Abstract
Flammable fuel-N2O mixtures raise safety and environmental protection issues in areas where these mixtures are used (such as: industry, research, internal combustion engines). Therefore, it is important to know their laminar combustion velocities and propagation speeds—important safety parameters for design of active protection devices against gas explosions and corresponding safety recommendations. In this paper, the laminar combustion velocities of N2-diluted CH4-N2O flames, obtained in experiments on outwardly propagating flames, at various initial pressures (within 0.5–2.0 bar) and room temperature, are reported. The experiments were made in a 0.5 L spherical cell with central ignition. The laminar combustion velocities were calculated from the constants of cubic law of flame propagation during the early stage of closed cell explosions and the expansion coefficients of unburned flammable mixtures, using the adiabatic model of the flame propagation. The expansion coefficients were determined from equilibrium calculations on flames propagating under isobaric conditions. The laminar combustion velocities were compared with data reported in the literature. Using the laminar combustion velocities and the expansion coefficients, the propagation speeds of N2-diluted CH4-N2O flames were calculated. Both laminar combustion velocities and propagation speeds decrease with the initial pressure increase.
1. Introduction
Determination of flammability properties of gaseous combustible mixtures in various conditions is the key to achieving the performance of combustion systems and the safety in their use. Special attention is paid to flammability limits, maximum explosion pressures, explosion indices (severity factors) and laminar combustion velocities of fuel-air gaseous mixtures. Presently, studies on the reaction characteristics were conducted not only on fuel-air mixtures but also on fuel-nitrous oxide mixtures, motivated by the recent interest in using nitrous oxide as an oxidant in propulsion systems [1,2,3]. Nitrous oxide is an oxidizer with enhanced oxidizing potential compared with air, but its handling is as critical as is handling pure oxygen, since N2O is extremely exothermic and an additional heat amount is released during its decomposition. The nitrous oxide can behave as both as oxidizer and it can act as an explosive when it dissociates exothermically [4]. It is generated by waste incineration, radiolysis, or chemical reactions. N2O can be observed in nuclear waste storage facilities, together with methane or hydrogen and therefore they can form easily a flammable mixture. Thus, flammable fuel-N2O mixtures raise safety and environmental protection issues in areas where these mixtures are used.
The combustion of fuels in presence of the nitrous oxide can produce highly unstable and turbulent deflagrations that rapidly self-accelerate and could change from deflagration to detonation regime. Bane et al. [5] highlighted that nitrous oxide flames become unstable immediately after ignition, even at low pressures and at lean compositions of flammable mixtures. Although there are many regulations to protect workers in the combustion field, there are accidental explosions related to fuel-N2O mixtures, some of them with disastrous consequences, due to the wider explosion limits and lower ignition energies of these mixtures. Therefore, such studies are needed to limit accidents. However, compared with fuel-air or fuel-oxygen mixtures, there are fewer studies on fuel-nitrous oxide reaction systems, especially on their laminar combustion velocities.
Several studies were conducted in the past to provide information on explosions in fuel-N2O mixtures. Some authors [6,7,8,9,10,11,12,13,14,15] reported the explosion parameters such as the flammability limits, the maximum explosion pressures, the explosion times, or the deflagration indices for explosions occurring in closed cells in the presence of N2O as oxidizer. The laminar combustion velocity of fuel-N2O and fuel-N2O-diluent mixtures (fuels: NH3, CO, H2, C2H4 and C2H2) was investigated mainly by burner method [16,17,18,19]. In addition, other authors used the closed vessel method to obtain the laminar combustion velocities of H2-N2O [20,21,22], C2H4-N2O or C2H4-N2O-N2 mixtures [23]. These results were helpful to provide data for testing and developing comprehensive chemical models of fuel-nitrous oxide combustion.
A widely examined fuel is methane, the main component of natural gas or mine gas. Methane is also the main flammable component of biogas, a low-cost, sustainable, renewable fuel produced by action of microorganisms on waste from vegetation, human and animal biological activity. Even if most previous research simply focused on the explosion of methane using air as oxidizer [24,25,26,27,28,29,30], the recent studies have also turned their attention to the explosive behavior of methane using N2O as oxidizer. Experiments using stationary flames (the burner method [31,32,33] or the counter-flow twin flames method [34]) or non-stationary flames (the constant volume method [35,36,37]) and numerical modeling were conducted using CH4-N2O gaseous mixtures (usually in the presence of diluents) to expand the available database regarding these flames.
Although the CH4-N2O-diluent flames were frequently studied, to date there is little data regarding their laminar combustion velocities and propagation speeds at pressures higher than ambient. In this research, the laminar combustion velocities of CH4-N2O-N2 flames are calculated using the constants of cubic law of the flame propagation during the early stage of explosion and the severity coefficients of unburned flammable mixtures, using the adiabatic model of the flame propagation [38]. The motivation of the present research rises from the necessity to predict and reduce the risks associated with fires and explosions in industrial facilities and research laboratories that handle and use nitrous oxide, an oxidizer with enhanced oxidizing potential.
The method approached in the present study is useful to evaluate the laminar combustion velocity of mixtures containing a composite fuel (i.e., diesel, gasoline, landfill gas or liquefied petroleum gas – LPG) or composite additives (i.e., flue gases), from records of pressure-time evolution during explosions in an enclosures, avoiding calculations performed using equations that contain parameters unavailable for mixtures of complex composition, such as the adiabatic flame temperature and pressure [38]. Because this calculation method involves only calculable parameters (the adiabatic compression coefficient and severity coefficient) along with the cubic law coefficients, it is suitable for determining the laminar combustion velocity for mixtures at different initial conditions.
Using the laminar combustion velocities and the severity coefficients, the propagation speeds of N2-diluted CH4-N2O flames were calculated. For both laminar combustion velocities and propagation speeds the pressure dependence is examined and discussed.
The data obtained in this research could share new perspectives regarding the explosion phenomena when nitrous oxide is used as oxidizer since N2O is more energetic compared with oxygen or air. On the other hand, the present results help develop explosion protection measures for handling, storage and use of these mixtures in industrial and domestic installations.
2. Experimental Procedure
Experiments were conducted in a spherical enclosure with a radius of 5 cm (V = 0.5 L) and central ignition, using stoichiometric and lean (φ = 0.8) CH4-N2O-N2 mixtures at different initial pressures between 0.5 and 2.0 bar and room temperature. In these small-scale explosions, the flame front of reactive mixtures does not develop significant cellular structures, a fact that supports the use of this calculation model. The studied CH4/N2O ratios, as shown by these equivalence ratios, were chosen as representative since they afford the complete fuel consumption and the comparison with other data sets from literature. For safety concerns, the CH4-N2O mixtures were diluted with various amounts of N2: 40, 50 and 60 vol% respectively, expressed as percentages of the total mixture. The mixtures prepared at a total pressure of 4 bar in a 10 L metallic cylinder according to the partial pressure method were used 48 h after mixing.
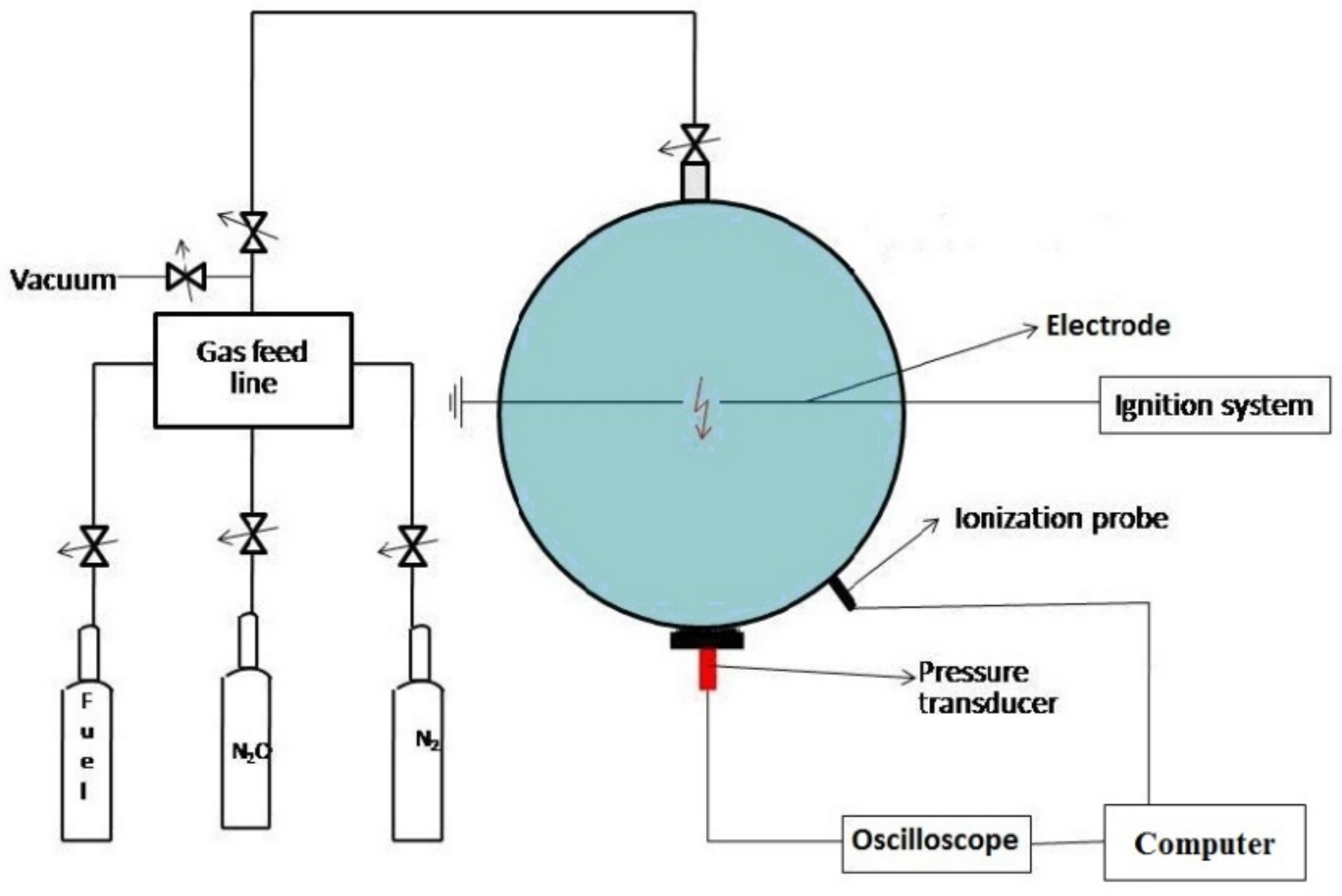
A schematic diagram of the experimental installation used in this research is illustrated in Figure 1.
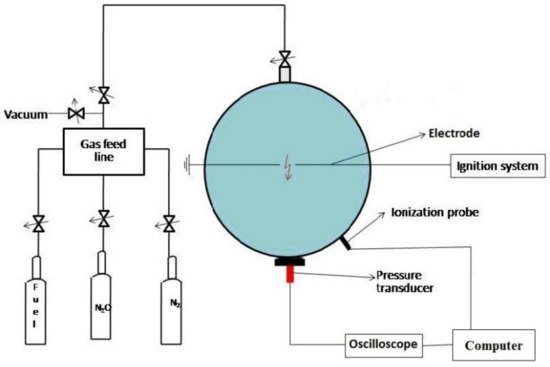
Figure 1.
Schematic diagram of the experimental installation.
The experimental enclosure is equipped with a control unit, a measurement and control system, a pressure measuring system, a vacuum pump, and an ignition device. Before each explosion test, the experimental cell was cleaned and vacuumed down to 0.1 mbar. After the mixture was added to the vessel, sparks produced between two stainless steel electrodes ignited the mixture. The spark gap located in the geometrical center of the vessel had a constant width of 3.5 mm. After ignition, the pressure transient during explosions is recorded using a piezoelectric pressure transducer (Kistler 601A, Kistler, Winterhur, Switzerland), coupled with a Charge Amplifier (Kistler 5001SN, Kistler, Winterhur, Switzerland) and an Acquisition Data System Tektronix TestLab 2505. Minimum three experiments were performed at each investigated initial pressure, in each case with fresh mixture, to ensure the accuracy and repeatability of the data. The errors in the pressure measurement were up to 3.5%. Methane (99.99%), N2O (99.999%) and nitrogen (99.99%), purchased from SIAD Italy, were used without further purification. Details of the experimental installation and procedure can also be found elsewhere [39,40,41].
3. Computing Program
The characteristic parameters of isobaric (the burned (end) gas volume and flame temperature) and isochoric (the flame temperature and peak explosion pressure) adiabatic combustion were obtained using COSILAB 0-D package [42]. The package allows the computation of the equilibrium composition of products for any fuel-oxidizer gaseous mixture using the minimum of free Gibbs energy (at constant pressure) and the minimum of the free Helmholtz energy (at constant volume) as thermodynamic criteria of chemical equilibrium. Fifty-three compounds were considered combustion products. The thermodynamic data were given according to the international standard format, put forward by Sandia National laboratories. The runs were performed for stoichiometric and lean (φ = 0.8) CH4-N2O flames in the presence of N2 at room temperature and various initial pressures (0.5–2.0 bar). Other details can be found in previous papers [43,44].
4. Results and Discussion
The laminar combustion velocity is defined as the linear velocity of the flame front normal to itself relative to unburned gas, or as the volume of unburned gas consumed per unit time divided by the area of the flame front in which that unburned gas volume is consumed. Straightforward procedures are necessary to obtain the laminar combustion velocity of stationary premixed flames, but their use is limited to experiments at ambient pressure. The determination of the laminar combustion velocities at pressures above 1 bar is made using experiments produced in closed cells, using methods for synchronous recording the pressure and flame radius, along with adequate correction procedures meant to consider the flame stretch [22,23]. A simple procedure for determining laminar combustion velocities only from the pressure variation during the early stage of closed cell explosions was earlier developed using either models of an isothermal or adiabatic propagation [38]. Most data were obtained from the isothermal model, which delivered reliable results for numerous gaseous mixtures, under variable initial composition, pressure, and temperature [37,38,39,45,46,47]. An alternative way is to use the adiabatic model of flame propagation that gives correlates the coefficient of cubic law of pressure variation during the early stage of flame propagation and its expansion coefficient with the laminar combustion velocity [37,48]. In the present research, this way was used to calculate the laminar combustion velocities of N2-diluted CH4-N2O mixtures and further on, their propagation speeds.
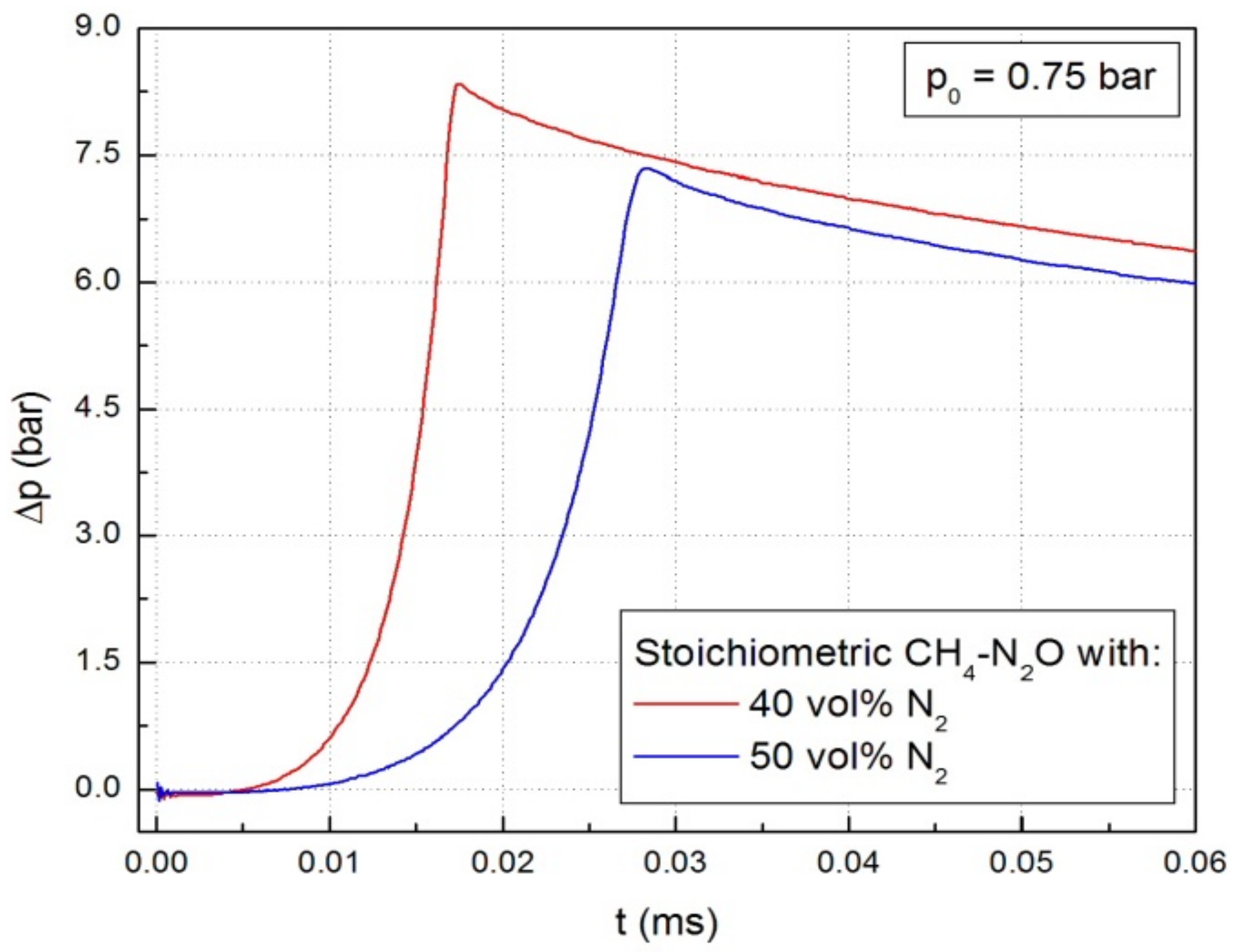
Typical diagrams of the pressure variation in the spherical vessel during the explosion of stoichiometric CH4-N2O mixture diluted with N2 (40% and 50%, respectively), at p0 = 0.75 bar and room temperature are illustrated in Figure 2. Similar graphs were obtained at all initial pressures.
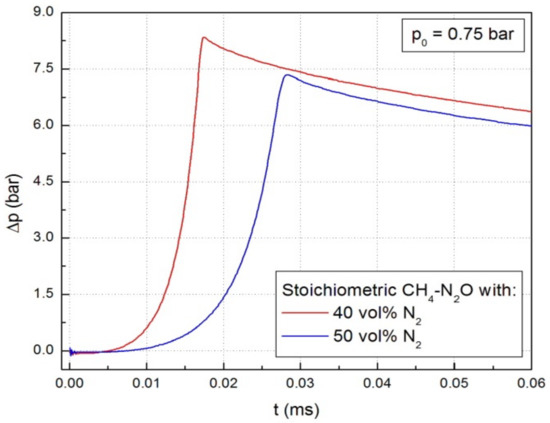
Figure 2.
Pressure variation during explosions in stoichiometric CH4-N2O mixture diluted with various N2 amounts, at p0 = 0.75 bar and room temperature.
Restricting the examination of the flame propagation only to the early stage, when the temperature gradients in both unburned and burned gas are small and can be neglected and when the flame maintains its spherical shape and the heat losses do not influence its development, a simple equation correlates the variation of pressure rise (Δp) with time from ignition (t) [38]:
where k is the cubic law constant; a and b are pressure and time corrections, respectively. These corrections are necessary to eliminate the errors in time and pressure measurements, respectively. Equation (1) is considered valid only for a restricted range of pressure variation: Δp ≤ p0 [38]. Considering an adiabatic compression of unburned gas ahead of the flame front, the laminar combustion velocity, Su, is obtained using the following relationship [38]:
here, R is the radius of the explosion enclosure; E is the expansion coefficient characteristic to the early (initial) stage of fuel-oxidizer explosion; p0 is the initial pressure and γu is the adiabatic compression coefficient of the unburned mixture.
It is worth mentioning that the present method requires the cubic law constants of pressure rise during the early stage of explosions, along with calculable properties such as the expansion coefficient and the adiabatic compression coefficient. This feature recommends this method for evaluation of early stage pressure variation in closed cells with various shapes and symmetry aspects, as used in many laboratory-scale explosion studies.
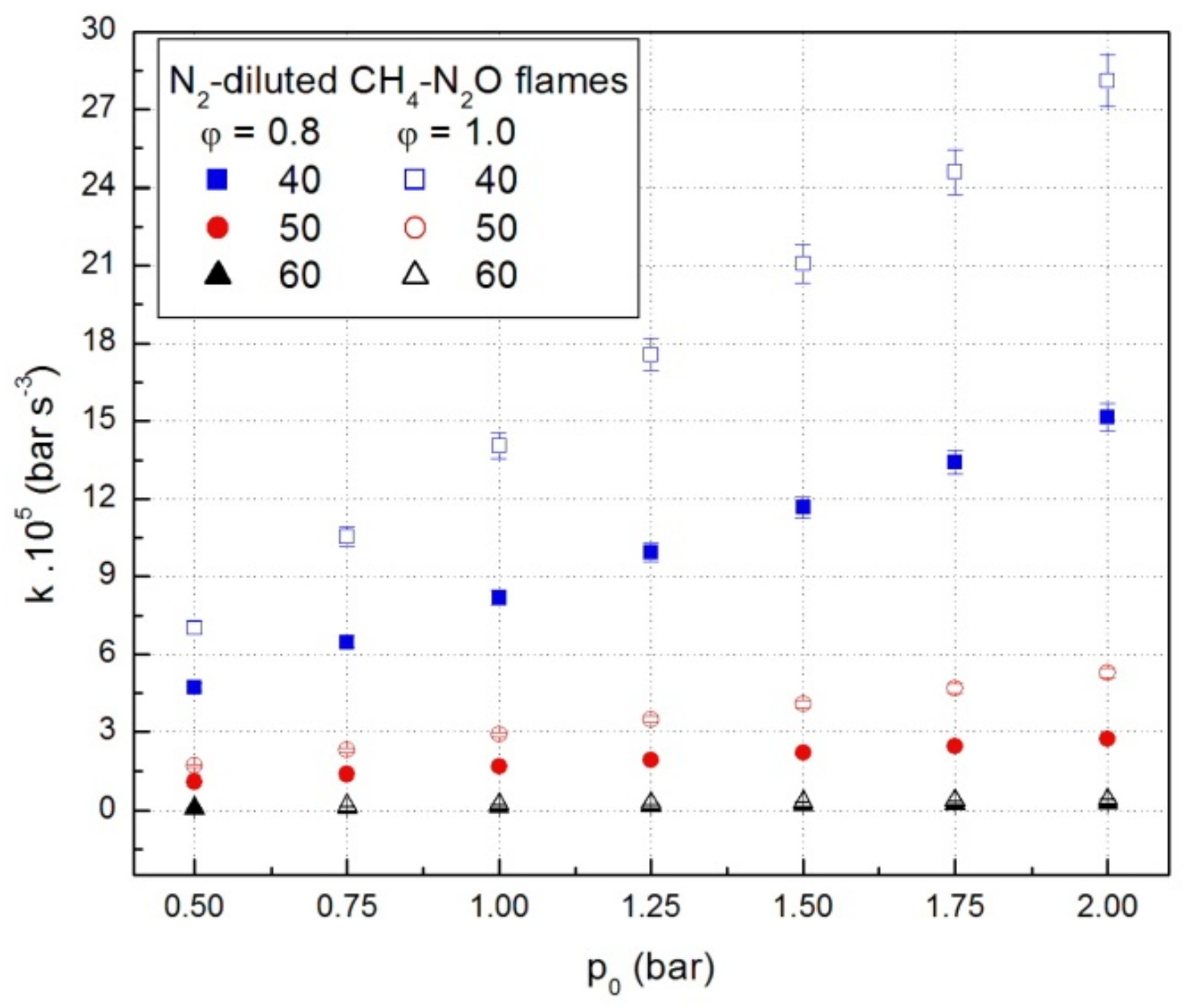
The coefficients k from Equation (1) were obtained for each experiment using a nonlinear regression method. Representative values of these coefficients, under various initial conditions, are given in Figure 3. They depend on many factors such as initial pressure, fuel, and diluent content. At constant initial temperature, fuel and dilution, the constants of cubic law increase with an increase in the initial pressure, as shown in Figure 3 for lean- and stoichiometric CH4-N2O mixtures diluted with N2.
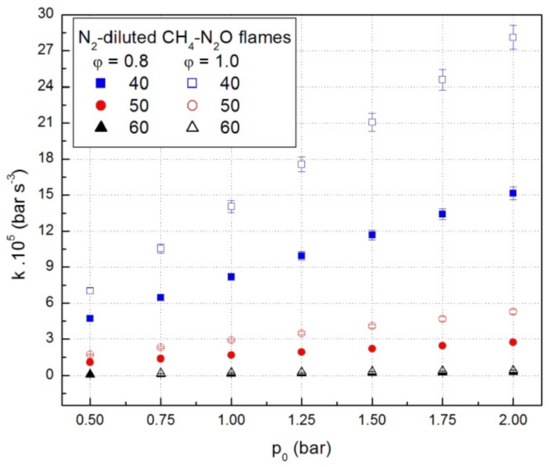
Figure 3.
Cubic law constants of deflagrations in CH4-N2O mixtures diluted with N2.
For any flammable mixture, the expansion coefficient defined as the ratio of burned and unburned gas volumes or the ratio of unburned to burned gas densities across the flame front can be obtained by equilibrium calculations:
where V0 is the volume of reactants and Ve is the volume of burned (“end”) gas generated by combustion of V0.
For N2-diluted CH4-N2O mixtures the expansion coefficients delivered by equilibrium calculations on their adiabatic isobaric combustion are given in Table 1. At each composition, the expansion coefficients increase slightly with the initial pressure; at constant initial pressure, the expansion coefficients decrease with the increase of the diluent content.

Table 1.
Expansion coefficients of deflagrations in N2-diluted CH4-N2O mixtures.
Flamm and Mache [49], Lewis and von Elbe [50], Babkin et al. [51], and Agrawal [52] suggested alternative relationships for calculating the expansion coefficients, when no equilibrium calculations are possible (e.g., flammable gases with complex composition). These equations are summarized in Table 2. They deliver approximate values of expansion coefficients, based on a parameter characteristic to the isochoric combustion, i.e., the maximum (end) pressure pe, reached in explosions at p0. It was noted that constants A and B (from Babkin’s equation) were found to be weakly dependent on the state of the mixture and its composition [51], so that Agrawal’s general equation is just a more simplified form of Babkin’s equation.

Table 2.
Approximate relationships for calculating the expansion coefficients, by data referring to the isochoric combustion of flammable mixtures.
A comparison of results obtained using these equations is given in Table 3, for two sets of initial conditions. For all data sets, the adiabatic explosion pressures were used instead of experimental peak explosion pressures, dependent on real, non-adiabatic conditions of propagation. This affords a better comparison of data given in Table 3. In all examples shown in this table the expansion coefficients obtained by equations Babkin-Agrawal are close to those delivered by the exact computations, while the values computed with the approximate equation of Flamm-Mache-Lewis-von Elbe are lower for all compositions. This prompted us to use only the expansion coefficients calculated by Equation (3) for calculating the laminar combustion velocities, using the constants of cubic law and the adiabatic compression model.

Table 3.
Expansion coefficients of CH4-N2O mixtures diluted with N2
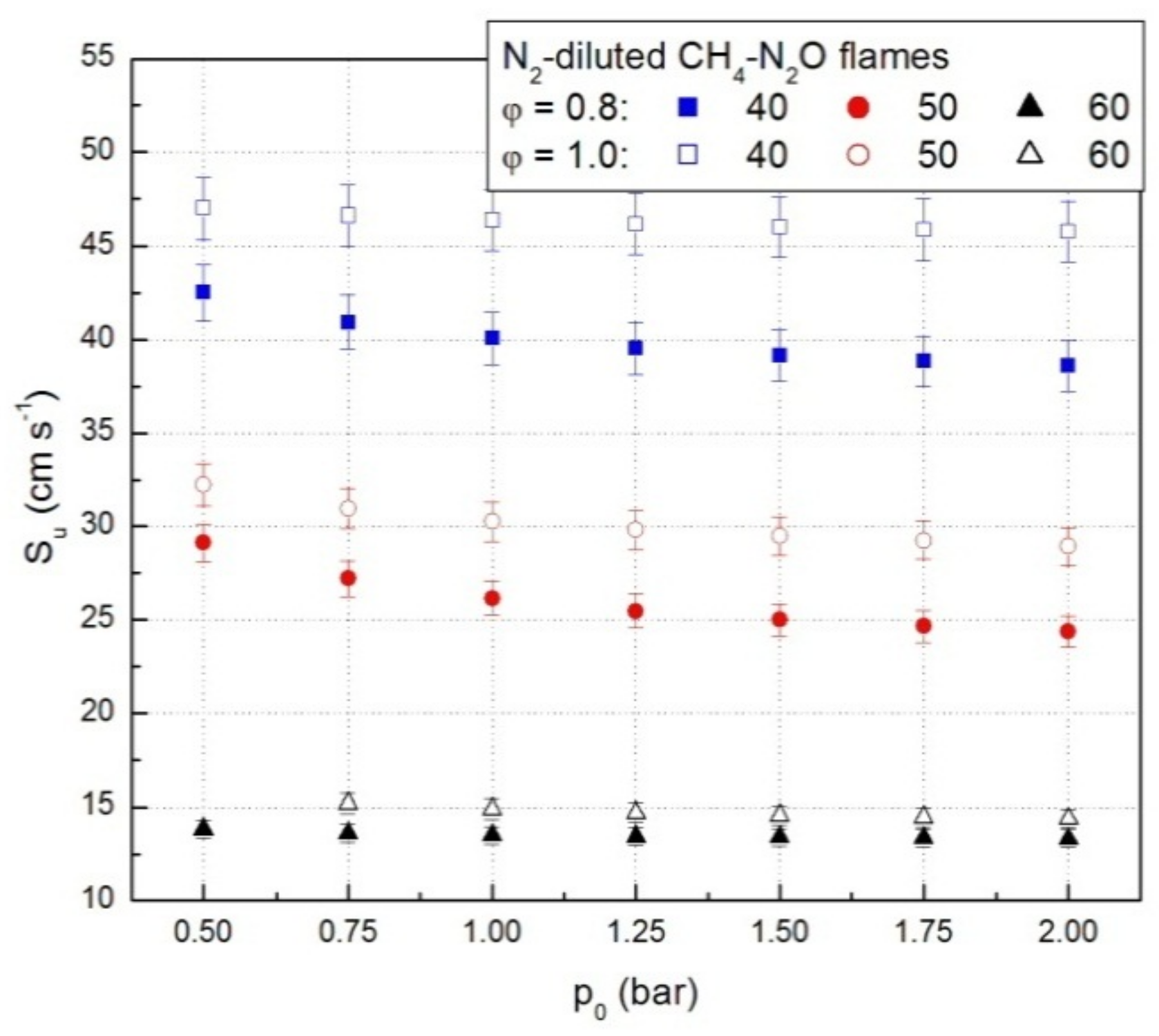
A set of representative results referring to lean CH4-N2O mixture diluted with N2 is plotted in Figure 4. Similar graphs were obtained for all N2-diluted CH4-N2O mixtures.
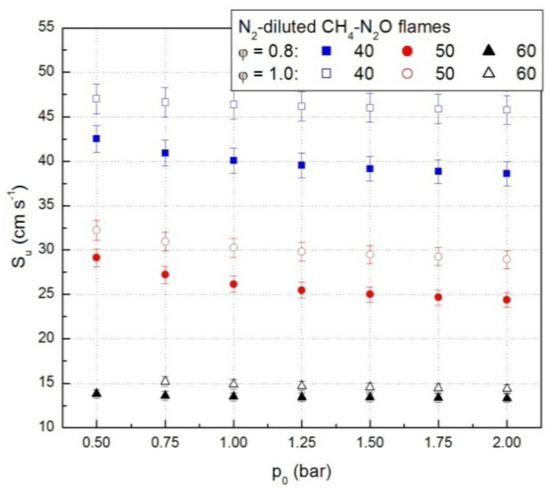
Figure 4.
Laminar combustion velocities of lean and stoichiometric CH4-N2O-N2 mixtures, from experimental p(t) data.
For all examined N2-diluted CH4-N2O mixtures the laminar combustion velocities decrease with initial pressure, within the examined range of initial conditions. A decrease of the laminar combustion velocity at initial pressure increase was already reported in the literature for other flammable gaseous mixtures, under various initial conditions [25,37,39,40,41,43,44,53].
The increase in the initial pressure usually decreases the radical concentration within the reaction zone, along with the increase of the flame temperature. These decreases in the radical concentrations are responsible in a large extent for the reduction in the laminar combustion velocities with initial pressure increase. Numerous computational studies on hydrocarbons burning pointed out that the main chain carriers are the radicals O, H and OH, which seem to most influence the fuel consumption reaction [37,40,45,54]. Another study [55] showed that CO and NO are also important for flame propagation in fuel-N2O mixtures.
The dependence of laminar combustion velocities on the initial pressure was analyzed using an empirical power law [38,43,44,45,46,47,56]:
where Su,ref is the laminar combustion velocity at reference pressure (pref) and ν is the baric coefficient. Choosing the atmospheric pressure as reference, pref = 1 bar, the corresponding baric coefficients of laminar combustion velocities of the studied mixtures were obtained using a non-linear regression analysis of Su = f(p). The results are presented in Table 4, where the baric coefficients of laminar combustion velocities delivered by the kinetic modeling of CH4-N2O-N2 flames by using GRI 3.0 mechanism [37] are also given.

Table 4.
The baric coefficients of laminar combustion velocities of the studied mixtures.
Excepting the lean CH4-N2O mixture diluted with 50% N2, a low pressure dependence of the laminar combustion velocities is observed. This behavior was already observed by Bane et al. [5] for undiluted H2-N2O flames at pressures between 1 and 3 bar. They found global reaction orders close to 2 for these mixtures, a fact that explains the observed low pressure dependency of the laminar combustion velocities. Furthermore, the authors noticed that for a given N2 dilution, there is no significant variation of the combustion speeds with the increase in the initial pressure. In addition, Movileanu et al. [46] reported a weak dependence of the laminar combustion velocity on the initial pressure for N2-diluted ethylene-air mixtures. The laminar combustion velocities reported in the present research can be compared with those reported in the literature.
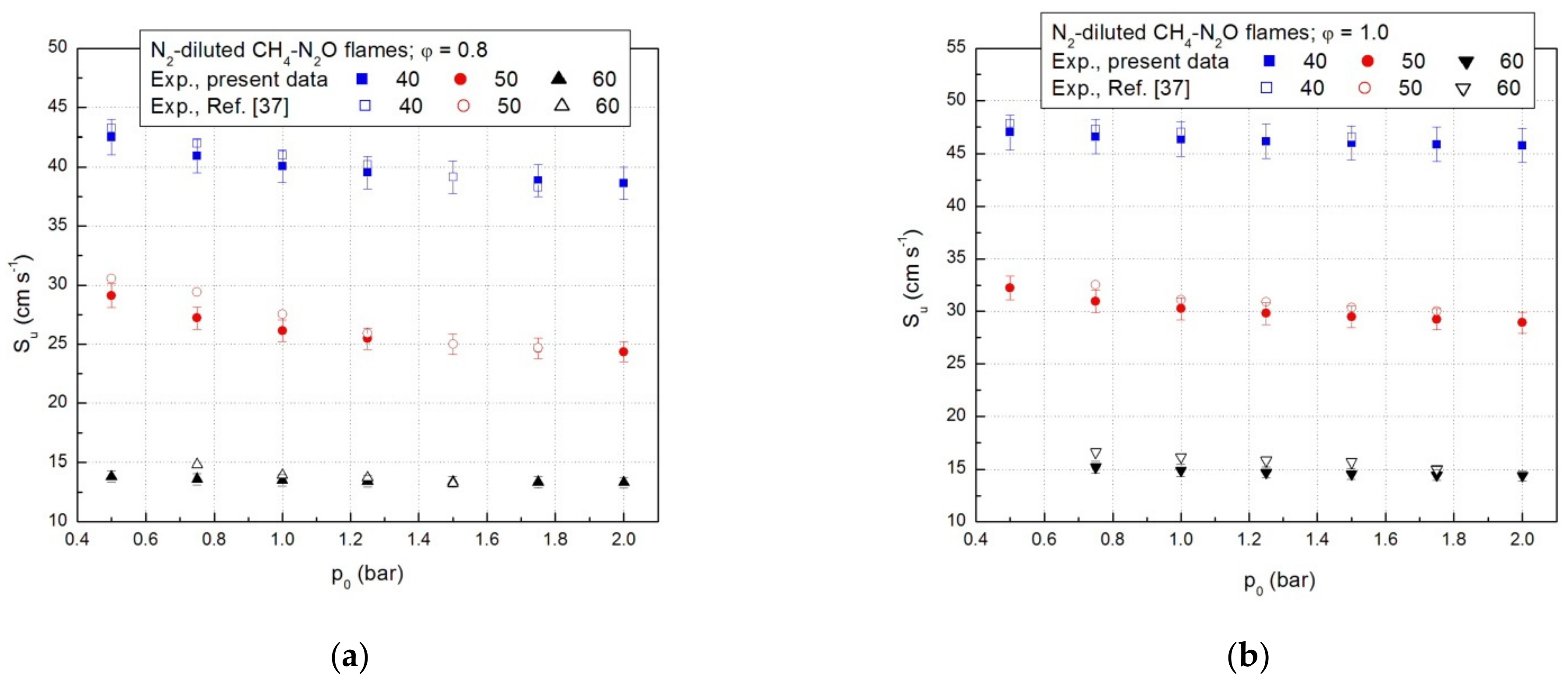
In a previous study [37], the laminar combustion velocities of CH4-N2O-N2 flames were calculated according to the model of isothermal flame propagation. A comparison between the present data and the laminar combustion velocities delivered by the isothermal model is given in Figure 5a,b. Within experimental errors, the laminar combustion velocities obtained from the adiabatic model of the flame propagation are close to those obtained by the isothermal compression model.
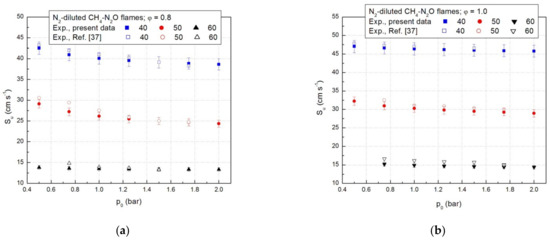
Figure 5.
Laminar combustion velocities of CH4-N2O mixtures diluted with N2; present results and literature data: (a) for equivalence ratio ϕ = 0.8 and (b) for equivalence ratio ϕ = 1.0.
Powell et al. [31,32,33] examined by experiments and modeling the laminar combustion velocities of stoichiometric CH4-N2O mixture in the presence of 52% N2 at near atmospheric pressure (0.8 bar). They reported Su = 24.2 cm s−1 from measurements using a flat flame burner and Su = 21.5 cm s−1 from modeling using a modified PPD mechanism. In comparison, we found a higher value: Su = 31.0 cm s−1 for the stoichiometric CH4-N2O mixture with 50% N2 at 0.75 bar (from experimental data).
Another study [57] was conducted on stoichiometric CH4-N2O mixture diluted with 40 and 50% N2 using a Bunsen burner. The results are given in Table 5 together with the present data referring to the same mixtures at ambient initial pressure; here, the two sets of data agree well.

Table 5.
Laminar combustion velocities of stoichiometric N2-diluted CH4-N2O mixtures.
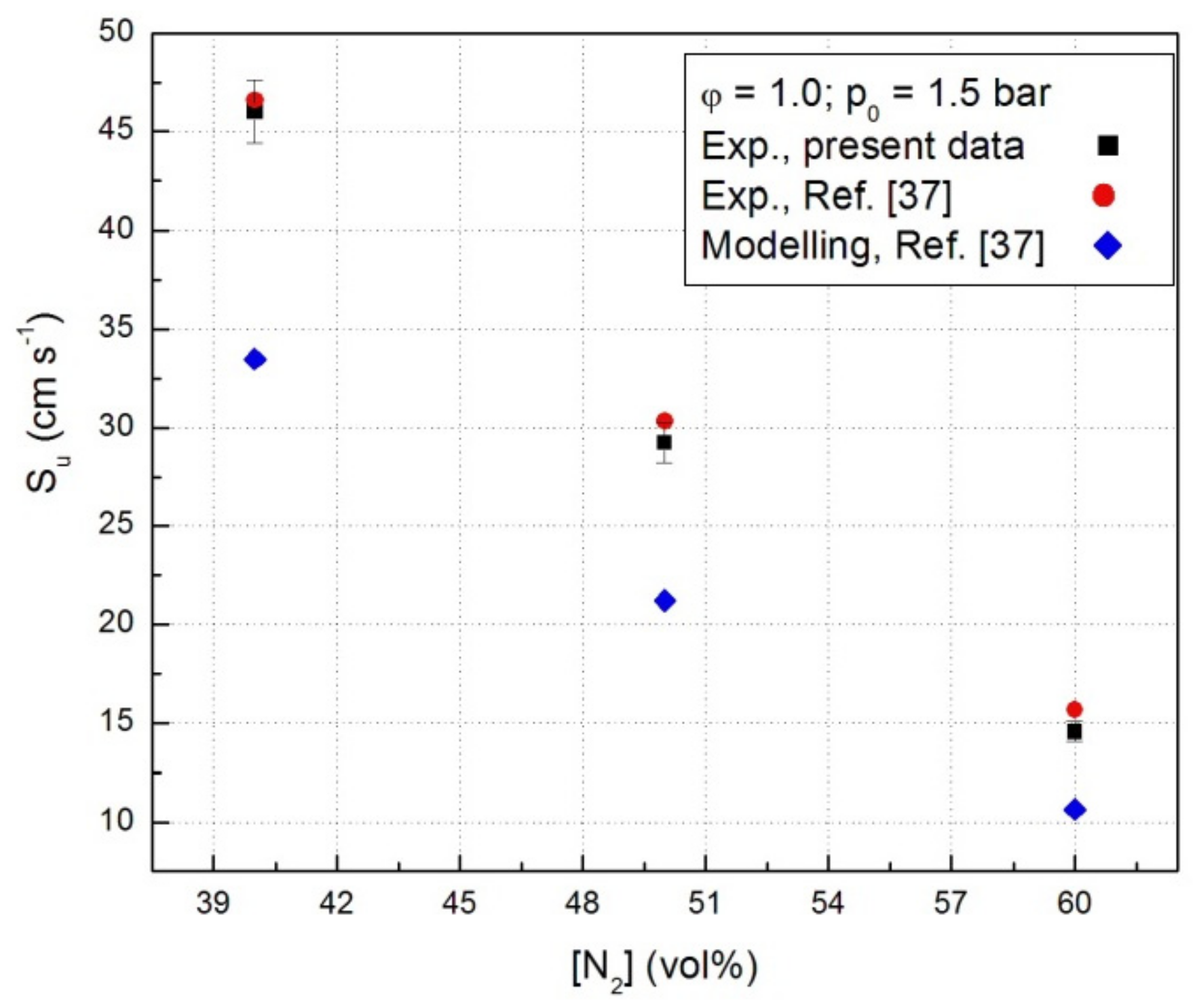
For all systems, the computed laminar combustion velocities of CH4-N2O-N2 flames were lower than the experimental Su as shown in Figure 6 where data referring to stoichiometric CH4-N2O mixture in the presence of N2 at constant initial pressure (p0 = 1.5 bar) are given. This was already reported for the laminar combustion velocities obtained for these mixtures from closed vessel experiments [37] or from flat flames, observed at 0.8 bar [32,33]. This trend was found for C2H4-N2O-N2 mixtures as well, when the kinetic modeling of these flames has been made with GRI 3.0 mechanism, but no discrepancies were observed using other mechanisms with improved rate constants [23]. The sensitivity analyses undertaken by Powell et al. [32,33] revealed that several reactions within the hydrogen-N2O sub-mechanism are most important with respect to the laminar combustion velocity:
N2O (+ M) ⇔ N2+ O (+M)
N2O + H ⇔ N2+ OH
NH + NO ⇔ N2O + H
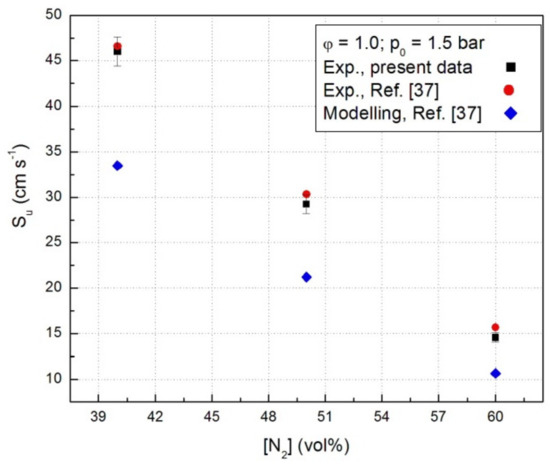
Figure 6.
Laminar combustion velocities of stoichiometric N2-diluted CH4-N2O flames at constant initial pressure.
The improvement of their rate constants could lead to better predictions of the laminar combustion velocities.
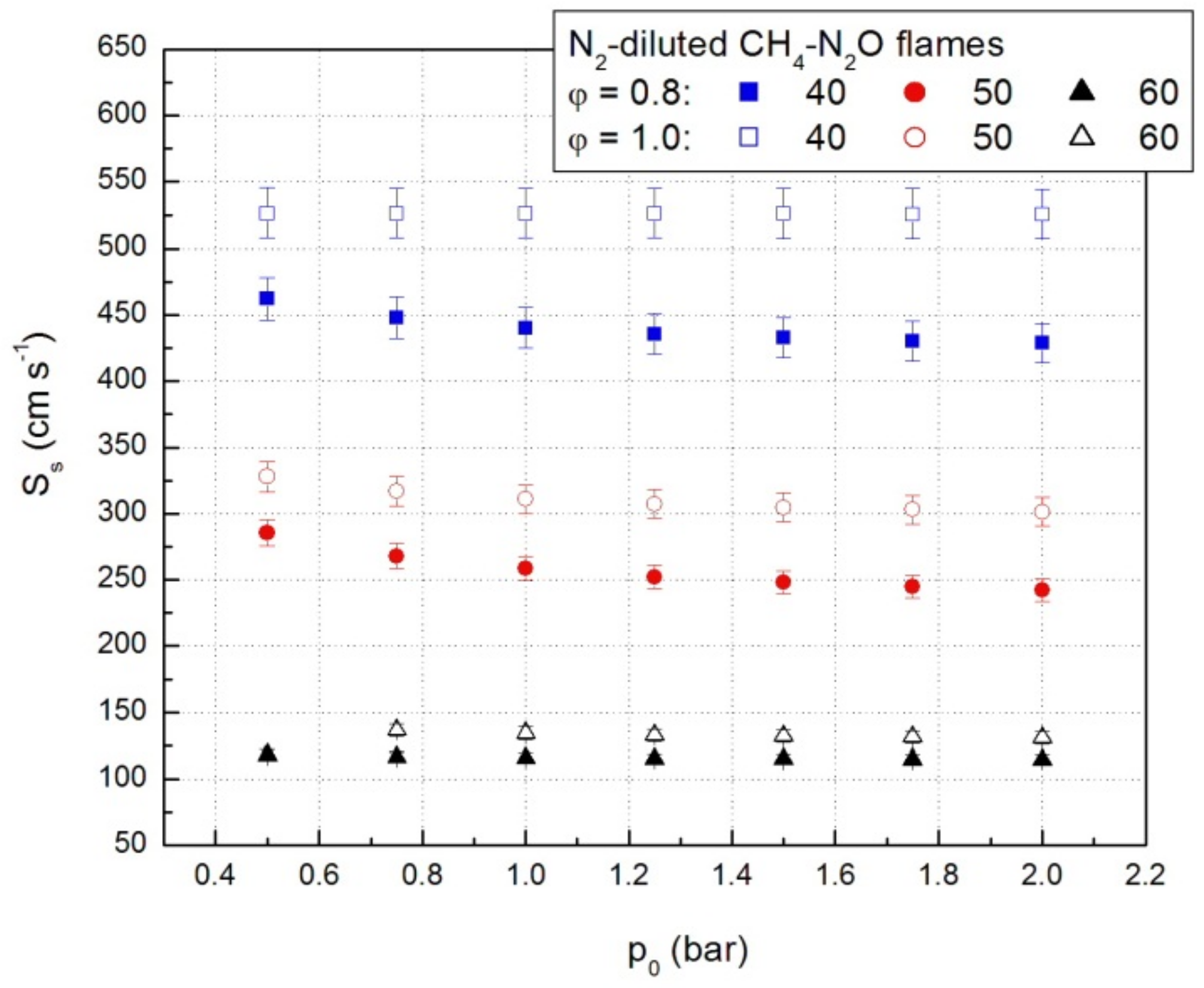
The presence of diluents in the flammable mixtures strongly affects both the kinetics and thermodynamics of the combustion process and consequently the laminar combustion velocity. As expected, the increase of the diluent content leads to the decrease of the laminar combustion velocity as it can be observed from Figure 6. The same trend was obtained at all initial pressures.
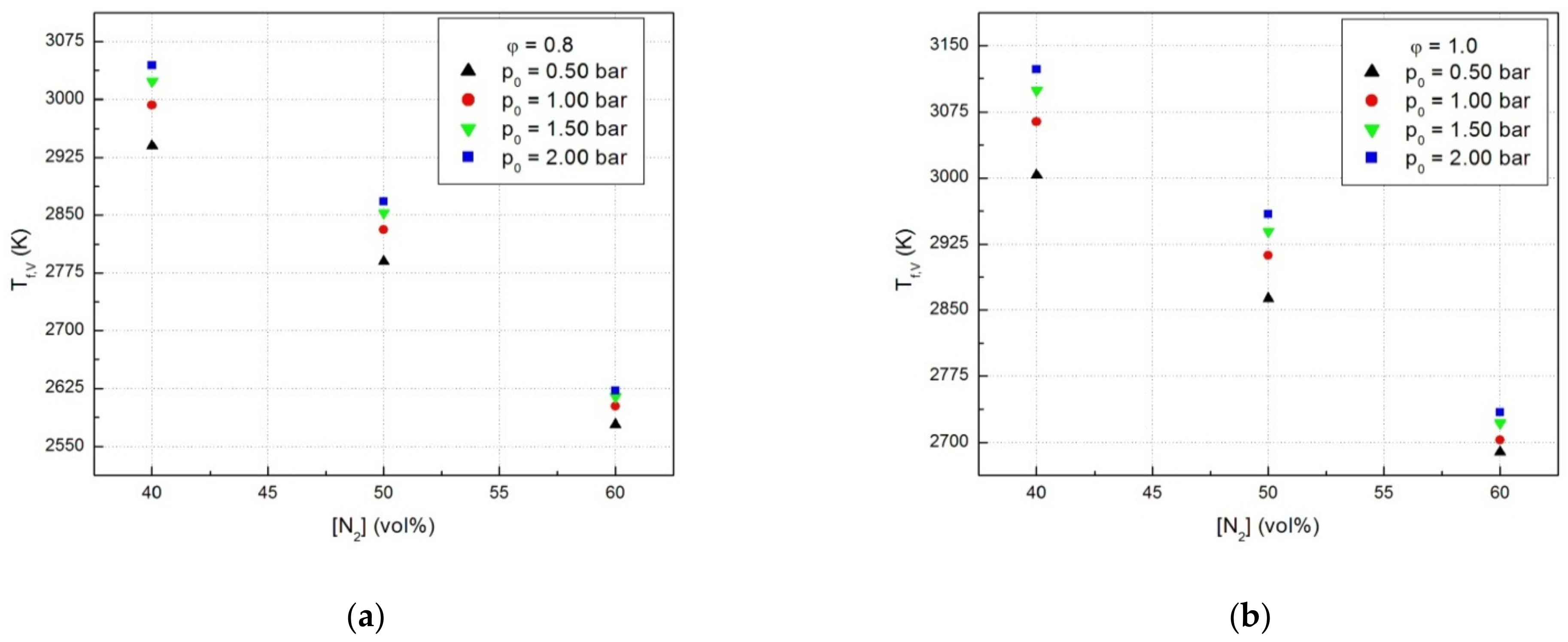
The decrease of the laminar combustion velocities determined by dilution with an inert gas can be linked with the decrease of the adiabatic flame temperatures of examined mixtures. The plots of adiabatic flame temperatures (Tf,V) in the isochoric combustion of N2-diluted CH4-N2O flames of constant equivalence ratio against the diluent content, shown in Figure 7a,b, have the same variation trend as the laminar combustion velocities, as already pointed out earlier [45].
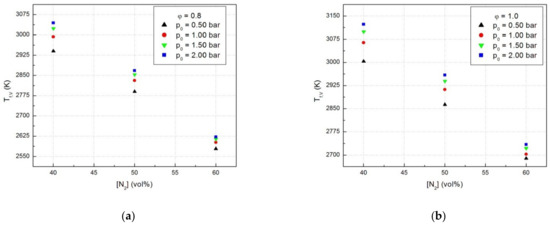
Figure 7.
Adiabatic flames temperatures of CH4-N2O mixtures in the presence of N2: (a) for equivalence ratio ϕ = 0.8 and (b) for equivalence ratio ϕ = 1.0.
At constant initial pressure and temperature, adding nitrogen to a flammable mixture influences flame propagation through concentration changes (diminution of CH4 and N2O concentrations), and through changes of reactivity. Dilution leads to diminution of available heat which sustain the explosion and hence, in the decrease of the temperature of the flame. Simultaneously, N2 addition significantly reduces the reaction rate, the rate of heat release and the total amount of radical species. Furthermore, in N2O atmosphere, nitrogen is not acting just as an inert component. As outlined by Shen et al. [12], N2 can participate to consume O, inhibiting thus the decomposition of N2O by reaction (R.4):
O + N2 (+ M) ⇔ N2O (+M)
An additional influence is exerted by N2 through its third-body collisional effect since it favors reaction (R.6) over the branching reaction (R.5), not mentioning the fact that HO2 is less reactive than O or OH [12]:
H + O2 ⇔ O + OH
H + O2 (+ M) ⇔ HO2 (+M)
The laminar combustion velocities obtained in the present research together with the expansion coefficients can be used to calculate the propagation speeds, Ss, using the following equation:
Relevant values of the propagation speeds obtained using Equation (5) are given in Figure 8. The pressure increase determines the decrease of propagation speeds. Simultaneously, the addition of a diluent also determines the decrease of the propagation velocities of the CH4-N2O-N2 mixtures by the decrease of the initial fuel concentration.
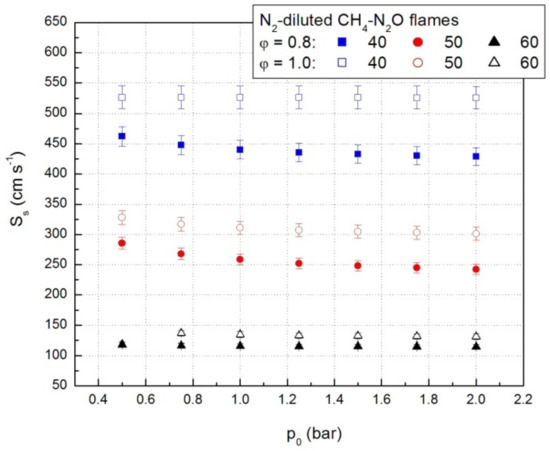
Figure 8.
Propagation speeds of lean and stoichiometric CH4-N2O-N2 mixtures.
The propagation speed depends on the same parameters as the laminar combustion velocity (initial pressure and temperature, fuel type, fuel-oxidizer ratio, presence of diluents) [58,59] and, additionally, on the flow pattern. A power law characteristic for flammable mixtures describes the dependence of the propagation speeds on the initial pressure [47]:
In Equation (6) Ss,ref is the propagation speed at reference conditions (here pref = 1 bar) and a is the baric coefficient. The baric coefficients of the propagation speeds, determined by a non-linear regression analysis of Ss = f(p) data, are presented in Table 6.

Table 6.
Baric coefficients of the propagation speeds of CH4-N2O-N2 mixtures.
As shown by data from Table 6, a low pressure dependence of the propagation speed is observed, as in the case of laminar combustion velocities. The baric coefficients of propagation speeds do not differ much from those of laminar combustion velocities, since the pressure has a reduced influence on expansion coefficients.
The flammable mixtures of CH4 and N2O raise much interest, as both components are present in the complex gaseous mixtures generated by nuclear wastes in the dome space of storage tanks. Moreover, the laminar combustion velocity and propagation speed, which are fundamental properties of any combustible mixture, are essential for the design of venting devices, combustors, engines (e.g., internal combustion or rocket engines), power plant burners etc. The laminar combustion velocity is also used in theoretical modeling of the pressure-time evolution inside a cell or a piping, which is important to consider during the design process.
5. Conclusions
The laminar combustion velocity and propagation speed are the most useful parameters for various applications important in industry and for safety, with impact on environmental protection. Simultaneously, the laminar combustion velocity and propagation speed are used to validate chemical reaction mechanisms and modeling the turbulent combustion. Therefore, the accurate determination of the laminar combustion velocity and propagation speed is of great interest.
The combustion burning velocities of lean and stoichiometric CH4-N2O mixtures in the presence of N2, at various initial pressures and room temperature, were obtained from the constants of cubic law of flame propagation during the early stage of closed cell explosions and from the expansion factors. The laminar combustion velocities and expansion coefficients were later used to calculate the propagation speeds of the N2 diluted CH4-N2O flames.
For the studied systems, a decrease of the laminar combustion velocities and hence of the propagation speeds was observed when the initial pressures increased. The laminar combustion velocity and the propagation speed dependence on initial pressure were examined by empirical power laws. The baric coefficients of these velocities were calculated by non-linear regression analysis of Su = f(p) and Ss = f(p), respectively.
The laminar combustion velocities were compared with those obtained from the isothermal model of flame propagation and with those obtained from the kinetic modeling of N2-diluted CH4-N2O flames. The results reveal that both the isothermal model and the adiabatic compression model deliver higher values of laminar combustion velocities than those computed with GRI 3.0 mechanism.
The increase of the diluent content leads to the decrease of the laminar combustion velocities and the propagation speeds for all studied initial pressures and mixtures composition. The decrease of the laminar combustion velocities determined by dilution with an inert gas can be linked with the decrease of the adiabatic flame temperatures of examined mixtures and hence by the diminution of available heat able to sustain the explosion as well as the rate of heat release.
As the present method of laminar combustion velocity calculation needs only calculable quantities (expansion coefficient and adiabatic compression coefficient) along with the cubic law coefficients, this method is suitable for the evaluation of data recorded in cylindrical enclosures (centrally ignited) frequently used in industry and laboratory research.
Author Contributions
Conceptualization, V.G. and M.M.; methodology, D.O.; software, V.G., M.M. and C.M.; validation, V.G. and D.R.; investigation, M.M. and C.M.; data curation, V.G., M.M. and C.M.; writing—original draft preparation, V.G. and D.R.; visualization, M.M., V.G. and C.M.; supervision, D.R.; D.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The present study was partially supported by the Romanian Academy under research project “Dynamics of fast oxidation and decomposition reactions in homogeneous systems” of Ilie Murgulescu Institute of Physical Chemistry.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tyll, J.S.; Herdy, R. Nitrous Oxide/Propane Rocket Engine; Report for BAA 99-22, No. GSL-TR-387; Microcraft Inc.: Huntsville, AL, USA, 2001. [Google Scholar]
- Taylor, R. Safety and performance advantages of nitrous oxide fuel blends (NOFBX) propellants for manned and unmanned spaceflight applications. In Proceedings of the 5th IAASS Conference: A Safer Space for Safer World, Versailles, France, 17–19 October 2011; p. 67. [Google Scholar]
- Perakis, N.; Hochheimer, B.; Werling, L.; Gernoth, A.; Schlechtriem, S. Development of an experimental demonstrator unit using nitrous oxide/ethylene premixed bipropellant for satellite applications. In Proceedings of the Meet the Space Conference, Krakow, Poland, 3–6 November 2014. [Google Scholar]
- Pfahl, U.; Shepherd, J.E. Nitrous oxide consumption and flammability limits of H2-N2-air and CH4-N2O-O2-N2 mixtures. Explosion Dynamic Laboratory Report FM 97-16. In Proceedings of the Combustion Institute Meeting Fall: Western States Section, East Hartford, CN, USA, 27–29 October 1997. [Google Scholar]
- Bane, S.P.M.; Mevel, R.; Coronel, S.A.; Shepherd, J.E. Flame burning speeds and combustion characteristics of undiluted and nitrogen-diluted hydrogen-nitrous oxide mixtures. Int. J. Hydrog. Energy 2011, 36, 10107–10116. [Google Scholar] [CrossRef]
- Holland, S.; Jones, D.T.; Gray, P. Combustion supported by nitrous oxide: Flame speeds and flammability limits in the hydrogen + ethane + nitrous oxide system. Combust. Flame 1971, 17, 31–35. [Google Scholar] [CrossRef]
- Konnov, A.A.; Dyakov, I.V. Nitrous oxide conversion in laminar premixed flames of CH4 + O2 + Ar. Proc. Combust. Inst. 2009, 32, 319–326. [Google Scholar] [CrossRef]
- Koshiba, Y.; Takigawa, T.; Matsuoka, Y.; Ohtani, H. Explosion characteristics of flammable organic vapors in nitrous oxide atmosphere. J. Hazard. Mat. 2010, 183, 746–753. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koshiba, Y.; Nishida, T.; Morita, N.; Ohtani, H. Explosion behavior of n-alkane/nitrous oxide mixtures. Process Saf. Environ. Prot. 2015, 98, 11–15. [Google Scholar] [CrossRef]
- Koshiba, Y.; Hasegawa, T.; Kim, B.; Ohtani, H. Flammability limits, explosion pressures, and applicability of Le Chatelier’s rule to binary alkane-nitrous oxide mixtures. J. Loss Prev. Process Ind. 2017, 45, 1–8. [Google Scholar] [CrossRef]
- Koshiba, Y.; Hasegawa, T.; Ohtani, H. Numerical and experimental study of the explosion pressures and flammability limits of lower alkenes in nitrous oxide atmosphere. Process Saf. Environ. Prot. 2018, 118, 59–67. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, N.; Shi, X.; Cheng, X. Experimental studies on pressure dynamics of C2H4/N2O mixtures explosion with dilution. Appl. Therm. Eng. 2019, 147, 74–80. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.Q.; Ma, H.H.; Pan, J.; Liu, R.; Shen, Z.W.; Feng, S.Y. Explosion characteristics of H2/CH4/N2O at fuel-lean and stoichiometric conditions. Comb. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Wang, L.; Ma, H.; Shen, Z. Explosion characteristics of H2/N2O and CH4/N2O diluted with N2. Fuel 2020, 260, 116355. [Google Scholar] [CrossRef]
- Wang, L.Q.; Ma, H.H.; Shen, Z.W.; Pan, J. A comparative study of the explosion behaviors of H2 and C2H4 with air, N2O and O2. Fire Saf. J. 2021, 119, 103260. [Google Scholar] [CrossRef]
- Van Wonterghem, J.; Van Tiggelen, A. Flame propagation in gaseous mixtures containing nitrous-oxide as oxidant. Bull. Soc. Chim. Belg. 1955, 64, 780–797. [Google Scholar] [CrossRef]
- Aldous, K.M.; Bailey, B.W.; Rankin, J.M. Burning velocity of the premixed nitrous-oxide/acetylene flame and its influence on burner design. Anal. Chem. 1972, 44, 191–194. [Google Scholar] [CrossRef]
- Linteris, G.T.; Rumminger, M.D.; Babushok, V.I. Premixed carbon monoxide-nitrous oxide-hydrogen flames: Measured and calculated burning velocities with and without Fe(CO)5. Combust. Flame 2000, 122, 58–75. [Google Scholar] [CrossRef]
- Naumann, C.; Kick, T.; Methling, T.; Braun-Unkhoff, M.; Riedel, U. Ethene/dinitrogen oxide-a green propellant to substitute hydrazine: Investigation on its ignition delay time and laminar flame speed. In Proceedings of the 26th ICDERS, Boston, MA, USA, 30 July–4 August 2017; p. 1075. [Google Scholar]
- Gray, P.; Mackinven, R.; Smith, D. Combustion of hydrogen and hydrazine with nitrous oxide and nitric oxide: Flame speeds and flammability limits of ternary mixtures at sub-atmospheric pressures. Combust. Flame 1967, 11, 217–226. [Google Scholar] [CrossRef]
- Gray, P.; Holland, S.; Smith, D. The effect of isotopic substitution on the flame speeds of hydrogen-oxygen and hydrogen-nitrous oxide flames. Combust. Flame 1970, 14, 361–374. [Google Scholar] [CrossRef]
- Mével, R.; Lafosse, F.; Chaumeix, N.; Dupré, G.; Paillard, C.E. Spherical expanding flames in H2-N2O-Ar mixtures: Flame speed measurements and kinetic modelling. Int. J. Hydrog. Energy 2009, 34, 9007–9018. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H. Laminar burning velocities of C2H4/N2O flames: Experimental study and its chemical kinetics mechanism. Combust. Flame 2019, 202, 362–375. [Google Scholar] [CrossRef]
- Cammarota, F.; Di Benedetto, A.; Russo, P.; Salzano, E. Experimental analysis of gas explosions at non-atmospheric initial conditions in cylindrical vessel. Process Saf. Environ. Prot. 2010, 88, 341–349. [Google Scholar] [CrossRef]
- Hinton, N.; Stone, R. Laminar burning velocity measurements of methane and carbon dioxide mixtures (biogas) over wide ranging temperatures and pressures. Fuel 2014, 116, 743–750. [Google Scholar] [CrossRef]
- Zheng, K.; Yu, M.; Wen, X. Comparative study of the propagation of methane/air and hydrogen/air flames in a duct using large eddy simulation. Process Saf. Environ. Prot. 2018, 120, 45–56. [Google Scholar] [CrossRef]
- Fan, W.P.; Gao, Y.; Zhang, Y.M.; Chow, C.L.; Chow, W.K. Experimental studies and modeling on flame velocity in turbulent deflagration in an open tube. Process Saf. Environ. Prot. 2019, 129, 291–307. [Google Scholar] [CrossRef]
- Sulaiman, S.Z.; Khan, N.A.M.H.; Izhab, I.; Shaarani, S.M.; Mudalip, S.K.A.; Man, R.C.; Arshad, Z.I.; Kasmani, R.M.; Sulaiman, S. Explosion characteristics assessment of premixed biogas/air mixture in a 20-L spherical vessel. Chem. Eng. Commun. 2021, 208, 583–591. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Zheng, L.; Yu, M.; Pan, R.; Shan, W. Study on the propagation characteristics of hydrogen/methane/air premixed flames in variable cross-section ducts. Process Saf. Environ. Prot. 2020, 135, 135–143. [Google Scholar] [CrossRef]
- Luo, Z.; Liang, H.; Wang, T.; Cheng, F.; Su, B.; Liu, L.; Liu, B. Evaluating the effect of multiple flammable gases on the flammability limit of CH4: Experimental study and theoretical calculation. Process Saf. Environ. Prot. 2021, 146, 369–376. [Google Scholar] [CrossRef]
- Powell, O.A.; Miller, J.E.; Dreyer, C.; Papas, P. Characterization of hydrocarbon/nitrous oxide propellant combinations. In Proceedings of the 46th AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 7–10 January 2008. [Google Scholar]
- Powell, O.A.; Papas, P.; Dreyer, C. Laminar burning velocities for hydrogen-, methane-, acetylene- and propane-nitrous oxide flames. Combust. Sci. Technol. 2009, 181, 917–936. [Google Scholar] [CrossRef]
- Powell, O.A.; Papas, P.; Dreyer, C.B. Hydrogen- and C1-C3 hydrocarbon-nitrous oxide kinetics in freely propagating and burner-stabilized flames, shock tubes, and flow reactors. Combust. Sci. Technol. 2010, 182, 252–283. [Google Scholar] [CrossRef]
- Newman-Lehman, T.; Grana, R.; Seshadri, K.; Williams, F. The structure and extinction of nonpremixed methane/nitrous oxide and ethane/nitrous oxide flames. Proc. Combust. Inst. 2013, 34, 2147–2153. [Google Scholar] [CrossRef]
- Zamaschikov, V.V.; Bunev, V.A.; Dubkov, K.A.; Panov, G.I.; Babkin, V.S. Combustion characteristics of mixtures of hydrocarbons with N2O. In Proceedings of the 5th International Seminar on Flame Structure, Novosibirsk, Russia, 11–14 July 2005. [Google Scholar]
- Shebeko, A.Y.; Shebeko, Y.N.; Zuban, A.V.; Navzenya, V.Y. An experimental investigation of an inertization effectiveness of fluorinated hydrocarbons in relation to premixed H2-N2O and CH4-N2O flames. J. Loss Prev. Proc. Ind. 2013, 26, 1639–1645. [Google Scholar] [CrossRef]
- Razus, D.; Mitu, M.; Giurcan, V.; Movileanu, C.; Oancea, D. Methane-unconventional oxidant flames. Laminar burning velocities of nitrogen-diluted methane-N2O mixtures. Process Saf. Environ. Prot. 2018, 114, 240–250. [Google Scholar] [CrossRef]
- Razus, D.; Oancea, D.; Movileanu, C. Burning velocity evaluation from pressure evolution during the early stage of closed-vessel explosions. J. Loss Prev. Process Ind. 2006, 19, 334–342. [Google Scholar] [CrossRef]
- Mitu, M.; Razus, D.; Giurcan, V.; Oancea, D. Normal burning velocity and propagation speed of ethane-air: Pressure and temperature dependence. Fuel 2015, 147, 27–34. [Google Scholar] [CrossRef]
- Movileanu, C.; Mitu, M.; Razus, D.; Giurcan, V.; Oancea, D. Propagation indexes of C2H4-N2O-N2 deflagrations in elongated closed vessels. Rev. Roum. Chim. 2017, 62, 357–363. [Google Scholar]
- Giurcan, V.; Mitu, M.; Razus, D.; Oancea, D. Experimental study and kinetic modeling of laminar flame propagation in premixed stoichiometric n-butane-air mixture. Rev. Chim. 2019, 70, 1125–1131. [Google Scholar] [CrossRef]
- COSILAB. Version 3.0.3; Rotexo-Softpredict-Cosilab GmbH & Co KG: Bad Zwischenhahn, Germany, 2012. [Google Scholar]
- Giurcan, V.; Razus, D.; Mitu, M.; Oancea, D. Numerical study of the laminar flame propagation in ethane-air mixtures. Cent. Eur. J. Chem. 2014, 12, 391–402. [Google Scholar] [CrossRef]
- Mitu, M.; Giurcan, V.; Razus, D.; Oancea, D. Influence of initial pressure and vessel’s geometry on deflagration of stoichiometric methane-air mixture in small-scale closed vessels. Energy Fuels 2020, 34, 3828–3835. [Google Scholar] [CrossRef]
- Razus, D.; Brinzea, V.; Mitu, M.; Oancea, D. Burning velocity of LPG (Liquefied petroleum gas)-air mixtures in the presence of exhaust gas. Energy Fuels 2010, 24, 1487–1494. [Google Scholar] [CrossRef]
- Movileanu, C.; Razus, D.; Oancea, D. Additive effects on the burning velocity of ethylene-air mixtures. Energy Fuels 2011, 25, 2444–2451. [Google Scholar] [CrossRef]
- Mitu, M.; Giurcan, V.; Razus, D.; Oancea, D. Inert gas influence on laminar burning velocity of methane-air mixtures. J. Hazard. Mater. 2017, 321, 440–448. [Google Scholar] [CrossRef]
- Perlee, H.; Fuller, F.; Saul, C. Constant-volume flame propagation. US Bur. Mines Rep. 2016, 61. [Google Scholar] [CrossRef]
- Flamm, L.; Mache, H. Combustion of an explosive gas mixture within a closed vessel. Wien Ber. Akad. Viss. 1917, 126, 9–16. [Google Scholar]
- Lewis, B.; von Elbe, G. Determination of the speed of flames and the temperature distribution in a spherical bomb from time-pressure explosion records. J. Chem. Phys. 1934, 2, 283–290. [Google Scholar] [CrossRef]
- Babkin, V.S.; V’yun, A.V.; Kozachenko, L.S. Effect of pressure on normal flame velocity investigated by the initial-section method in a constant-volume vessel. Fiz. Gorenyia Vzryva 1966, 2, 52–60. [Google Scholar] [CrossRef]
- Agrawal, D. Experimental determination of burning velocity of methane-air mixtures in a constant volume vessel. Combust. Flame 1981, 42, 243–252. [Google Scholar] [CrossRef]
- Han, P.; Checkel, M.D.; Fleck, B.A.; Nowicki, N.L. Burning velocity of methane/diluent mixture with reformer gas addition. Fuel 2007, 86, 585–596. [Google Scholar] [CrossRef]
- Biet, J.; Delfau, J.L.; Seydi, A.; Vovelle, C. Experimental and modeling study of lean premixed atmospheric-pressure propane/O2/N2 flames. Combust. Flame 2005, 142, 197–209. [Google Scholar] [CrossRef]
- Powell, O.A.; Papas, P. Flame structure measurements of nitrous oxide in hydrocarbon-nitrous-oxide flames. J. Prop. Power 2012, 28, 1052–1059. [Google Scholar] [CrossRef]
- Dahoe, A.E.; de Goey, L.P.H. On the determination of the laminar burning velocity from closed vessel gas explosions. J. Loss Prev. Process Ind. 2003, 16, 457–478. [Google Scholar] [CrossRef]
- D’Olieslager, J.; van Tiggelen, A. Kinetical study of hydrocarbon-nitrous oxide flames. Bull Soc. Chim. Belg. 1964, 73, 135–153. [Google Scholar] [CrossRef]
- Lewis, B.; von Elbe, G. Combustion, Flames and Explosion of Gases, 3rd ed.; Academic Press: New York, NY, USA, 1987. [Google Scholar]
- Hattwig, M.; Steen, H. Handbook of Explosion Prevention and Protection; Wiley VCH: Weinheim, Germany, 2004. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).