Kinetics on Chromium-Bearing Vanadia-Titania Magnetite Smelting with High-Basicity Pellet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
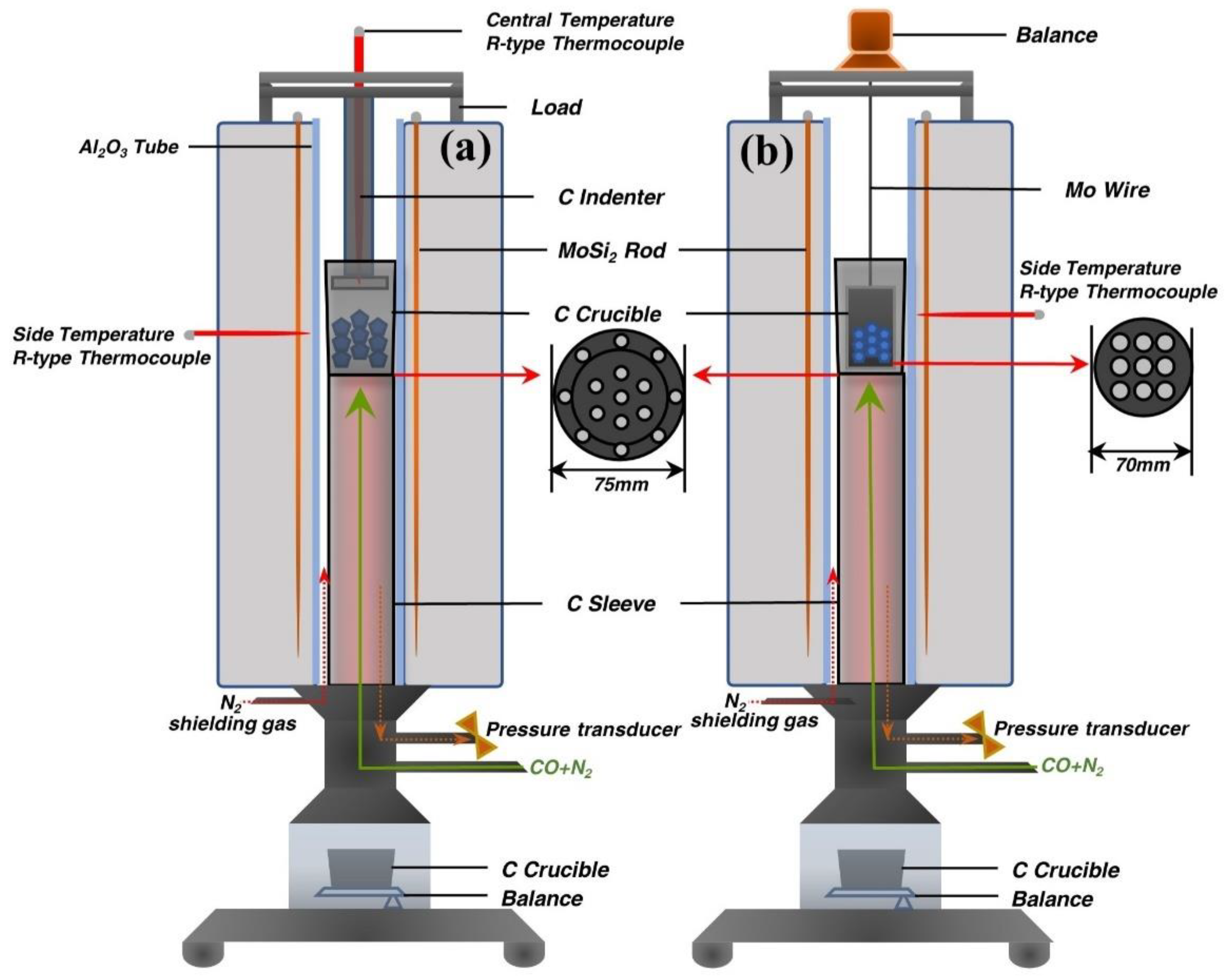
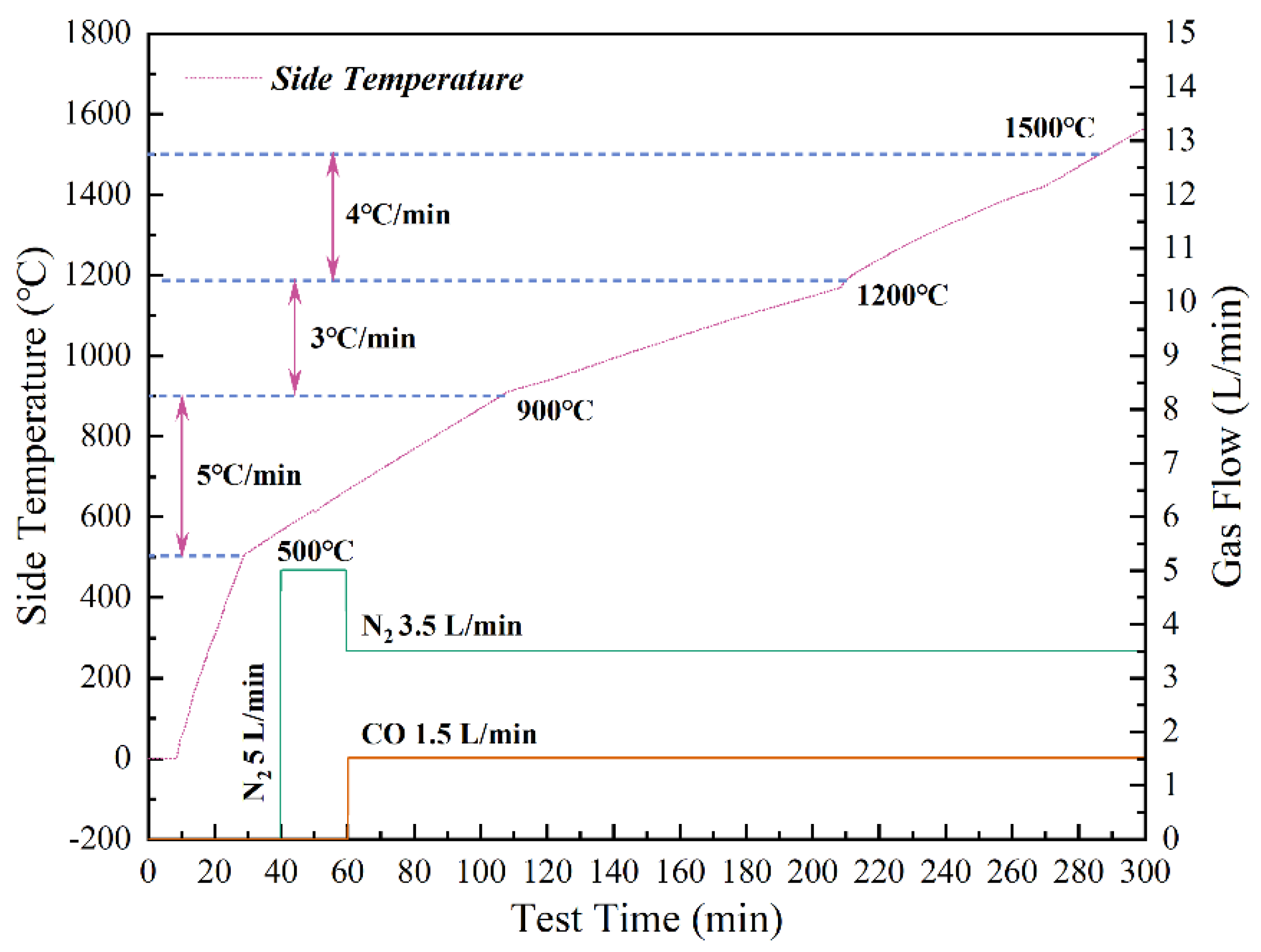
2.2. Apparatus and Methods
2.3. Characterizations
3. Results and Discussion
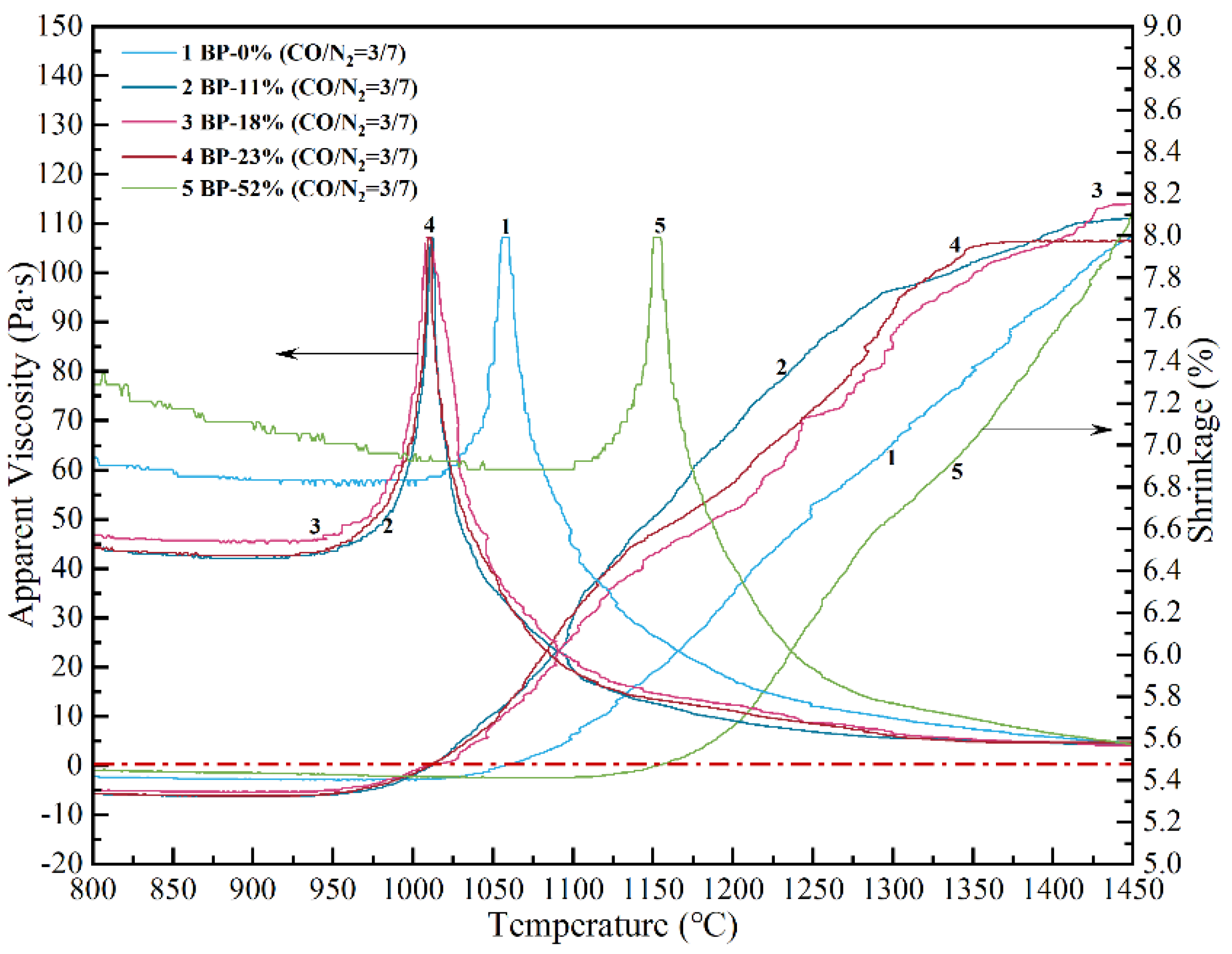
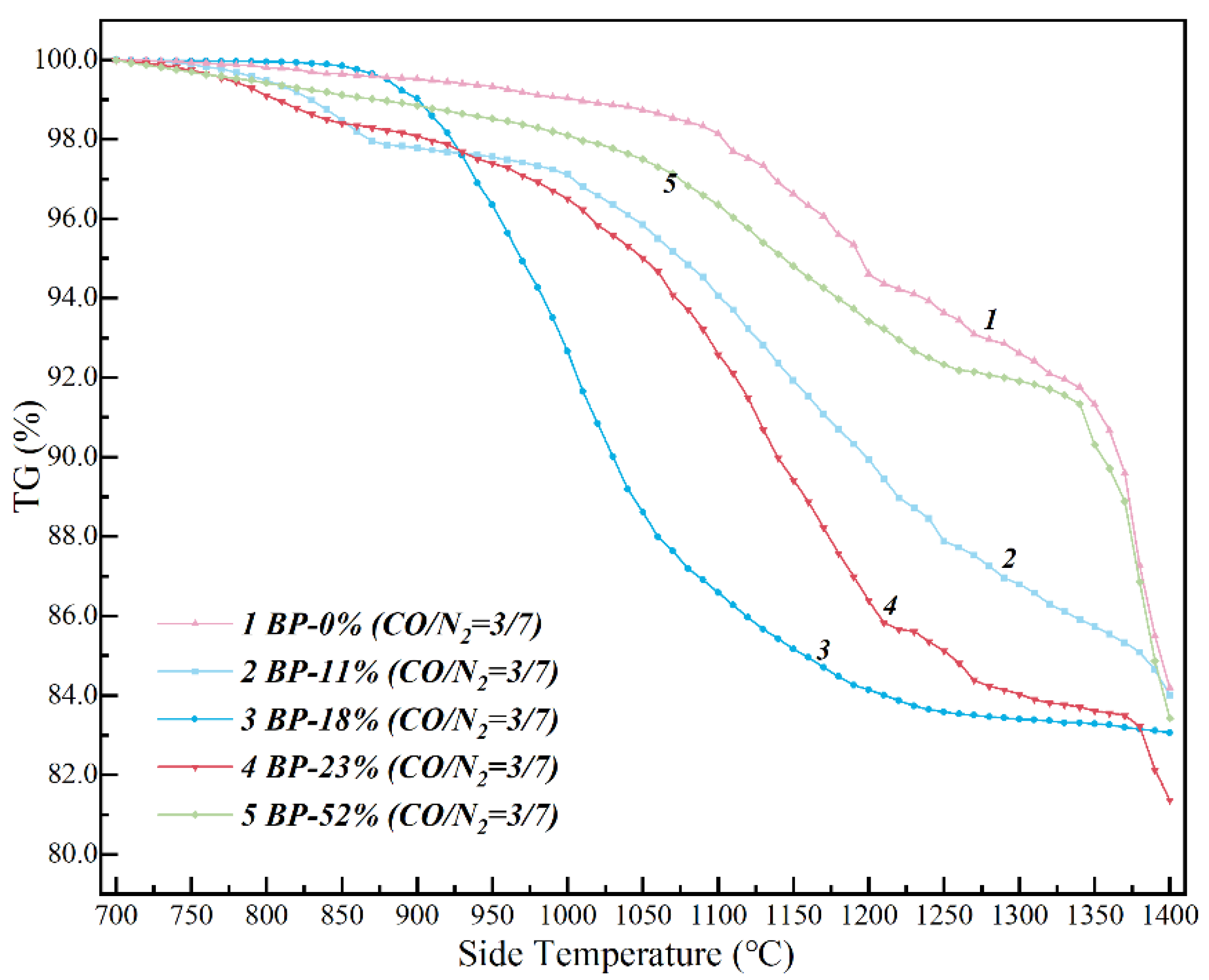
3.1. Effect of High-Basicity Pellet in the Thermodynamic Smelting Process
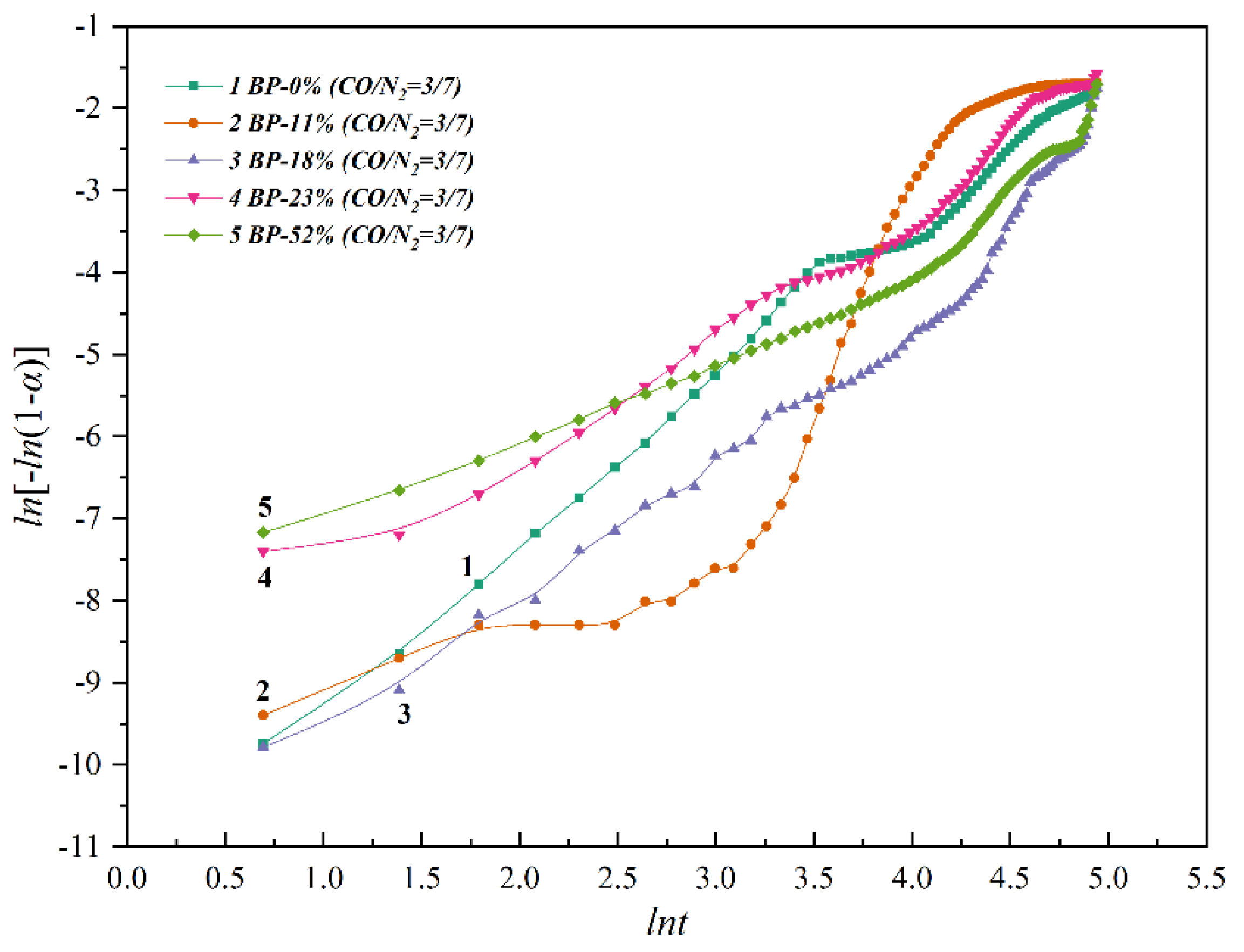
3.2. Non-Isothermal Kinetics of High-Basicity Pellet in the Smelting Process
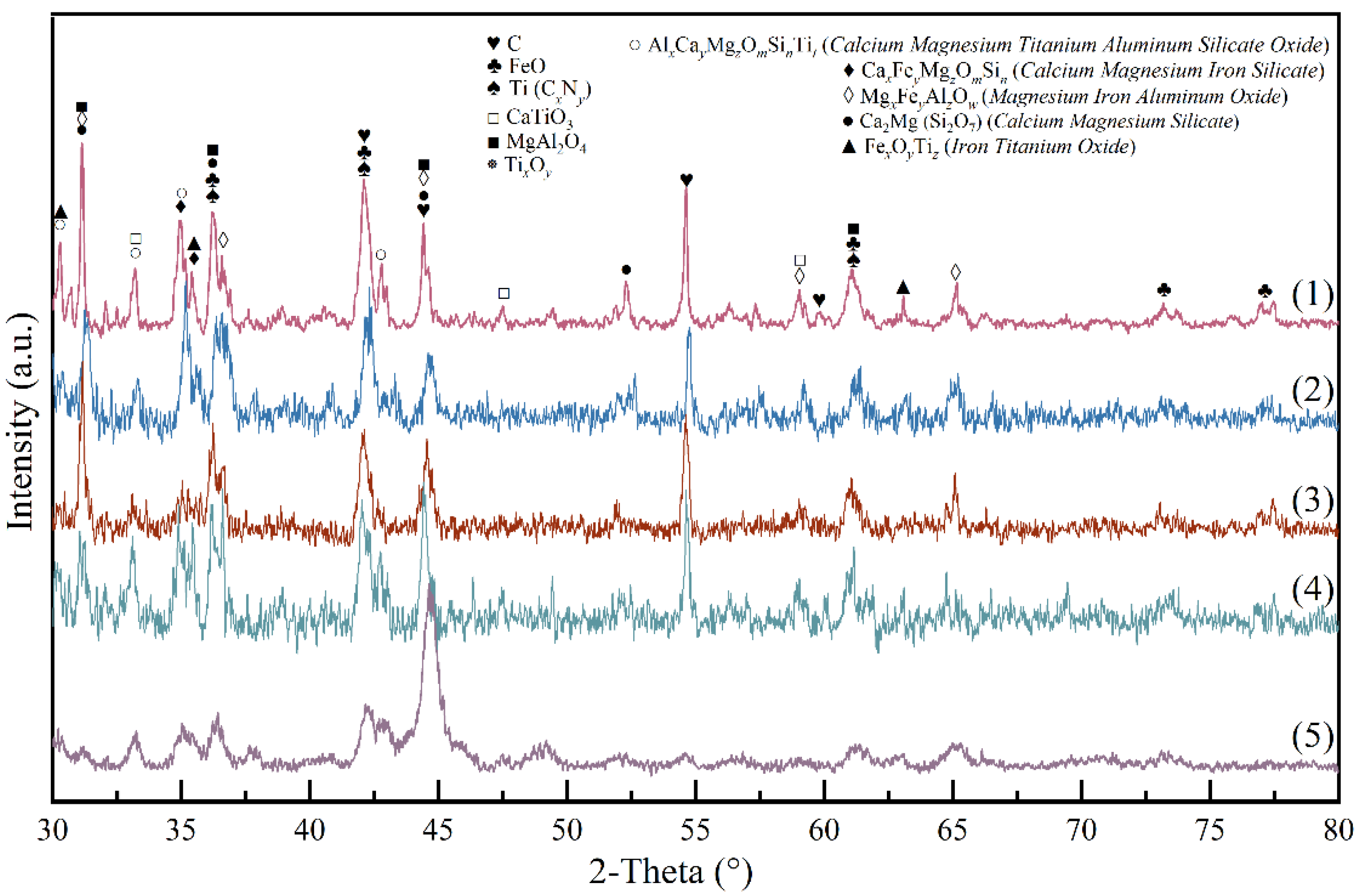
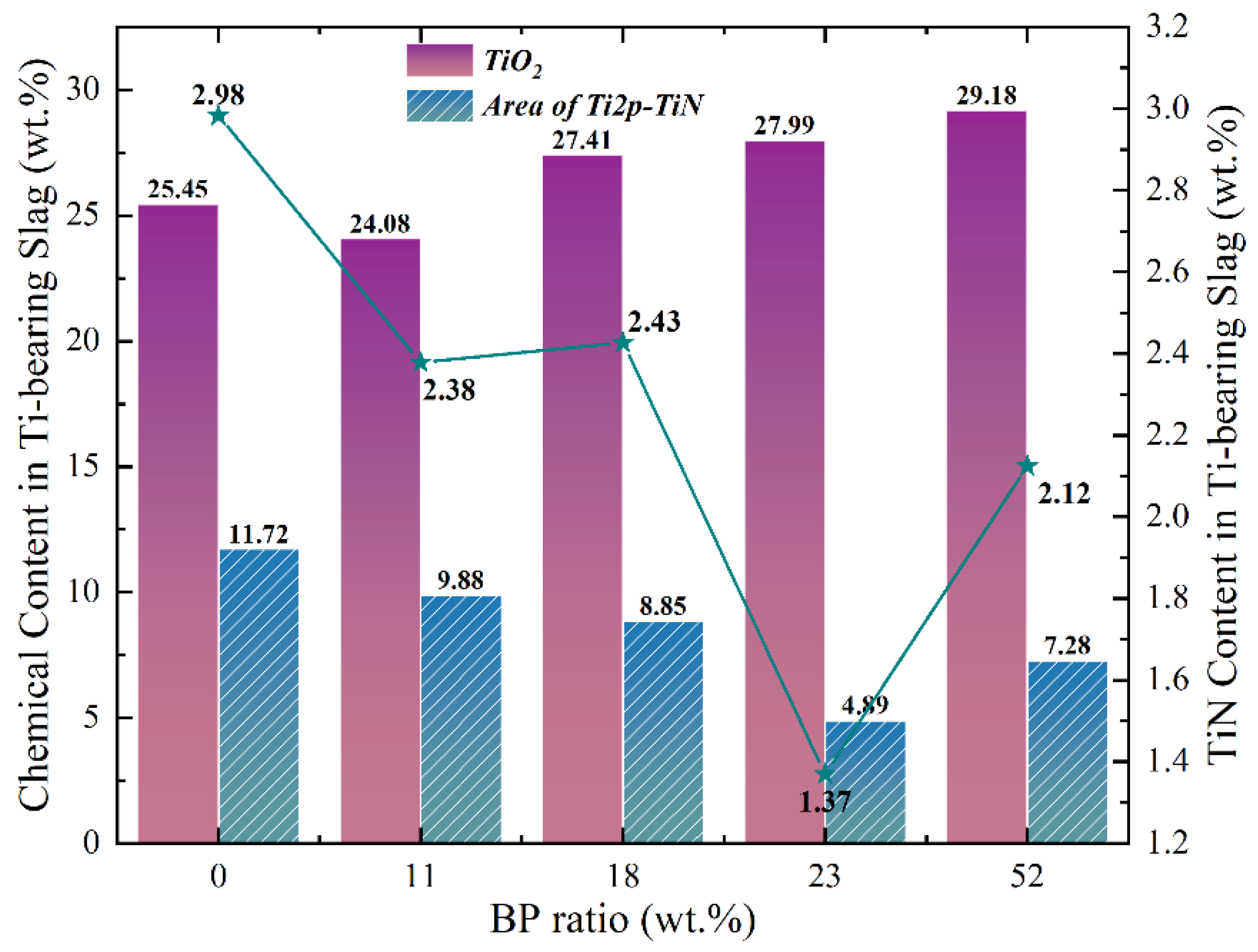
3.3. Reaction Mechanism of High-Basicity Pellet in the Smelting Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, H.G. Principle of Smelting Vanadium-Titanium Magnetite, 1st ed.; Science Press: Beijing, China, 1996. [Google Scholar]
- Xue, X.Y.; Yang, S.T.; Zhang, Y. Blast Furnace Process Smelting Chromium-Containing Vanadia-Titania Magnetite: Theory and Practice, 1st ed.; Science Press: Beijing, China, 2020. [Google Scholar]
- Cheng, G.J.; Xue, X.X.; Jiang, T.; Duan, P.N. Effect of TiO2 on the Crushing Strength and Smelting Mechanism of High-Chromium Vanadium-Titanium Magnetite Pellets. Metall. Mater. Trans. B 2016, 47, 1713–1726. [Google Scholar] [CrossRef]
- Li, T.L.; Sun, C.Y.; Lan, D.; Song, J.; Song, S.; Wang, Q. Effect of Mineral Elements Migration on Softening-melting Properties of Ti-bearing High Basicity Sinter. ISIJ Int. 2019, 59, 245–252. [Google Scholar] [CrossRef]
- Liu, J.X.; Cheng, G.J.; Liu, Z.G.; Chu, M.S.; Xue, X.X. Softening and melting properties of different burden structures containing high chromic vanadium titano-magnetite. Int. J. Miner. Process. 2015, 142, 113–118. [Google Scholar] [CrossRef]
- Yue, H.R.; He, Z.W.; Jiang, T.; Duan, P.N.; Xue, X.X. Rheological Evolution of Ti-Bearing Slag with Different Volume Fractions of TiN. Metall. Mater. Trans. B 2018, 49, 2118–2127. [Google Scholar] [CrossRef]
- Halli, P.; Taskinen, P.; Eric, R.H. Mechanisms and Kinetics of Solid State Reduction of Titano Magnetite Ore with Methane. J. Sustain. Metall. 2017, 3, 191–206. [Google Scholar] [CrossRef]
- Liu, S.S.; Guo, Y.F.; Qiu, G.Z.; Jiang, T.; Chen, F. Solid-state reduction kinetics and mechanism of pre-oxidized vanadium-titanium magnetite concentrate. Trans. Nonferrous Met.. Soc. 2014, 24, 3372–3377. [Google Scholar] [CrossRef]
- Lu, C.Y.; Zou, X.L.; Lu, X.G.; Xie, X.L.; Zheng, K.; Xiao, W.; Cheng, H.W.; Li, G.S. Reductive kinetics of Panzhihua ilmenite with hydrogen. Trans. Nonferrous Met.. Soc. 2016, 26, 3266–3273. [Google Scholar] [CrossRef]
- Lv, W.; Lv, X.W.; Zhang, Y.Y.; Li, S.P.; Tang, K.; Song, B. Isothermal oxidation kinetics of ilmenite concentrate powder from Panzhihua in air. Powder Technol. 2017, 320, 239–248. [Google Scholar] [CrossRef]
- Pan, F.; Zhu, Q.S.; Du, Z.; Sun, H.Y. Oxidation Kinetics, Structural Changes and Element Migration during Oxidation Process of Vanadium-titanium Magnetite Ore. J. Iron Steel Res. Int. 2016, 23, 1160–1167. [Google Scholar] [CrossRef]
- Sarkar, B.K.; Samanta, S.; Dey, R.; Das, G.C. A study on reduction kinetics of titaniferous magnetite ore using lean grade coal. Int. J. Miner. Process. 2016, 152, 36–45. [Google Scholar] [CrossRef]
- Sui, Y.L.; Guo, Y.F.; Jiang, T.; Qiu, G.Z. Reduction kinetics of oxidized vanadium titano-magnetite pellets using carbon monoxide and hydrogen. J. Alloys Compd. 2017, 706, 546–553. [Google Scholar] [CrossRef]
- Zhang, J.L.; Xing, X.D.; Cao, M.M.; Jiao, K.X.; Wang, C.L.; Ren, S. Reduction Kinetics of Vanadium Titano-Magnetite Carbon Composite Pellets Adding Catalysts Under High Temperature. J. Iron Steel Res. Int. 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Liu, Z.G.; Chu, M.S.; Wang, H.T.; Zhao, W.; Xue, X.X. Effect of MgO content in sinter on the softening-melting behavior of mixed burden made from chromium-bearing vanadium-titanium magnetite. Int. J. Min. Met. Mater. 2016, 23, 25–32. [Google Scholar] [CrossRef]
- Zhao, W.; Chu, M.S.; Wang, H.T.; Liu, Z.G.; Tang, Y.T. Novel blast furnace operation process involving charging with low-titanium vanadium-titanium magnetite carbon composite hot briquette. Int. J. Min. Met. Mater. 2016, 23, 501–510. [Google Scholar] [CrossRef]
- Song, H.L.; Zhang, J.P.; Cheng, G.J.; Yang, S.T.; Xue, X.X. The Effect of Abandoned Basic Oxygen Furnace Gas Blowing on the Softening-Melting-Dripping Performance of Full High Cr-Bearing Vanadia-Titania Magnetite Pellets. Steel Res. Int. 2020, 91, 1900501. [Google Scholar] [CrossRef]
- Song, H.L.; Zhang, J.P.; Cheng, G.J.; Yang, S.T.; Xue, X.X. CO2 injection improves the high-temperature performances of Cr-bearing vanadia-titania magnetite smelting in blast furnace. J. CO2 Util. 2021, 43, 101363. [Google Scholar] [CrossRef]
- An, X.W.; Wang, J.S.; Lan, R.Z.; Han, Y.H.; Xue, Q.G. Softening and Melting Behavior of Mixed Burden for Oxygen Blast Furnace. J. Iron Steel Res. Int. 2013, 20, 11–16. [Google Scholar] [CrossRef]
- Nandy, B.; Chandra, S.; Bhattacharjee, D.; Ghosh, D. Assessment of blast furnace behaviour through softening-melting test. Ironmak. Steelmak. 2006, 33, 111–119. [Google Scholar] [CrossRef]
- Qie, Y.N.; Lyu, Q.; Liu, X.J.; Li, J.P.; Lan, C.C.; Zhang, S.H.; Yan, C.J. Effect of Hydrogen Addition on Softening and Melting Reduction Behaviors of Ferrous Burden in Gas-Injection Blast Furnace. Metall. Mater. Trans. B 2018, 49, 2622–2632. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, F.; Wang, J.; Yan, Z.M.; Pei, G.S.; Qiu, G.B.; Lv, X.W. Effect of Cr2O3 content on viscosity and phase structure of chromium-containing high-titanium blast furnace slag. J. Mater. Res. Technol. 2020, 9, 12673–12681. [Google Scholar] [CrossRef]
- Liu, X.L.; Honeyands, T.; Evans, G.; Zulli, P.; O’Dea, D. A review of high-temperature experimental techniques used to investigate the cohesive zone of the ironmaking blast furnace. Ironmak. Steelmak. 2019, 46, 953–967. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Wang, J.; Li, Q.J.; Shan, C.; Qiu, G.B.; Yu, W.Z.; Lv, X.W. Slag-foaming phenomenon originating from reaction of titanium-bearing blast furnace slag: Effects of TiO2 content and basicity. Can. Metall. Quart. 2020, 59, 151–158. [Google Scholar] [CrossRef]
- Hu, K.; Tang, K.; Lv, X.W.; Safarian, J.; Yan, Z.M.; Song, B. Modeling Viscosity of High Titania Slag. Metall. Mater. Trans. B 2021, 52, 245–254. [Google Scholar] [CrossRef]
- Li, W.; Wang, N.; Fu, G.Q.; Chu, M.S.; Zhu, M.Y. Influence of TiO2 addition on the oxidation induration and reduction behavior of Hongge vanadium titanomagnetite pellets with simulated shaft furnace gases. Powder Technol. 2018, 326, 137–145. [Google Scholar] [CrossRef]
- Zhao, L.S.; Wang, L.N.; Chen, D.S.; Zhao, H.X.; Liu, Y.H.; Qi, T. Behaviors of vanadium and chromium in coal-based direct reduction of high-chromium vanadium-bearing titanomagnetite concentrates followed by magnetic separation. Trans. Nonferrous Met. Soc. 2015, 25, 1325–1333. [Google Scholar] [CrossRef]
- Malinauskaite, J.; Jouhara, H.; Czajczynska, D.; Stanchev, P.; Katsou, E.; Rostkowski, P.; Thorne, R.J.; Colon, J.; Ponsa, S.; Al-Mansour, F.; et al. Municipal solid waste management and waste-to-energy in the context of a circular economy and energy recycling in Europe. Energy 2017, 141, 2013–2044. [Google Scholar] [CrossRef]
- Li, H.T.; Zhang, H.; Li, K.J.; Zhang, J.L.; Sun, M.M.; Su, B.X. Catalytic graphitization of coke carbon by iron: Understanding the evolution of carbon Structure, morphology and lattice fringes. Fuel 2020, 279, 118531. [Google Scholar] [CrossRef]







| CVTM | TFe | CaO | MgO | SiO2 | Al2O3 | TiO2 | V2O5 | Cr2O3 |
|---|---|---|---|---|---|---|---|---|
| Concentrate fines | 53.350 | 0.960 | 3.330 | 4.710 | 2.820 | 11.600 | 1.143 | 0.810 |
| BS (R = 1.90) | 46.669 | 9.540 | 3.324 | 5.016 | 2.920 | 10.145 | 0.975 | 1.003 |
| BP (R = 2.01) | 48.346 | 9.380 | 3.044 | 4.675 | 2.672 | 10.512 | 1.035 | 0.734 |
| AP (R = 0.19) | 52.822 | 0.991 | 3.326 | 5.108 | 2.920 | 11.485 | 1.131 | 0.802 |
| Conditions | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Slope | 1.9298 | 1.8773 | 2.5804 | 1.5929 | 1.3347 |
| Intercept | −12.087 | −10.953 | −13.871 | −9.5373 | −9.0386 |
| R2 | 0.9603 | 0.9906 | 0.893 | 0.9751 | 0.9397 |
| Type | G(α) | n |
|---|---|---|
| D1 | α2 = kt | 0.62 |
| D2 | (1 − α) ln(1 − α) + α = kt | 0.57 |
| D3 | [1 − (1 − α)1/3]2 = kt | 0.54 |
| D4 | (1 − 2/3α) − (1 − α)2/3 = kt | 0.57 |
| CG2 | 1 − (1 − α)1/2 = kt | 1.11 |
| CG3 | 1 − (1 − α)1/3 = kt | 1.07 |
| R1 | −ln(1 − α) = kt | 1.00 |
| A2 | [−ln(1 − α)]1/2 = kt | 2.00 |
| A3 | [−ln(1 − α)]1/3 = kt | 3.00 |
| Conditions | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Slope | −12017 | −10800 | −10217 | −9445 | −7786 |
| Intercept | −9.47 | −9.47 | −9.46 | −10.23 | −12.05 |
| R2 | 0.94 | 0.81 | 0.84 | 0.91 | 0.96 |
| Ea | 99.91 | 89.79 | 84.94 | 78.53 | 64.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Zhang, J.; Xue, X. Kinetics on Chromium-Bearing Vanadia-Titania Magnetite Smelting with High-Basicity Pellet. Processes 2021, 9, 811. https://doi.org/10.3390/pr9050811
Song H, Zhang J, Xue X. Kinetics on Chromium-Bearing Vanadia-Titania Magnetite Smelting with High-Basicity Pellet. Processes. 2021; 9(5):811. https://doi.org/10.3390/pr9050811
Chicago/Turabian StyleSong, Hanlin, Jinpeng Zhang, and Xiangxin Xue. 2021. "Kinetics on Chromium-Bearing Vanadia-Titania Magnetite Smelting with High-Basicity Pellet" Processes 9, no. 5: 811. https://doi.org/10.3390/pr9050811
APA StyleSong, H., Zhang, J., & Xue, X. (2021). Kinetics on Chromium-Bearing Vanadia-Titania Magnetite Smelting with High-Basicity Pellet. Processes, 9(5), 811. https://doi.org/10.3390/pr9050811





