Extraction and Quality Evaluation of Biodiesel from Six Familiar Non-Edible Plants Seeds †
Abstract
1. Introduction
2. Description of Plant Sources for Biodiesel Feedstock
2.1. Sapindus mukorossi (Soapnut, SP)
2.2. Verniciafordii (Tung, TO)
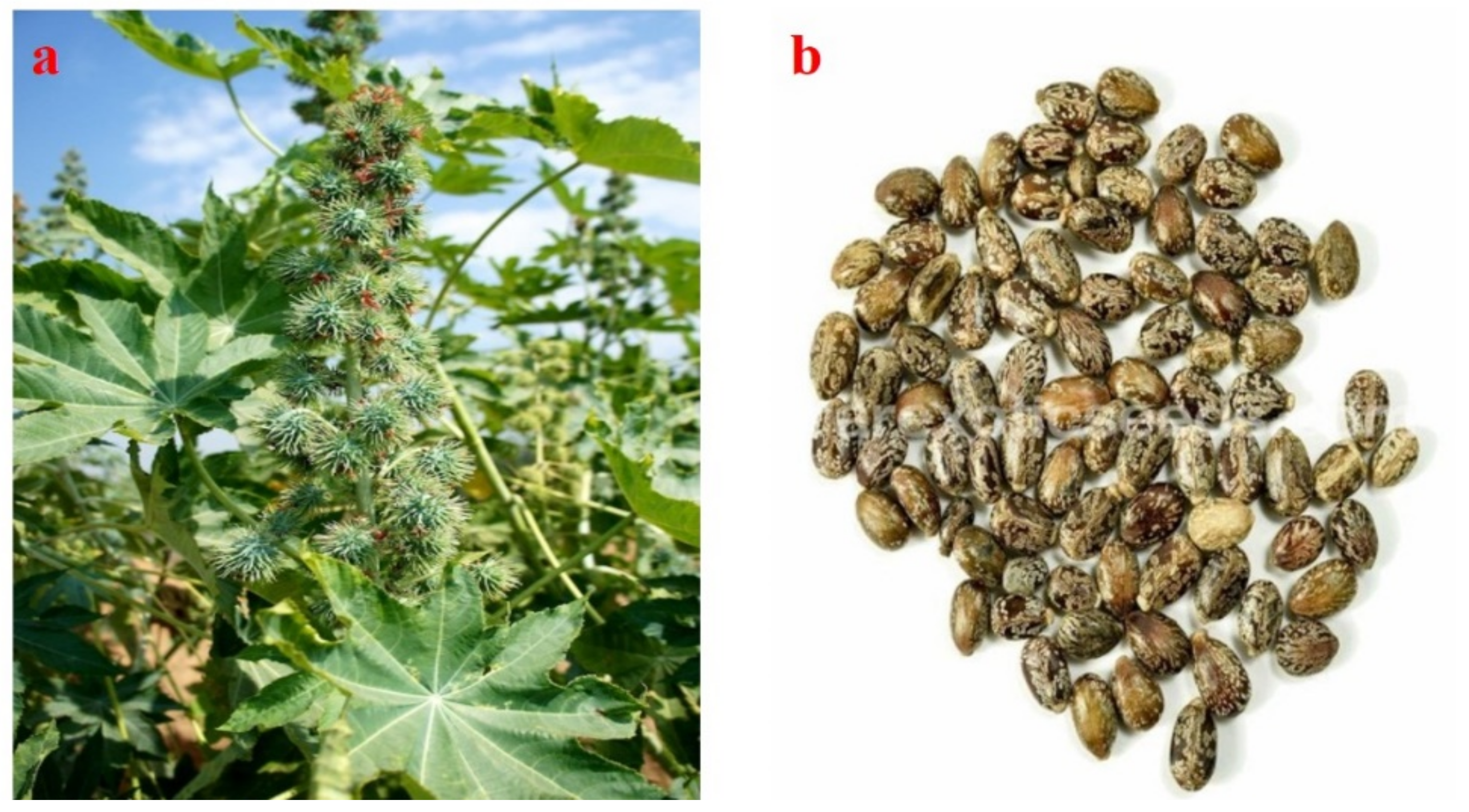
2.3. Ricinus communis (Castor, CA)
2.4. Toona sinensis (Juss.,TS)
2.5. Ailanthus altissima (Heaven Tree, AA)
2.6. Linum usitatissimum L. (Linseed, LS)
3. Materials and Methods
3.1. Plant Materials
3.2. Preparation of Seeds for Feed Stocking
3.3. Oil Extraction
3.4. Evaluation of Potential of the Six Plants as Biodiesel Resources
3.5. Acid-Catalyzed Esterification Process
3.6. Base-Catalyzed Transesterification Process
3.7. Fourier Transform Infrared (FT-IR) Study
3.8. Nuclear Magnetic Resonance (NMR) Study
3.9. Gas Chromatography–Mass Spectrometry (GC-MS) Study
3.10. Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) and Elemental Analysis Study of Biodiesel
4. Results and Discussion
4.1. Process of Variables/Optimization
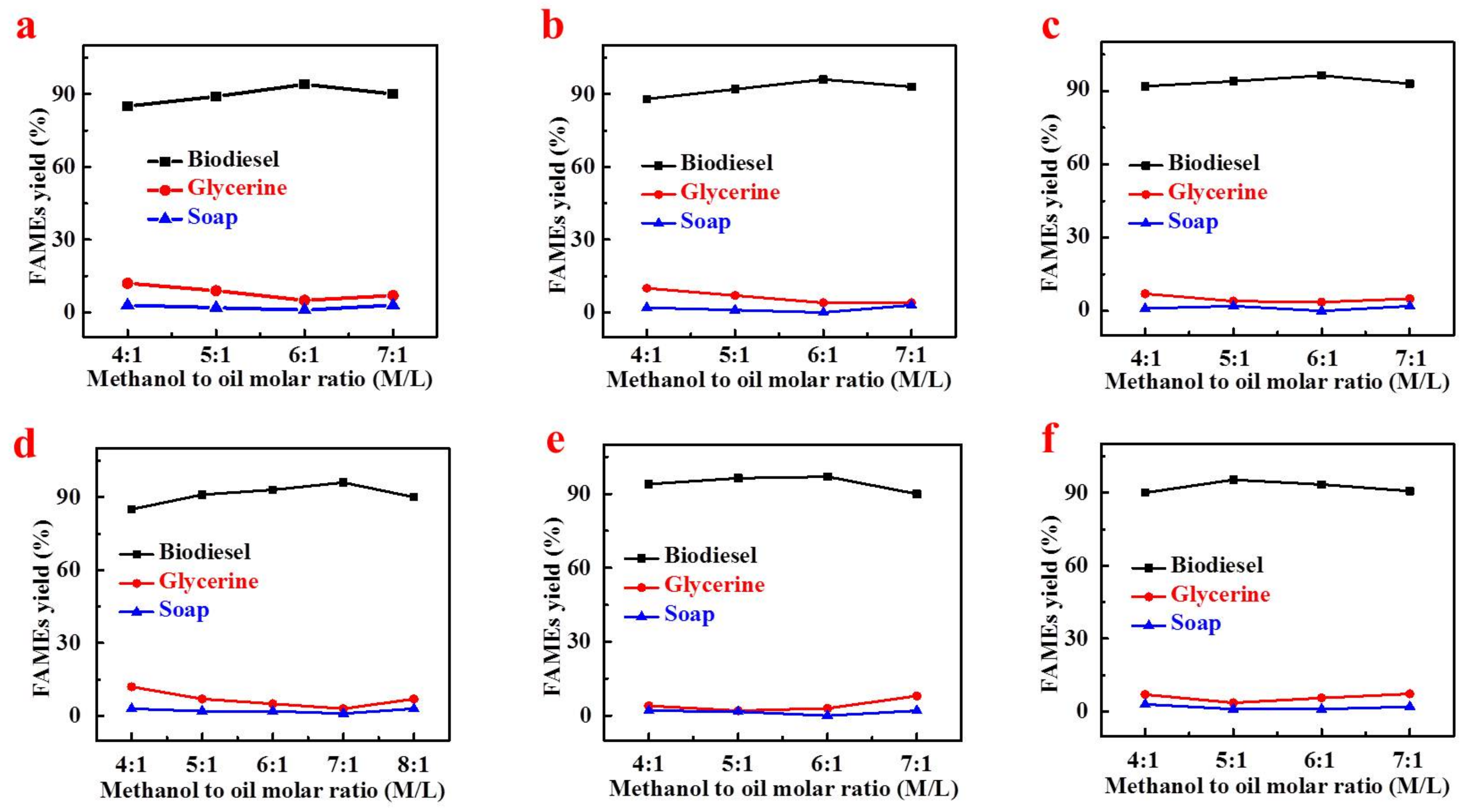
4.1.1. Effect of Methanol to Oil Molar Ratio on FAMEs Yield
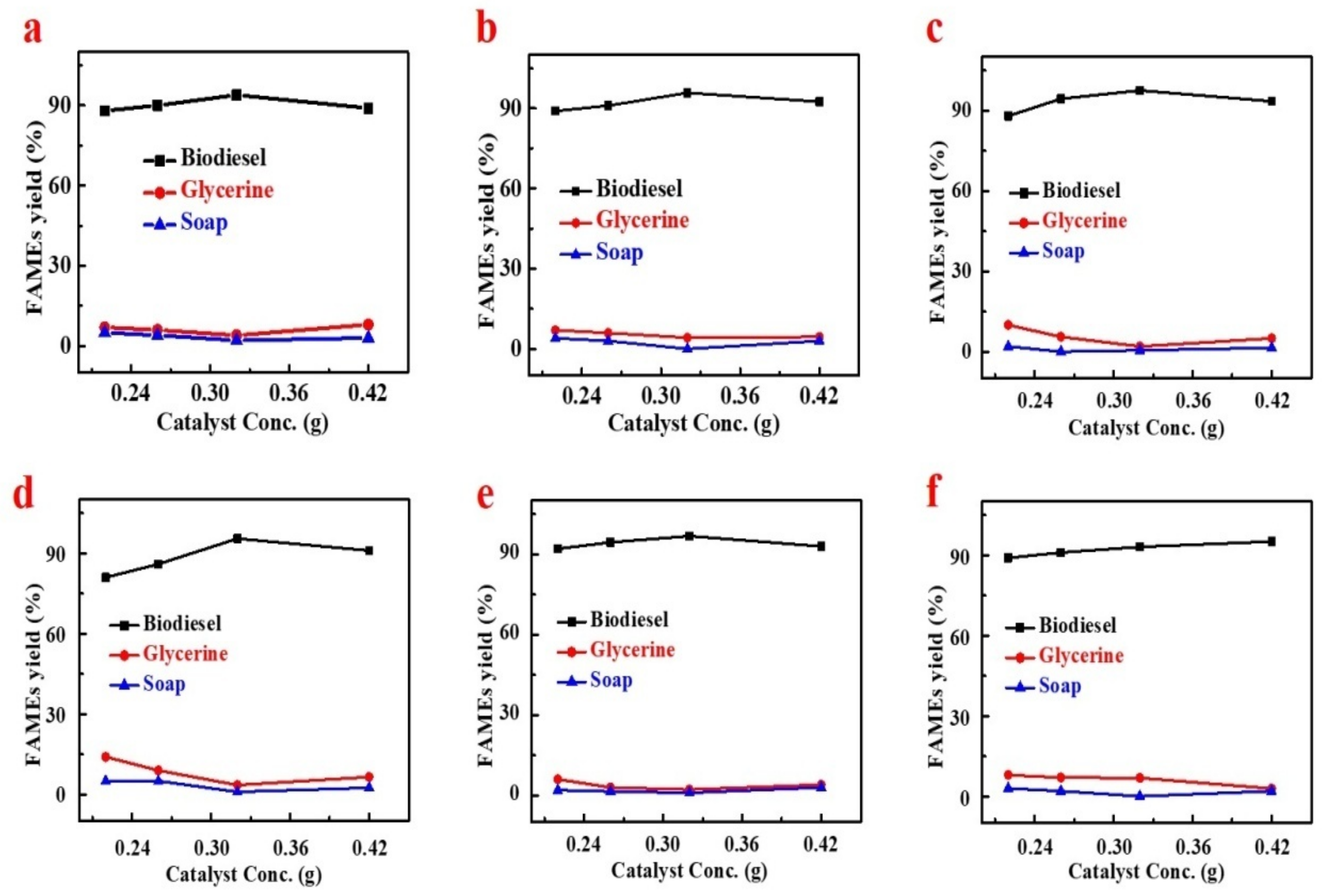
4.1.2. Effect of Catalyst Concentration on FAMEs Yield
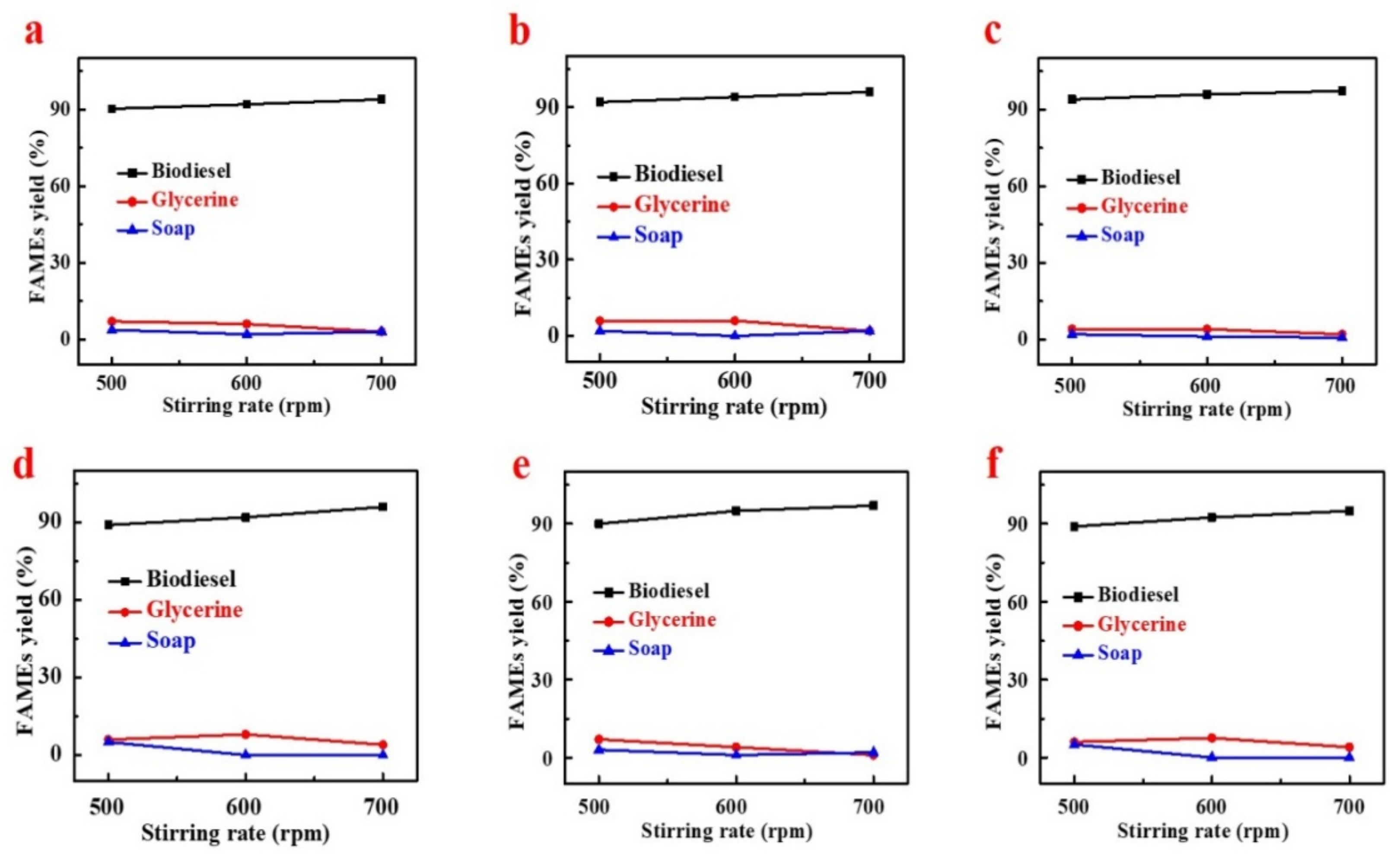
4.1.3. Effect of Temperature and Stirring Intensity on Fames Yield
4.1.4. Effect of Reaction Time on FAMEs Yield
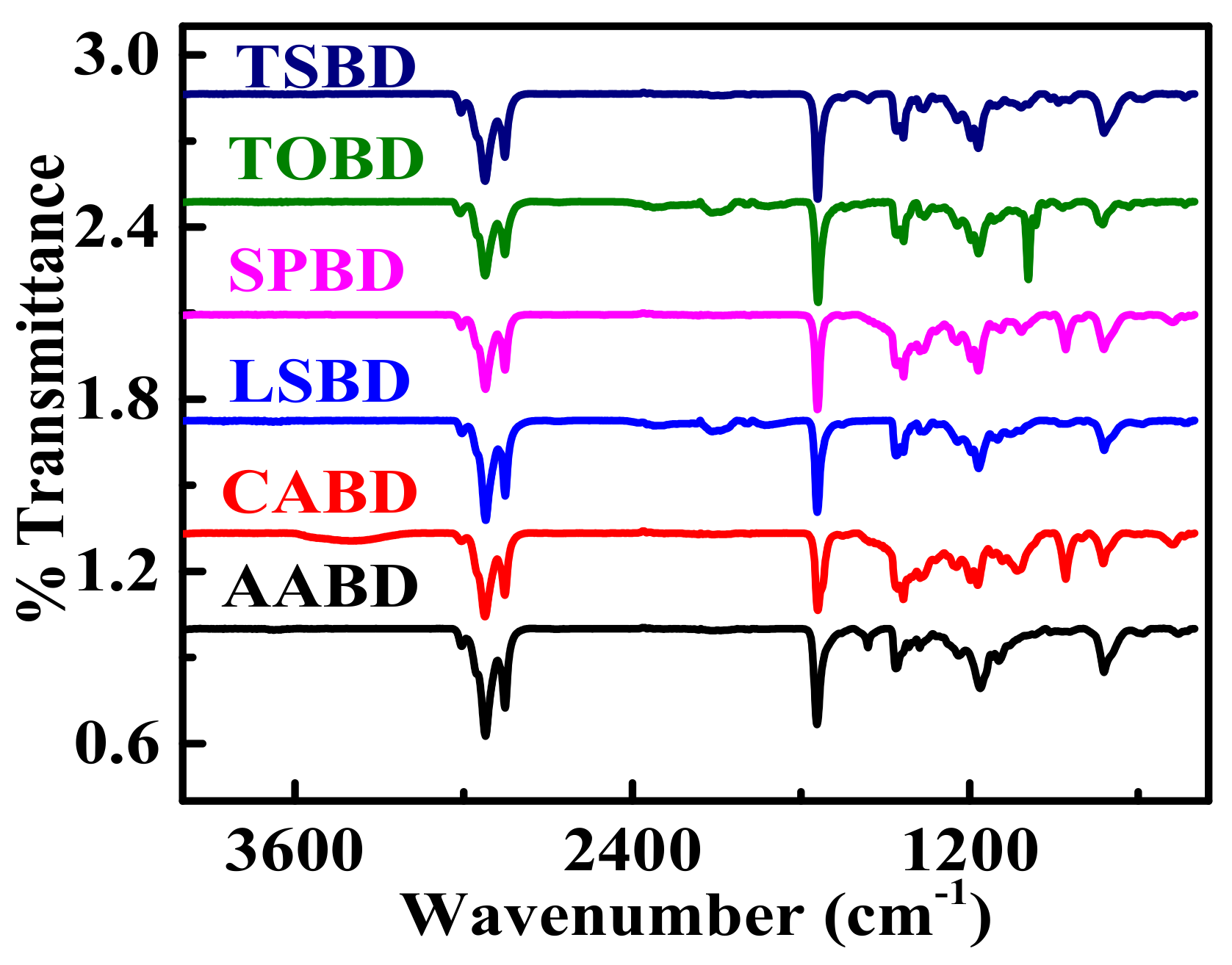
4.2. FTIR Analysis of Non-Edible Seed Oil Sources
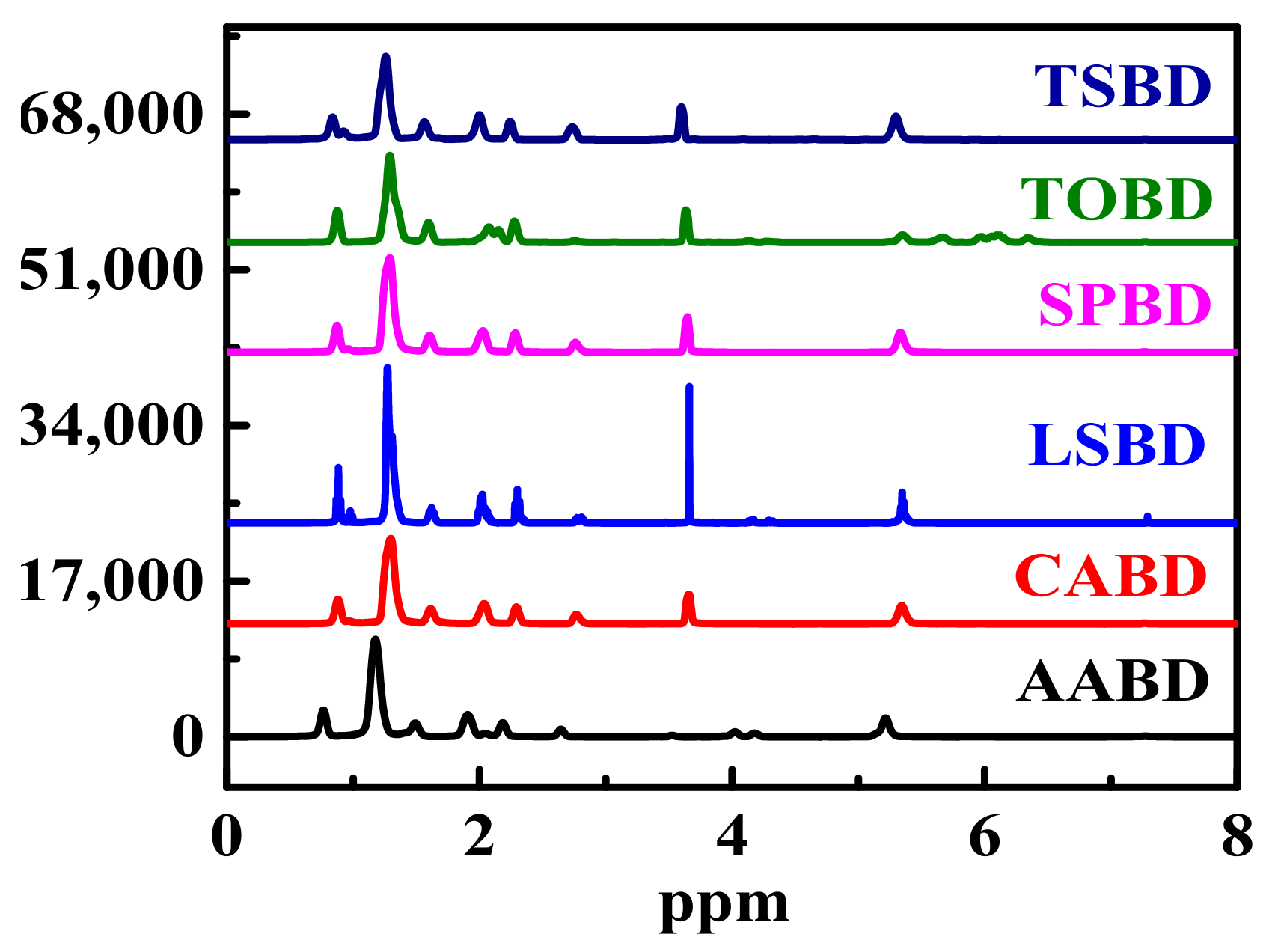
4.3. NMR Analysis of Non-Edible Seed Oil Sources FAMEs
4.3.1. 1H NMR Analysis of Non-Edible Seed Oil Sources FAMEs
4.3.2. 13C NMR Analysis of Non-Edible Seed Oil Sources Fames
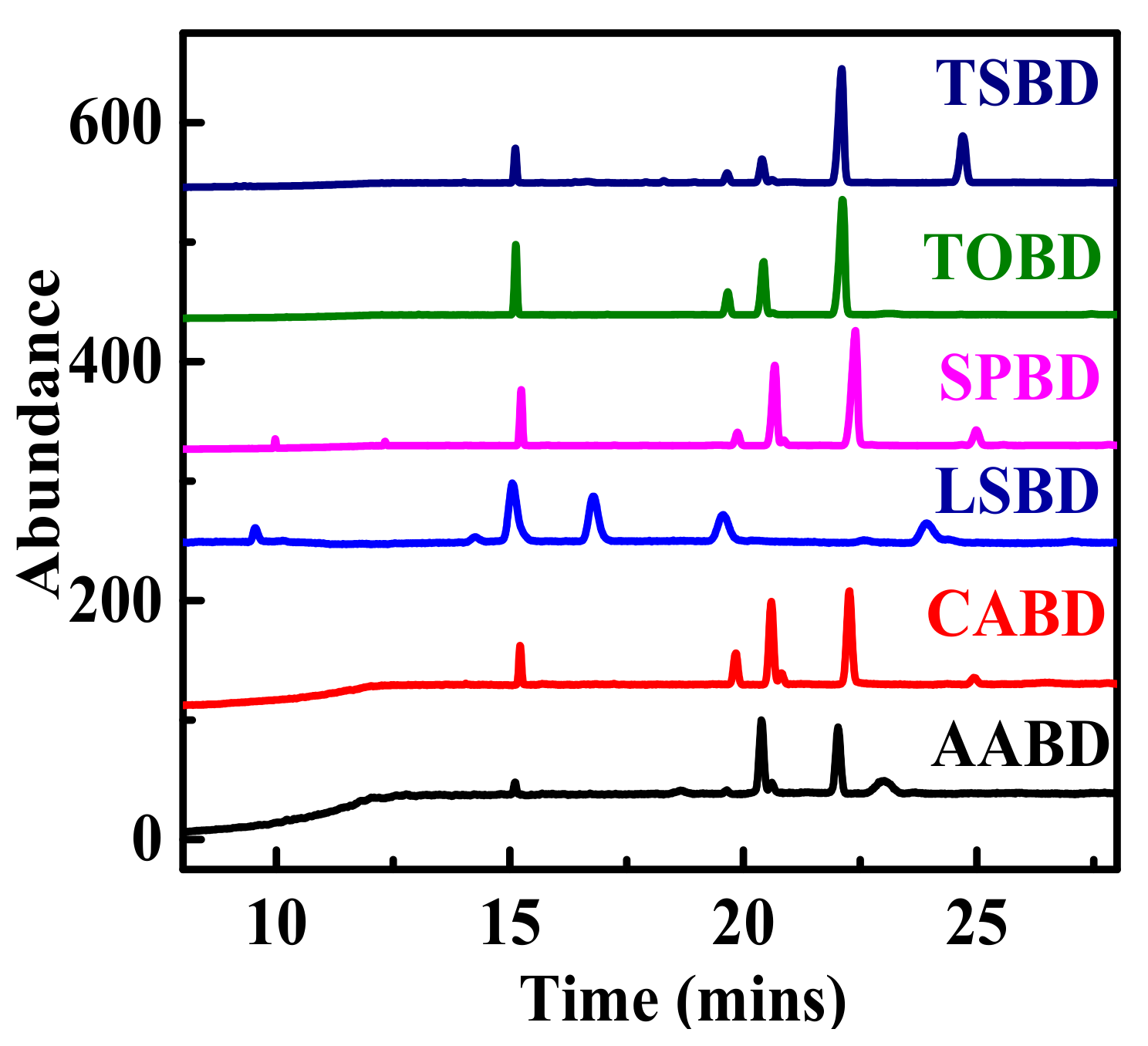
4.4. GC-MS Analysis of Non-Edible Seed Oil Sources FAMEs
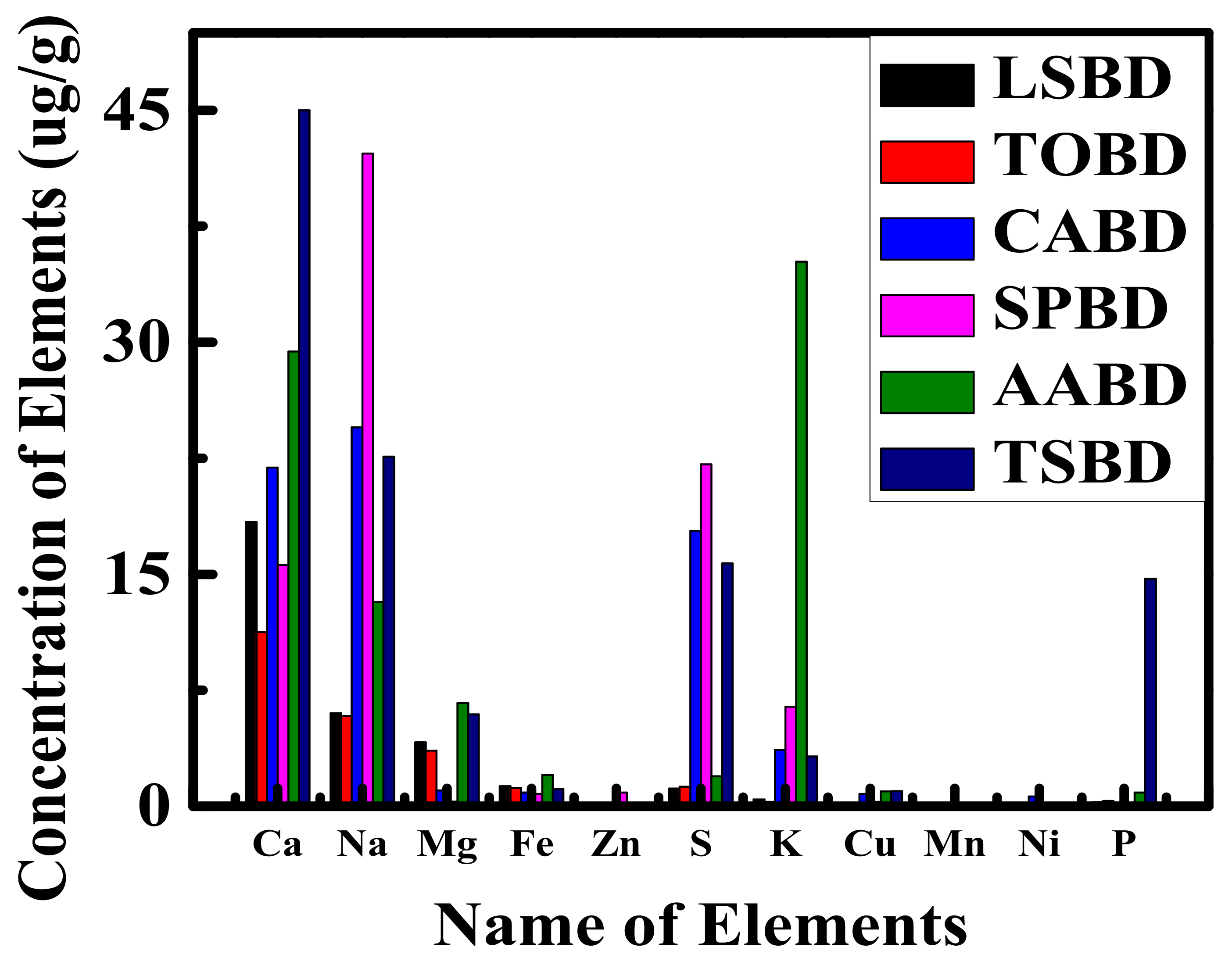
4.5. ICP-OES and Elemental Analyzer Study of Non-Edible Seed Oil Sources FAMEs
4.6. Physiochemical Characterization of Non-Edible Seed Oil Sources Fames
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Yaşar, F.; Altun, Ş. Biodiesel properties of microalgae (Chlorella protothecoides) oil for use in diesel engines. Int. J. Green Energy 2018, 15, 941–946. [Google Scholar] [CrossRef]
- Woolley, K.E.; Dickinson-Craig, E.; Bartington, S.E.; Oludotun, T.; Kirenga, B.; Mariga, S.T.; Kabera, T.; Coombe, A.; Pope, F.D.; Singh, A. Effectiveness of interventions to reduce household air pollution from solid biomass fuels and improve maternal and child health outcomes in low-and middle-income countries: A systematic review protocol. Syst. Rev. 2021, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef]
- Naylor, R.L.; Higgins, M.M. The political economy of biodiesel in an era of low oil prices. Renew. Sustain. Energy Rev. 2017, 77, 695–705. [Google Scholar] [CrossRef]
- Atabani, A.; Mahlia, T.; Badruddin, I.A.; Masjuki, H.; Chong, W.; Lee, K.T. Investigation of physical and chemical properties of potential edible and non-edible feedstocks for biodiesel production, a comparative analysis. Renew. Sustain. Energy Rev. 2013, 21, 749–755. [Google Scholar] [CrossRef]
- Anwar, M. Biodiesel feedstocks selection strategies based on economic, technical, and sustainable aspects. Fuel 2021, 283, 119204. [Google Scholar] [CrossRef]
- Krisnangkura, K.; Yimsuwan, T.; Pairintra, R. An empirical approach in predicting biodiesel viscosity at various temperatures. Fuel 2006, 85, 107–113. [Google Scholar] [CrossRef]
- Lee, C.S.; Park, S.W.; Kwon, S.I. An experimental study on the atomization and combustion characteristics of biodiesel-blended fuels. Energy Fuels 2005, 19, 2201–2208. [Google Scholar] [CrossRef]
- Graboski, M.S.; McCormick, R.L. Combustion of fat and vegetable oil derived fuels in diesel engines. Prog. Energy Combust. Sci. 1998, 24, 125–164. [Google Scholar] [CrossRef]
- Da Rocha Filho, G.; Brodzki, D.; Djéga-Mariadassou, G. Formation of alkanes, alkylcycloalkanes and alkylbenzenes during the catalytic hydrocracking of vegetable oils. Fuel 1993, 72, 543–549. [Google Scholar] [CrossRef]
- McMullin, T.; Bamber, A.; Flores, J.; Vigil, D. Oil and Gas Health Information and Response Program; Department of Public Health & Environment: Denver, CO, USA, 2017; pp. 15–27.
- Patrick, S.O.; Abdullahi, Z.; Bello, I. Biodisel Development in Nigeria: Prospects and Challenges. Int. J. Mod. Bot. 2013, 3, 4–9. [Google Scholar]
- Holt-Giménez, E. Biofuels: Five myths of the agro-fuels transition. Rev. Nera 2012, 10, 151–164. [Google Scholar]
- Silalertruksa, T.; Gheewala, S.H. Food, Fuel, and Climate Change: Is Palm-Based Biodiesel a Sustainable Option for Thailand? J. Ind. Ecol. 2012, 16, 541–551. [Google Scholar] [CrossRef]
- Janaun, J.; Ellis, N. Perspectives on biodiesel as a sustainable fuel. Renew. Sustain. Energy Rev. 2010, 14, 1312–1320. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; García-Martínez, N.; del Mar Durán-del-Amor, M.; Quesada-Medina, J. Advances on kinetics and thermodynamics of non-catalytic supercritical methanol transesterification of some vegetable oils to biodiesel. Energy Convers. Manag. 2018, 173, 187–196. [Google Scholar] [CrossRef]
- Heisner, B. Utilizing the 3/27 Conversion Test to Measure the Effects of Temperature on the Base-Catalyzed Transesterification of Waste Vegetable Oils into Fatty Acid Methyl Esters. J. Automot. Technol. Educ. 2020, 13, 184–197. [Google Scholar]
- Dash, S.K.; Lingfa, P. A review on production of biodiesel using catalyzed transesterification. AIP Conf. Proc. 2017. [Google Scholar] [CrossRef]
- Demirbas, A. Comparison of transesterification methods for production of biodiesel from vegetable oils and fats. Energy Convers. Manag. 2008, 49, 125–130. [Google Scholar] [CrossRef]
- Khan, I.U.; Yan, Z.; Chen, J. Production and characterization of biodiesel derived from a novel source Koelreuteria paniculata seed oil. Energies 2020, 13, 791. [Google Scholar] [CrossRef]
- Guo, M.; Jiang, W.; Chen, C.; Qu, S.; Lu, J.; Yi, W.; Ding, J. Process optimization of biodiesel production from waste cooking oil by esterification of free fatty acids using La3+/ZnO-TiO2 photocatalyst. Energy Convers. Manag. 2021, 229, 113745. [Google Scholar] [CrossRef]
- Ucciani, E.; Mallet, J.; Zahra, J. Cyanolipids and fatty acids of Sapindus trifoliatus L. (Sapindaceae) Seed Oil. Lipid/Fett 1994, 96, 69–71. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Tango, M.S.; Budge, S.M.; Watts, K.C.; Islam, M.R. Non-edible plant oils as new sources for biodiesel production. Int. J. Mol. Sci. 2008, 9, 169–180. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chiang, T.-H.; Chen, J.-H. Properties of soapnut (Sapindus mukorossi) oil biodiesel and its blends with diesel. Biomass Bioenergy 2013, 52, 15–21. [Google Scholar] [CrossRef]
- Chaudhary, S.; Mandal, A.; Bhar, R.; Gopi, M.; Kannan, A.; Jadhav, S.; Rokade, J. Effect of graded levels of soapnut (Sapindus mukorossi) shell powder on reproductive performance in broiler breeders. Asian Australas. J. Anim. Sci. 2019, 32, 118. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, A.; Watts, K.; Rahman, M.; Islam, M. Soapnut extract as a natural surfactant for enhanced oil recovery. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 31, 1893–1903. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. Potential non-edible oil resources as biodiesel feedstock: An Indian perspective. Renew. Sustain. Energy Rev. 2011, 15, 1791–1800. [Google Scholar] [CrossRef]
- Yu, X.-W.; Sha, C.; Guo, Y.-L.; Xiao, R.; Xu, Y. High-level expression and characterization of a chimeric lipase from Rhizopus oryzae for biodiesel production. Biotechnol. Biofuels 2013, 6, 1–12. [Google Scholar] [CrossRef]
- Zhuang, D.; Jiang, D.; Liu, L.; Huang, Y. Assessment of bioenergy potential on marginal land in China. Renew. Sustain. Energy Rev. 2011, 15, 1050–1056. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, L.; Tan, X.; Long, H.; Shockey, J.M. Identification, classification and differential expression of oleosin genes in tung tree. FASEB J. 2014, 9, e88409. [Google Scholar]
- Moser, B.R. Biodiesel production, properties, and feedstocks. Biofuels 2011, 45, 285–347. [Google Scholar]
- Ozcanli, M.; Gungor, C.; Aydin, K. Biodiesel fuel specifications: A review. Energy Sources Part A Recovery Util. Environ. Eff. 2013, 35, 635–647. [Google Scholar] [CrossRef]
- Liao, J.W.; Yeh, J.Y.; Lin, Y.C.; Wei, M.M.; Chung, Y.C. Mutagenicity and safety evaluation of water extract of fermented Toona sinensis Roemor leaves. J. Food Sci. 2009, 74, T7–T13. [Google Scholar] [CrossRef]
- Peng, W.; Liu, Y.; Hu, M.; Zhang, M.; Yang, J.; Liang, F.; Huang, Q.; Wu, C. Toona sinensis: A comprehensive review on its traditional usages, phytochemisty, pharmacology and toxicology. Rev. Bras. Farmacogn. 2019, 29, 111–124. [Google Scholar] [CrossRef]
- Kakumu, A.; Ninomiya, M.; Efdi, M.; Adfa, M.; Hayashi, M.; Tanaka, K.; Koketsu, M. Phytochemical analysis and antileukemic activity of polyphenolic constituents of Toona sinensis. Bioorg. Med. Chem. Lett. 2014, 24, 4286–4290. [Google Scholar] [CrossRef]
- Chia, Y.-C.; Rajbanshi, R.; Calhoun, C.; Chiu, R.H. Anti-neoplastic effects of gallic acid, a major component of Toona sinensis leaf extract, on oral squamous carcinoma cells. Molecules 2010, 15, 8377–8389. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Chang, W.-H.; Chen, C.-S.; Liao, J.-W.; Huang, C.-J.; Lu, F.-J.; Chia, Y.-C.; Hsu, H.-K.; Wu, J.-J.; Yang, H.-L. Antioxidant activities of Toona Sinensis leaves extracts using different antioxidant models. Food Chem. Toxicol. 2008, 46, 105–114. [Google Scholar] [CrossRef]
- Ouyang, J.; Wu, Y.-W.; Lu, X.-R. Comparison of anti-bacterial activities of the extracts from young and old leaves of Toona sinensis. Nat. Prod. Res. Dev. 2008, 20, 427–430. [Google Scholar]
- Ding, J.; Wu, Y.; Zheng, H.; Fu, W.; Reardon, R.; Liu, M. Assessing potential biological control of the invasive plant, tree-of-heaven, Ailanthus altissima. Biocontrol Sci. Technol. 2006, 16, 547–566. [Google Scholar] [CrossRef]
- Herrick, N.; McAvoy, T.; Snyder, A.; Salom, S.; Kok, L. Host-range testing of Eucryptorrhynchus brandti (Coleoptera: Curculionidae), a candidate for biological control of tree-of-heaven, Ailanthus altissima. Environ. Entomol. 2012, 41, 118–124. [Google Scholar] [CrossRef]
- Hoseini, S.; Najafi, G.; Ghobadian, B.; Mamat, R.; Ebadi, M.; Yusaf, T. Novel environmentally friendly fuel: The effects of nanographene oxide additives on the performance and emission characteristics of diesel engines fuelled with Ailanthus altissima biodiesel. Renew. Energy 2018, 125, 283–294. [Google Scholar] [CrossRef]
- Kowarik, I.; Säumel, I. Biological flora of central Europe: Ailanthus altissima (Mill.) swingle. Perspect. Plant Ecology Evol. Syst. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Kozuharova, E.; Benbassat, N.; Berkov, S.; Ionkova, I. Ailanthus altissima and Amorpha fruticosa–invasive arboreal alien plants as cheap sources of valuable essential oils. Pharmacia 2020, 67, 71. [Google Scholar] [CrossRef]
- Kanakraj, S.; Dixit, S.; Rehman, A. Biofuel derived from enzymatic degummed linum usitatissimum seed oil: As a potential resource for diesel engine. Bangladesh J. Sci. Ind. Res. 2014, 49, 13–24. [Google Scholar] [CrossRef]
- Kpikpi, W. Jatropha curcas as vegetable source of renewable energy. In Proceedings of the ANSTI Sub-Network Meeting on Renewable Energy, Kumasi, Ghana, 18–22 February 2002. [Google Scholar]
- Tyagi, O.S.; Atray, N.; Kumar, B.; Datta, A. Production, characterization and development of standards for biodiesel—A review. Mapan 2010, 25, 197–218. [Google Scholar] [CrossRef]
- McCabe, W.L.; Smith, J.C.; Harriott, P. Unit Operations of Chemical Engineering; McGraw-Hill: New York, NY, USA, 1993; Volume 5. [Google Scholar]
- Moser, B.R. Influence of blending canola, palm, soybean, and sunflower oil methyl esters on fuel properties of biodiesel. Energy Fuels 2008, 22, 4301–4306. [Google Scholar] [CrossRef]
- Ahmad, M.; Khan, M.A.; Zafar, M.; Sultana, S. Practical Handbook on Biodiesel Production and Properties; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Antolın, G.; Tinaut, F.; Briceno, Y.; Castano, V.; Perez, C.; Ramırez, A. Optimisation of biodiesel production by sunflower oil transesterification. Bioresour. Technol. 2002, 83, 111–114. [Google Scholar] [CrossRef]
- Shahla, S.; Cheng, N.G.; Yusoff, R. An overview on transesterification of natural oils and fats. Biotechnol. Bioprocess Eng. 2010, 15, 891–904. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef]
- Al Basir, F.; Roy, P.K. Effects of Temperature and Stirring on Mass Transfer to Maximize Biodiesel Production from Jatropha curcas Oil: A Mathematical Study. Int. J. Eng. Math. 2015, 2015, 278275. [Google Scholar] [CrossRef][Green Version]
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol--A byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.; Van Gerpen, J.; Thompson, J. Soap and glycerin removal from biodiesel using waterless processes. Trans. ASABE 2011, 54, 535–541. [Google Scholar] [CrossRef]
- Okwundu, O.S.; El-Shazly, A.H.; Elkady, M. Comparative effect of reaction time on biodiesel production from low free fatty acid beef tallow: A definition of product yield. SN Appl. Sci. 2019, 1, 140. [Google Scholar] [CrossRef]
- Soares, I.P.; Rezende, T.F.; Silva, R.C.; Castro, E.V.R.; Fortes, I.C. Multivariate calibration by variable selection for blends of raw soybean oil/biodiesel from different sources using Fourier transform infrared spectroscopy (FTIR) spectra data. Energy Fuels 2008, 22, 2079–2083. [Google Scholar] [CrossRef]
- Safar, M.; Bertrand, D.; Robert, P.; Devaux, M.; Genot, C. Characterization of edible oils, butters and margarines by Fourier transform infrared spectroscopy with attenuated total reflectance. J. Am. Oil Chem. Soc. 1994, 71, 371–377. [Google Scholar] [CrossRef]
- Wang, L.; Yu, H. Biodiesel from Siberian apricot (Prunus sibirica L.) seed kernel oil. Bioresour. Technol. 2012, 112, 355–358. [Google Scholar] [CrossRef]
- Betha, R.; Balasubramanian, R. Emissions of particulate-bound elements from biodiesel and ultra low sulfur diesel: Size distribution and risk assessment. Chemosphere 2013, 90, 1005–1015. [Google Scholar] [CrossRef]










| Parameter | Descriptions |
|---|---|
| Column | QP2010SE, Shimadzu PEG-20M Length: 30 m Internal diameter: 0.32 mm Film thickness: 1 μm |
| Injector temperature | 220 °C |
| Detector temperature (EI 250) | 210 °C |
| Carrier gas | Helium, flow rate = 1.2 mL min−1 |
| Injection | V = 1 μL |
| Split | Flow rate = 40:1 |
| Temperature program | Initial temperature = 100 °C Rate of progression = 10 °C min−1. Final temperature = 210 °C, 20 min. |
| Source Name/ Optimized Condition | Molar Ratio of Methanol to Oil | Temperature (°C) | Stirring Intensity (rpm) | Reaction Time (min) | Amount of Catalyst Used | Amount of Methanol CH3OH (mL) | Percentage Yield of Various Products | ||
|---|---|---|---|---|---|---|---|---|---|
| KOH (g) | Biodiesel (% v/v) | Glycerol (% v/v) | Soap (% v/v) | ||||||
| TOBD | 6:1 | 65 | 700 | 80 | 0.32 | 8.33 | 97.2 | 1.8 | 1 |
| AABD | 6:1 | 65 | 700 | 60 | 0.32 | 8.33 | 93.9 | 6 | 0.1 |
| LSBD | 6:1 | 65 | 700 | 80 | 0.32 | 8.33 | 98 | 2 | 0 |
| TSBD | 5:1 | 65 | 700 | 80 | 0.42 | 10 | 95.2 | 3.8 | 1 |
| CABD | 6:1 | 65 | 700 | 60 | 0.32 | 8.33 | 96 | 4 | 0 |
| SPBD | 7:1 | 65 | 700 | 60 | 0.32 | 8.33 | 96 | 3 | 1 |
| Fatty Acids/Exp. Results | TSBD | TOBD | SPBD | LSBD | CABD | AABD | Carbon and Double Bonds | Chemical Name | Chemical Structure | Molecular Weight |
|---|---|---|---|---|---|---|---|---|---|---|
| Palmitic acid | PA (15.115) | PA (9.537) | PA (15.235) | PA (9.545) | PA (15.213) | PA (15.115) | C16:0 | Hexadecanoic acid, methyl ester |  | 270 |
| Stearic acid | SA (18.288) | SA (14.255) | SA (19.863) | SA (14.248) | SA (19.840) | SA (19.637) | C18:0 | methyl stearate | 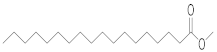 | 298 |
| Oleic acid | OA (18.947) | OA (15.050) | OA (20.853) | OA (15.042) | OA (20.583) | OA (20.387) | C18:1 | 9-Octadecenoic acid (Z(-, methyl ester | 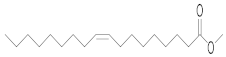 | 296 |
| Linoleic acid | LA (22.098) | LA (16.760) | LA (22.397) | LA (16.797) | LA (22.262) | LA (22.022) | C18:2 | 9, 12-Octadecadienoic acid (Z, Z)-, methyl ester | 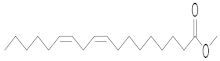 | 294 |
| α-Linolenic acid | LiA (24.708) | -- | LiA (24.985) | LiA (19.565) | LiA (24.940) | -- | C18:3 | α-Linolenic acid |  | 292 |
| Arachidic acid | -- | -- | AA (27.820) | AA (22.587) | -- | -- | C20:0 | Eicosanoic acid, methyl ester | 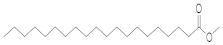 | 326 |
| Gondoic acid | -- | GA (23.938) | GA (29.065) | GA (23.922) | GA (29.058) | -- | C20:1 | CiS- 11- Eicosenoic acid, methyl ester | 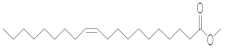 | 324 |
| Behenic acid | -- | -- | -- | -- | -- | BA (18.647) | C22:0 | Docosanoic acid/ Methyl behenate | 354 |
| Properties | TOBD % | AABD % | SPBD % | TSBD % | CABD % | LSBD % | Petro-Diesel |
|---|---|---|---|---|---|---|---|
| C | 76.47 | 74.88 | 76.44 | 75.83 | 71.71 | 76.25 | 86.5 |
| H | 11.65 | 13.24 | 12.13 | 12.42 | 11.43 | 13.01 | 13.5 |
| N | 1.96 | 1.49 | ND | 1.72 | ND | 1.41 | - |
| O | 9.92 | 10.39 | 11.43 | 10.03 | 11.43 | 9.33 | 0 |
| Properties | LSBD | AABD | SPBD | CABD | TOBD | TSBD | EN 14214 | ASTM D6751 |
|---|---|---|---|---|---|---|---|---|
| Oil Content (%) | 45 | 38 | 51 | 48 | 54.4 | 35 | - | - |
| Density at 15 °C (g/cm3) | 0.9323 | 0.873 | 0.929 | 0.924 | 0.8858 | 0.995 | 0.86–0.90 | 0.86–0.90 |
| Kinematic Viscosity at 40 °C (cSt) | 3.34 | 4.74 | 5.0 | 11.13 | 4.60 | 2.32 | 3.5–5.0 | 1.9–6.0 |
| Flash Point (°C) | 172 | 169 | 160 | 170 | 194 | 165 | Min. 120 | Min. 130 |
| Pour Point (°C) | −3 | −4 | −7 | −10 | −6 | −7 | −15 to 16 | −5 to 5 |
| Cetane Index | 54 | 49 | 51 | 54.53 | 52 | 48 | −51 | −47 |
| Sulphur Content (%) | 0.0093 | 0.0021 | 0.0032 | 0.0082 | 0.0031 | 0.0042 | ˂0.01 | ˂0.01 |
| Free Fatty Acid, FFA (%) | 2.7 | 1.9 | 1.1 | 0.80 | 0.12 | 2.1 | Max. 0.50 | ˂1 |
| Calorific Value (MJ/kg) | 41.85 | 37 | 39 | 41 | 39 | - | - | - |
| Water Content (% by vol.) | 0.05 | 0.09 | 0.03 | 0.31 | 0.02 | 0.06 | - | - |
| Cloud Point (°C) | −6 | 2 | −9 | −3 | −2 | −5 | −3 to 12 | −3 to 12 |
| Total Acid Number (mg KOH/g) | 0.75 | 0.37 | 0.59 | 1.19 | 0.42 | 0.83 | - | - |
| Carbon residue (%, w/w) | 0.051 | 0.031 | 0.011 | 0.0150 | 0.021 | - | - | |
| Ash content (%) | 0.01 | 0.002 | 0.001 | 0.02 | 0.01 | - | - | |
| Fire point (°C) | 182 | 178 | 165 | 177 | 180 | 169 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I.U.; Chen, H.; Yan, Z.; Chen, J. Extraction and Quality Evaluation of Biodiesel from Six Familiar Non-Edible Plants Seeds. Processes 2021, 9, 840. https://doi.org/10.3390/pr9050840
Khan IU, Chen H, Yan Z, Chen J. Extraction and Quality Evaluation of Biodiesel from Six Familiar Non-Edible Plants Seeds. Processes. 2021; 9(5):840. https://doi.org/10.3390/pr9050840
Chicago/Turabian StyleKhan, Inam Ullah, Hang Chen, Zhenhua Yan, and Jun Chen. 2021. "Extraction and Quality Evaluation of Biodiesel from Six Familiar Non-Edible Plants Seeds" Processes 9, no. 5: 840. https://doi.org/10.3390/pr9050840
APA StyleKhan, I. U., Chen, H., Yan, Z., & Chen, J. (2021). Extraction and Quality Evaluation of Biodiesel from Six Familiar Non-Edible Plants Seeds. Processes, 9(5), 840. https://doi.org/10.3390/pr9050840







