An Experimental Investigation of Water Vapor Condensation from Biofuel Flue Gas in a Model of Condenser, (1) Base Case: Local Heat Transfer without Water Injection
Abstract
:1. Introduction
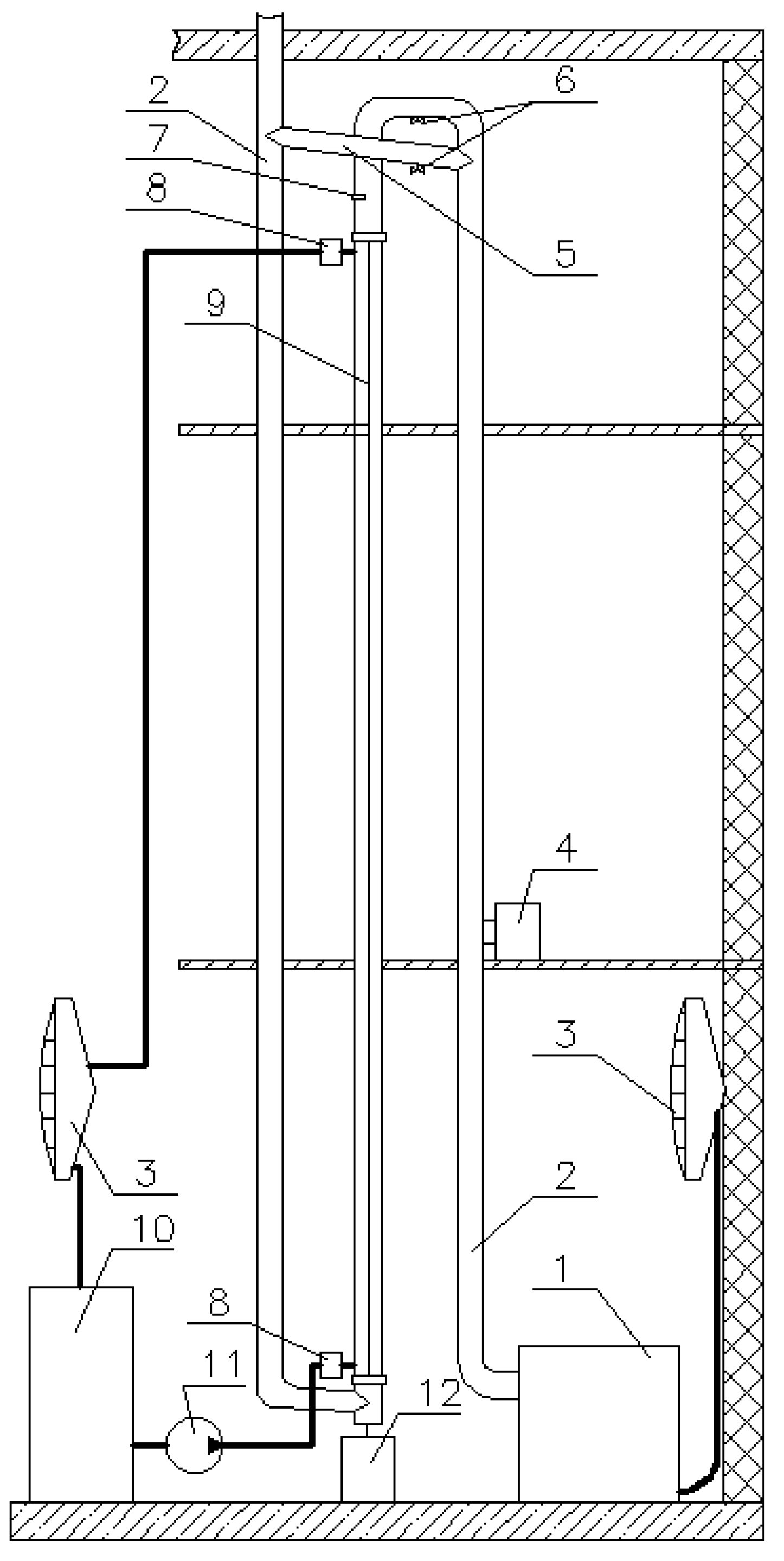
2. Experimental Setup
3. Methodology
- Twenty thermocouples were installed in the center of the calorimetric tube (tc) to measure the flue gas temperature along the tube. The distance between the thermocouples was approximately 0.28 m.
- Twenty thermocouples were installed at the inner wall of the calorimetric tube (tw) along the length of the tube. The distance between the thermocouples was approximately 0.28 m.
- Ten thermocouples were installed between the inner and the outer tubes to measure the cooling water temperature (tcw) along the space between the tubes. The distance between the thermocouples was approximately 0.56 m.
- Three thermocouples were installed in each of the water mixers to measure cooling water temperatures at the inlet and the outlet of the test section.
4. Results Analysis
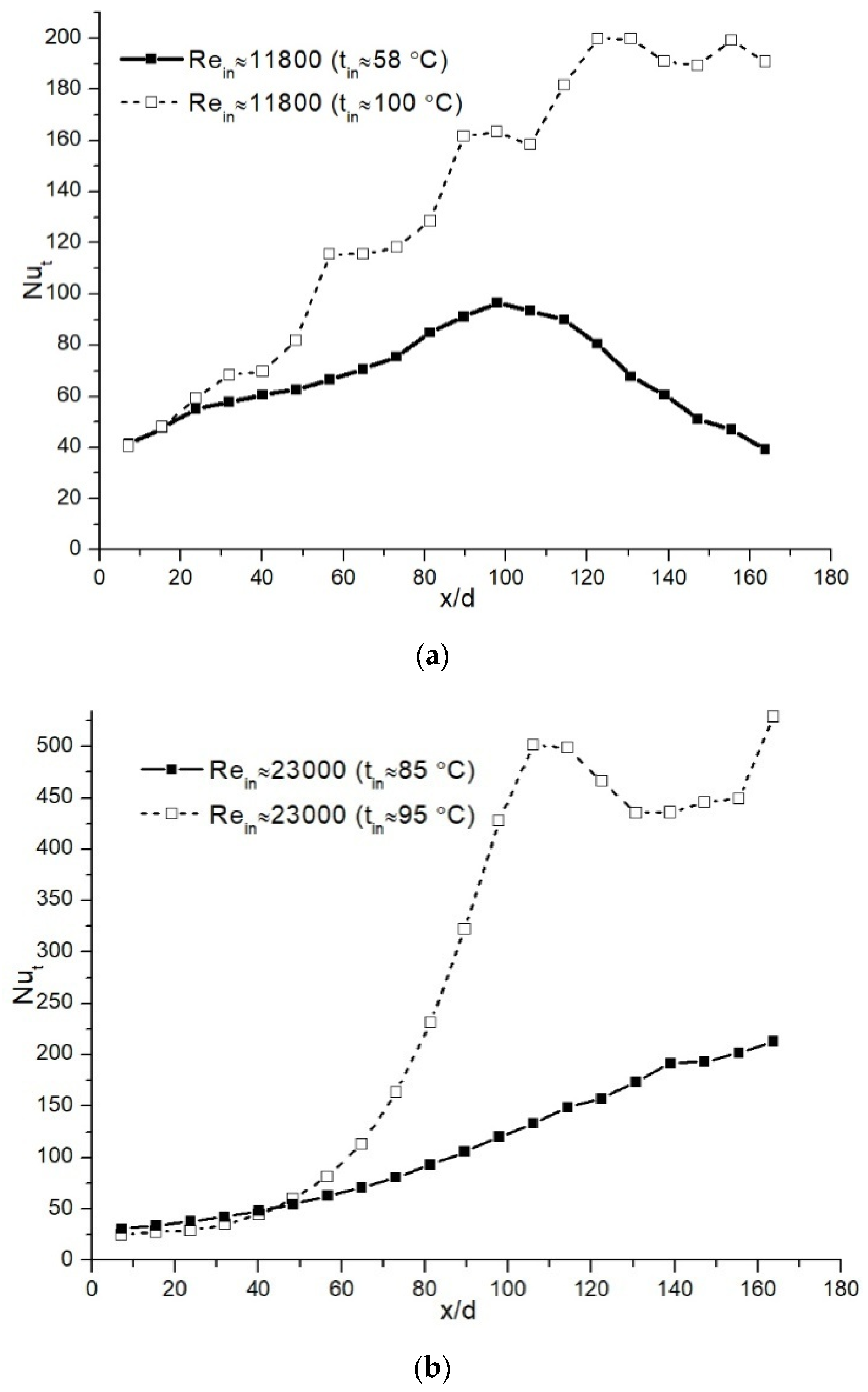
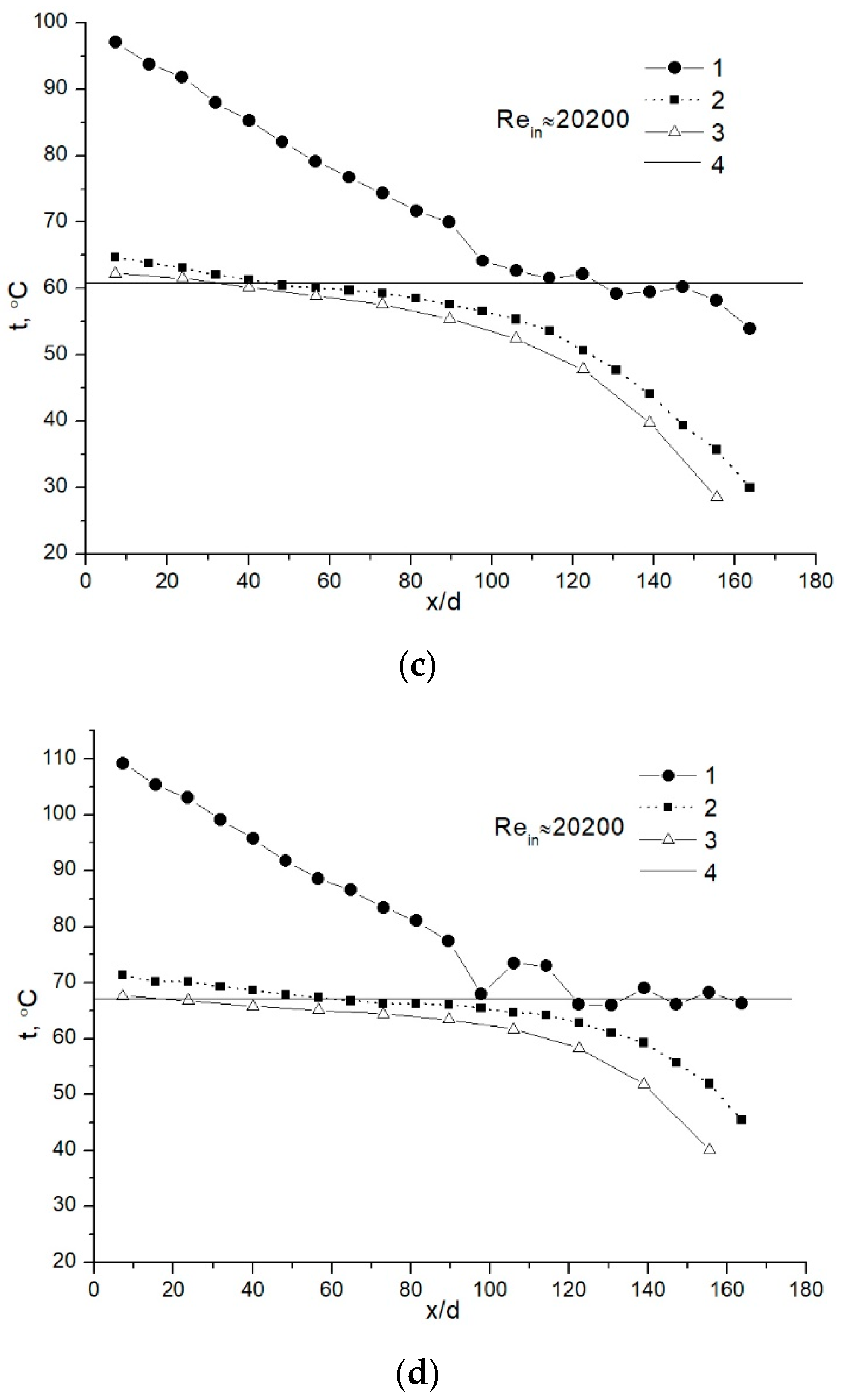
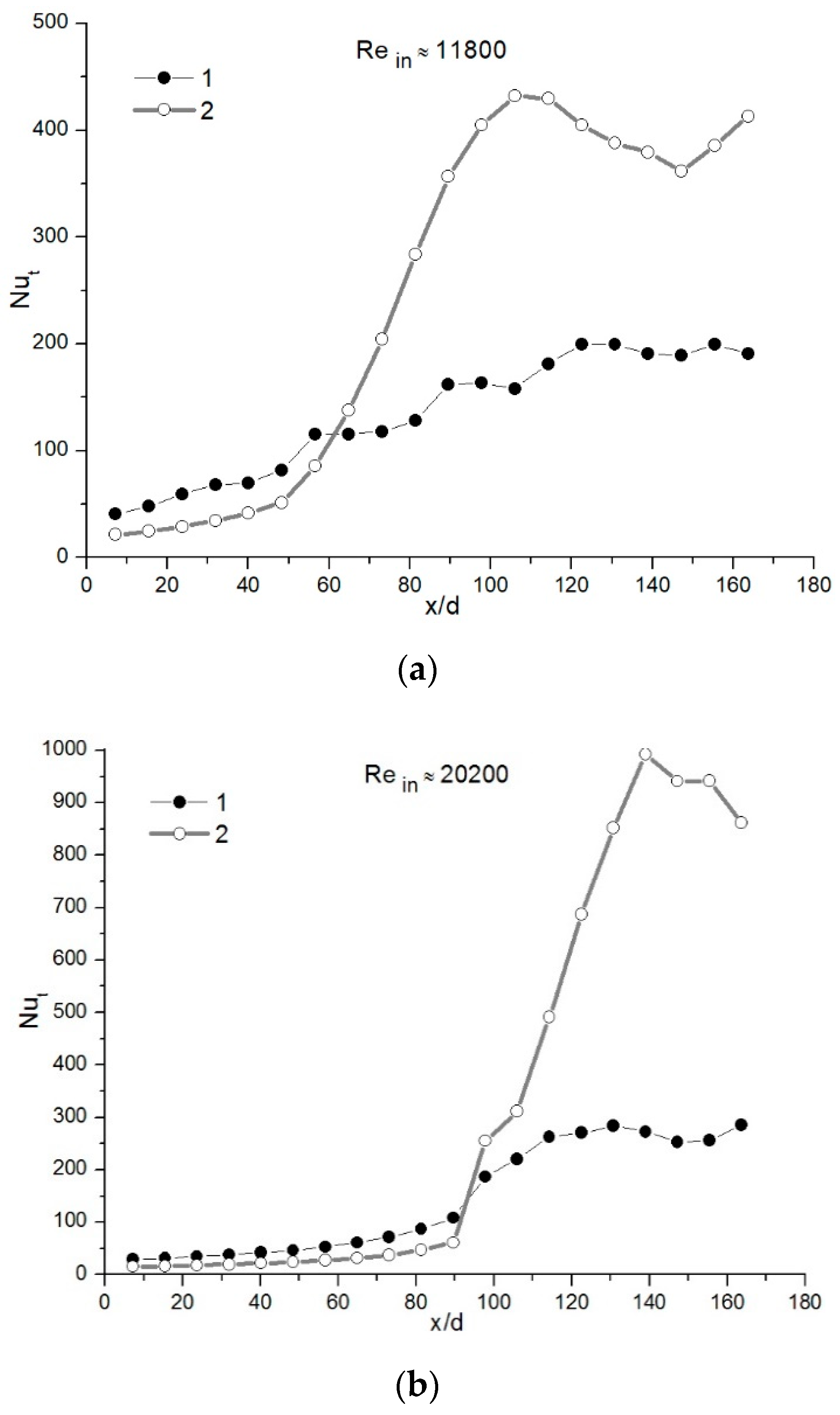
4.1. Water-Vapor Mass Fraction 7%
4.2. Water-Vapor Mass Fraction 17%
4.3. Comparison of the Results for Different Water-Vapor Mass Fractions
5. Conclusions
- Performed analysis revealed the influence of inlet parameters on heat transfer processes occurring in condensing heat exchangers.
- The investigations of the local heat transfer along the tube (the model of the condensing heat exchanger) provided important findings related to the optimization of the operation of heat exchangers of such type.
- The investigations showed that, in some cases in the initial part of the condensing heat exchanger model, the heat from flue gas is recovered by convection. Only after that, a sharp increase is noticed in heat transfer due to water vapor condensation.
- The analysis showed that, in the case of water-vapor mass fraction ≈ 7%, the condensation efficiency was in the range between ≈ 15% and 26%. When the condensable gas mass fraction was increased until ≈ 17%, the condensation efficiency was in the range between ≈ 39% and 56%.
- To use the condensing heat exchangers efficiently, there is a need to adjust certain flue gas flow rate, temperature and humidity and other parameters in such a way that the condensation of water vapor would be from the beginning of the condensing heat exchanger.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thulukkanam, K. Heat Exchanger Design Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Kasurinen, S.; Jalava, P.I.; Tapanainen, M.; Uski, O.; Happo, M.S.; Mäki-Paakkanen, J.; Lamberg, H.; Koponen, H.; Nuutinen, I.; Kortelainen, M.; et al. Toxicological effects of particulate emissions—A comparison of oil and wood fuels in small- and medium-scale heating systems. Atmos. Environ. 2015, 103, 321–330. [Google Scholar] [CrossRef]
- Survey of the Present State of Particle Precipitation Devices for Residential Biomass Combustion with a Nominal Capacity up to 50 KW in IEA Bioenergy Task32; Final Version; Institute for Process and Particle Engineering, Graz University of Technology: Graz, Austria, 2013; Available online: https://www.bios-bioenergy.at/uploads/media/Filter-study-IEA-Dez-2011.pdf (accessed on 21 January 2021).
- Che, D.; Liu, Y.; Gao, C. Evaluation of retrofitting a conventional natural gas fired boiler into a condensing boiler. Energy Convers. Manag. 2004, 45, 3251–3266. [Google Scholar] [CrossRef]
- Satyavada, H.; Baldi, S. A novel modelling approach for condensing boilers based on hybrid dynamical systems. Machines 2016, 4, 10. [Google Scholar] [CrossRef]
- Siddique, M.; Golay, M.W.; Kazimi, M.S. Local heat transfer coefficients for forced-convection condensation of steam in a vertical tube in the presence of a noncondensable gas. Nucl. Technol. 1993, 102, 386–402. [Google Scholar] [CrossRef]
- Park, H.S.; No, H.C. A condensation experiment in the presence of noncondensables in a vertical tube of a passive containment cooling system and its assessment with RELAP5/MOD3.2. Nucl. Technol. 1999, 127, 160–169. [Google Scholar] [CrossRef]
- Maheshwari, N.; Saha, D.; Sinha, R.; Aritomi, M. Investigation on condensation in presence of a noncondensable gas for a wide range of Reynolds number. Nucl. Eng. Des. 2004, 227, 219–238. [Google Scholar] [CrossRef]
- Caruso, G.; Giannetti, F.; Naviglio, A. Experimental investigation on pure steam and steam-air mixture condensation inside tubes. Int. J. Heat Technol. 2012, 30, 77–84. [Google Scholar] [CrossRef]
- Park, I.; Kim, S.-M.; Mudawar, I. Experimental measurement and modeling of downflow condensation in a circular tube. Int. J. Heat Mass Transf. 2013, 57, 567–581. [Google Scholar] [CrossRef]
- Oh, S.; Revankar, S.T. Experimental and theoretical investigation of film condensation with noncondensable gas. Int. J. Heat Mass Transf. 2006, 49, 2523–2534. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Kim, M.H. Experimental and empirical study of steam condensation heat transfer with a noncondensable gas in a small-diameter vertical tube. Nucl. Eng. Des. 2008, 238, 207–216. [Google Scholar] [CrossRef]
- Jia, L.; Peng, X.; Yan, Y.; Sun, J.; Li, X. Effects of water vapor condensation on the convection heat transfer of wet flue gas in a vertical tube. Int. J. Heat Mass Transf. 2001, 44, 4257–4265. [Google Scholar] [CrossRef]
- Delalić, N.; Ganić, E.; Džaferović, E. Experimental and numerical study on vapor condensation of wet flue gas in chimney. Defect Diffus. Forum 2008, 273, 119–125. [Google Scholar] [CrossRef]
- Chantana, C.; Kumar, S. Experimental and theoretical investigation of air-steam condensation in a vertical tube at low inlet steam fractions. Appl. Therm. Eng. 2013, 54, 399–412. [Google Scholar] [CrossRef]
- Shi, X.; Che, D.; Agnew, B.; Gao, J. An investigation of the performance of compact heat exchanger for latent heat recovery from exhaust flue gases. Int. J. Heat Mass Transf. 2011, 54, 606–615. [Google Scholar] [CrossRef]
- Li, J.-D.; Saraireh, M.; Thorpe, G. Condensation of vapor in the presence of non-condensable gas in condensers. Int. J. Heat Mass Transf. 2011, 54, 4078–4089. [Google Scholar] [CrossRef]
- Siddique, M. The Effects of Noncondensable Gases on Steam Condensation under Forced Convection Conditions. Ph.D. Thesis, Department of Nuclear Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA, 1992. [Google Scholar]
- Jeong, K.; Kessen, M.J.; Bilirgen, H.; Levy, E.K. Analytical modeling of water condensation in condensing heat exchanger. Int. J. Heat Mass Transf. 2010, 53, 2361–2368. [Google Scholar] [CrossRef]
- Barford, N.C. Experimental Measurements: Precision, Error and Truth, 2nd ed.; John Willey & Sons: Hoboken, NJ, USA, 1985. [Google Scholar]
- American Meteorological Society. Available online: https://journals.ametsoc.org/doi/pdf/10.1175/BAMS-86-2-225 (accessed on 21 January 2021).




| Temperature | x/d |
|---|---|
| tc | 7.2; 15.4; 23.7; 31.9; 40.1; 48.4; 56.6; 64.9; 73.1; 81.3; 89.6; 97.8; 106.0; 114.3; 122.5; 130.7; 139.0; 147.2; 155.4; 163.7. |
| tw | 7.2; 23.7; 40.1; 56.6; 73.1; 89.6; 106.0; 122.5; 139.0; 155.4. |
| tcw | 7.2; 15.4; 23.7; 31.9; 40.1; 48.4; 56.6; 64.9; 73.1; 81.3; 89.6; 97.8; 106.0; 114.3; 122.5; 130.7; 139.0; 147.2; 155.4; 163.7. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poškas, R.; Sirvydas, A.; Kulkovas, V.; Poškas, P. An Experimental Investigation of Water Vapor Condensation from Biofuel Flue Gas in a Model of Condenser, (1) Base Case: Local Heat Transfer without Water Injection. Processes 2021, 9, 844. https://doi.org/10.3390/pr9050844
Poškas R, Sirvydas A, Kulkovas V, Poškas P. An Experimental Investigation of Water Vapor Condensation from Biofuel Flue Gas in a Model of Condenser, (1) Base Case: Local Heat Transfer without Water Injection. Processes. 2021; 9(5):844. https://doi.org/10.3390/pr9050844
Chicago/Turabian StylePoškas, Robertas, Arūnas Sirvydas, Vladislavas Kulkovas, and Povilas Poškas. 2021. "An Experimental Investigation of Water Vapor Condensation from Biofuel Flue Gas in a Model of Condenser, (1) Base Case: Local Heat Transfer without Water Injection" Processes 9, no. 5: 844. https://doi.org/10.3390/pr9050844
APA StylePoškas, R., Sirvydas, A., Kulkovas, V., & Poškas, P. (2021). An Experimental Investigation of Water Vapor Condensation from Biofuel Flue Gas in a Model of Condenser, (1) Base Case: Local Heat Transfer without Water Injection. Processes, 9(5), 844. https://doi.org/10.3390/pr9050844





