072 | 5.4 | 90 | [54] |
| Polyether sulphone | 10 kDa | Hg(II) | Polyvinylamine | 2 | 0.05 wt% | 10 | >90 | [55] | |
| Polyether sulphone | 60 kDa | Cu(II) | Polyethylenimine (PEI) | 1.7 | 25 mM | 230 | 3 | 94 | [56] |
| Polysulphone | 8 kDa, 15 kDa | Cd(II) | Poly(ammonium) acrylate | 2 | 46 | 4 | 98 | [57] | |
| Ceramic | 10 kDa | Cr(VI) | poly(diallyldimethylammonium chloride) (PDADMAC) | 4 | 0.1 wt% | 50 | 9 | 99 | [58] |
| Polyethersulphone | 10 kDa | Cu(II) Ni(II) Cr(III) | Carboxy methyl cellulose | 1 | 1 g/L | 10 | 7 | 97.6 99.1 99.5 | [59] |
| Polyether sulphone | 10 kDa | Hg(II) | Polyvinylamine | 2 | 0.1 wt% | 10 | 6–7 | 99 | [60] |
| Ceramic | 10 kDa | Cu(II) | Partially ethoxylated polyethylenimine (PEPEI) | 4 | 0.06 wt% | 208 mg Cu/g PEPEI | 6 | 97 | [61] |
| Ceramic | 10 kDa | Pb(II) | Poly(acrylic) acid (PAA) | 4 | 0.036% | 100 | 6 | 100 | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, B.; Balomajumder, C.; Sabapathy, M.; Gumfekar, S.P. Pressure-Driven Membrane Process: A Review of Advanced Technique for Heavy Metals Remediation. Processes 2021, 9, 752. https://doi.org/10.3390/pr9050752
Verma B, Balomajumder C, Sabapathy M, Gumfekar SP. Pressure-Driven Membrane Process: A Review of Advanced Technique for Heavy Metals Remediation. Processes. 2021; 9(5):752. https://doi.org/10.3390/pr9050752
Chicago/Turabian StyleVerma, Bharti, Chandrajit Balomajumder, Manigandan Sabapathy, and Sarang P. Gumfekar. 2021. "Pressure-Driven Membrane Process: A Review of Advanced Technique for Heavy Metals Remediation" Processes 9, no. 5: 752. https://doi.org/10.3390/pr9050752
APA StyleVerma, B., Balomajumder, C., Sabapathy, M., & Gumfekar, S. P. (2021). Pressure-Driven Membrane Process: A Review of Advanced Technique for Heavy Metals Remediation. Processes, 9(5), 752. https://doi.org/10.3390/pr9050752







