Efficient Removal of Cr (VI) with Biochar and Optimized Parameters by Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Response Surface Optimization
3. Results and Discussion
3.1. Response Surface Methodology
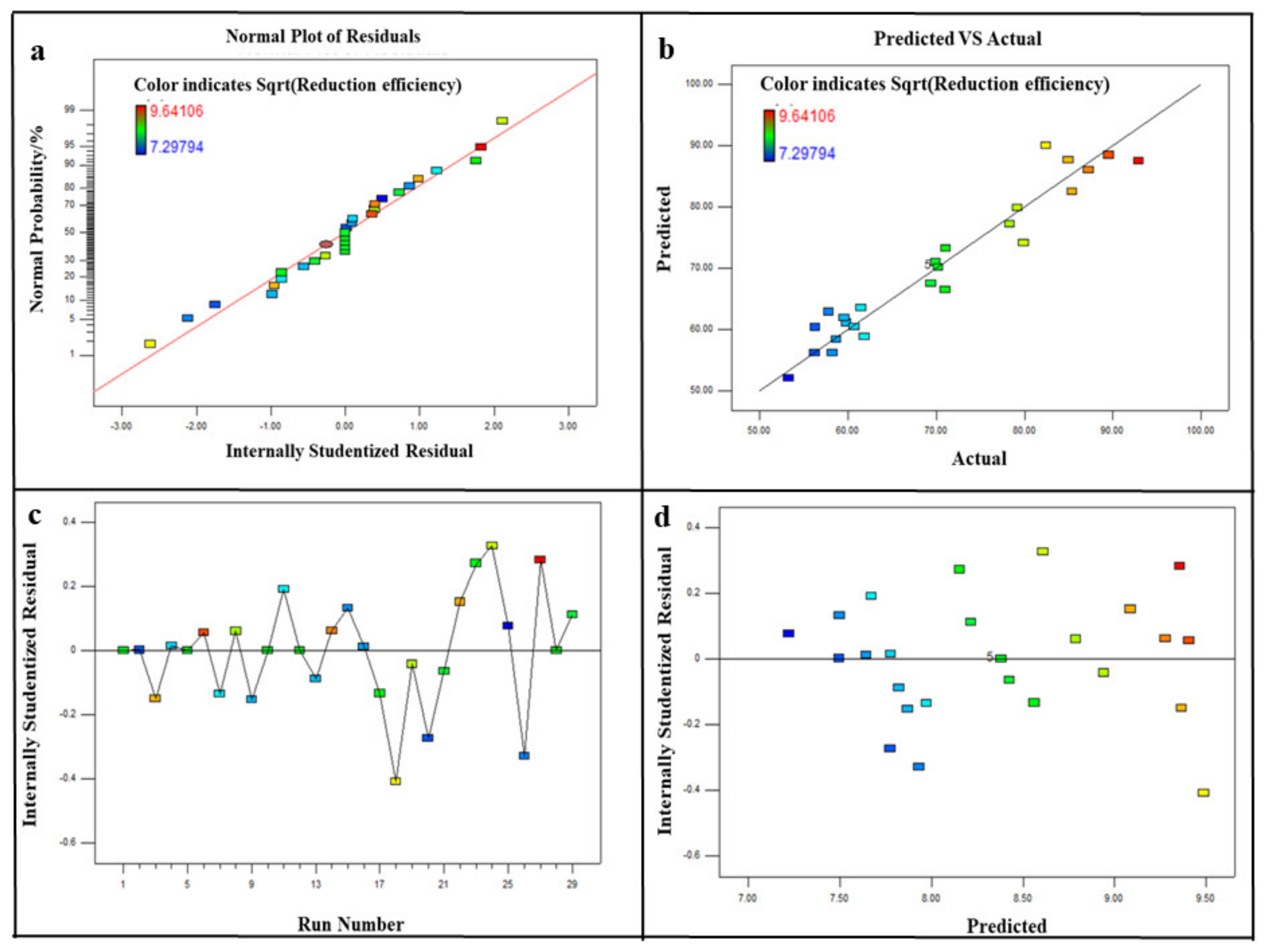
3.1.1. Model Fitting
+ 0.25*BD + 0.20*CD −0.03*A2 + 0.25*B2 + 0.02*C2−0.30*D2
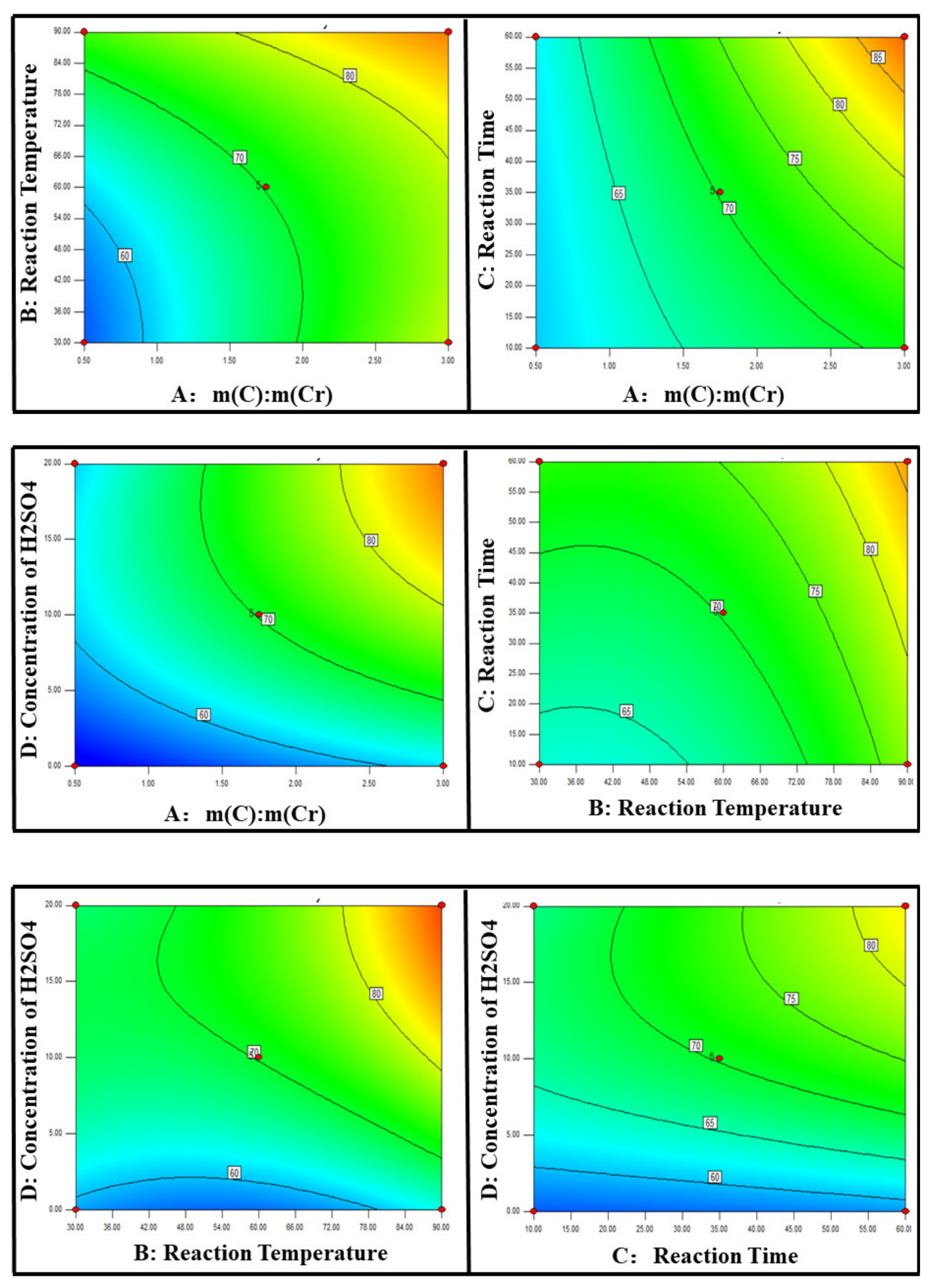
3.1.2. Response Surface Analysis
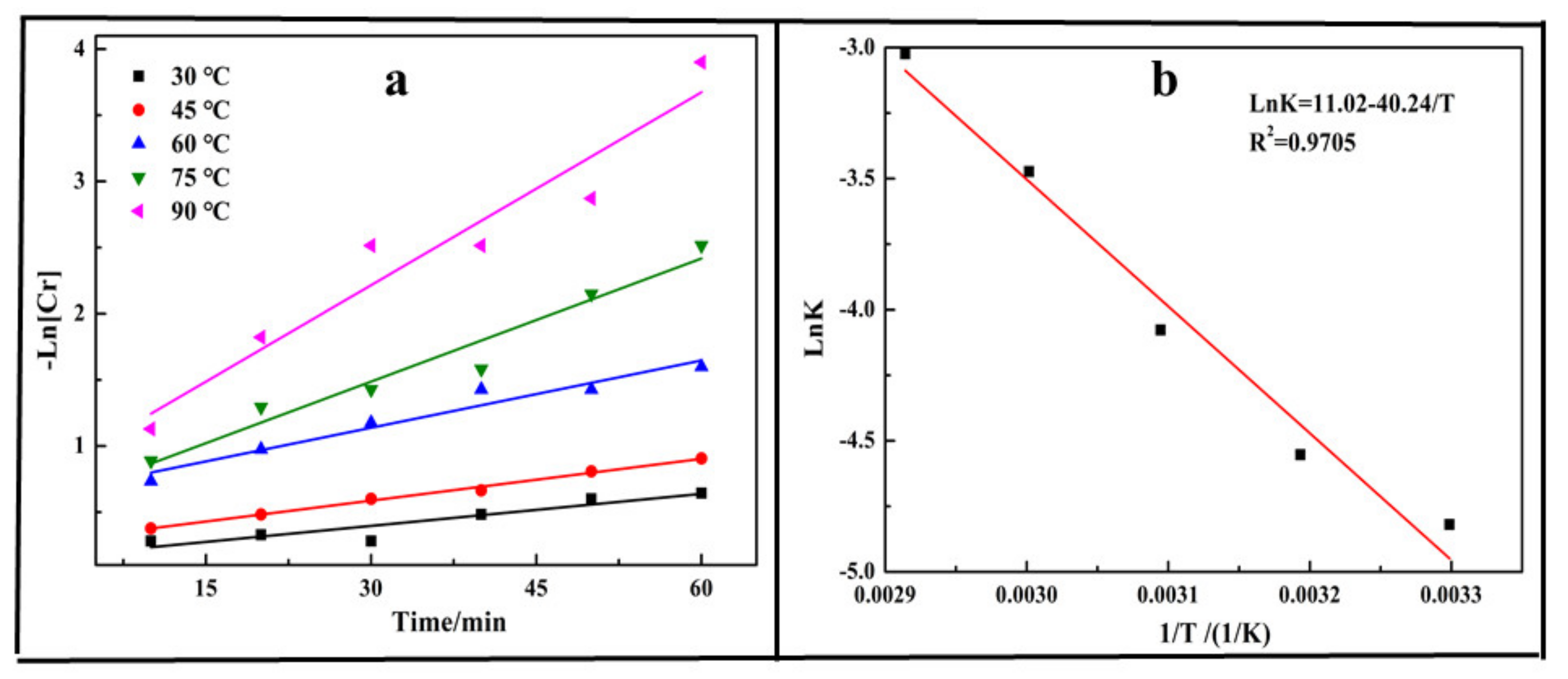
3.2. Reduction Kinetics Analysis
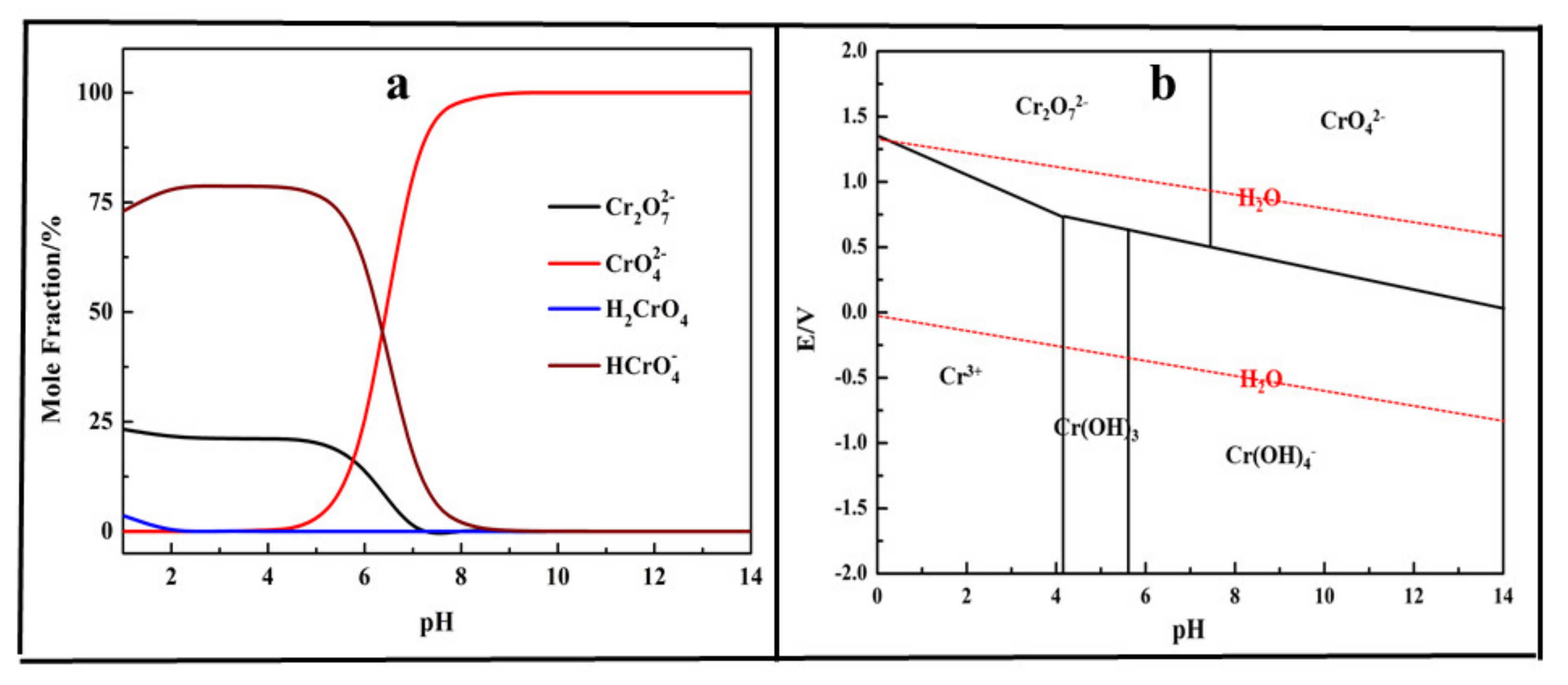
3.3. Removal of Chromium (III)
4. Conclusions
- (1)
- Cr (VI) was easily reduced by biochar at a high reaction temperature with a high dosage of biochar in a strong acidic medium. Nearly 100% of Cr (VI) was reduced at the selected reaction conditions: Dosage of biochar at m (C)/m(Cr) = 3.0, reaction temperature of 90 °C, reaction time of 60 min, and concentration of H2SO4 of 20 g/L.
- (2)
- The reduction kinetics analysis indicated that the reduction behavior of Cr (VI)with biochar fitted well with the pseudo-first-order model, and the apparent activation energy was calculated as 40.24 kJ/mol, which was much larger than the apparent energy calculated for reduction with oxalic acid (22.49 kJ/mol) and electrochemical reduction (4.74 kJ/mol).
- (3)
- Response surface methodology confirmed that all of the experimental parameters had a positive effect on the reduction of Cr (VI). The influence of each factor on the reduction process followed the order: A (dosage of biochar (m (C)/m(Cr)) > D (concentration of H2SO4) > B (reaction temperature) > C (reaction time). In particular, the dosage of biochar and the concentration of H2SO4 had the greatest influence on the reduction process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, J.; Kong, H.; Jang, J. Adsorption of heavy metal ions from aqueous solution by polyrhodanine-encapsulated magnetic nanoparticles. J. Colloid. Interf. Sci. 2011, 359, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Matis, K.A. Nanoadsorbents for pollutants removal: A review. J. Mol. Liq. 2015, 203, 159–168. [Google Scholar] [CrossRef]
- Nogueira, V.; Lopes, I.; Rocha-Santos, T.; Gonçalves, F.; Pereira, R. Toxicity of solid residues resulting from wastewater treatment with nanomaterials. Aquat. Toxicol. 2015, 165, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Adhoum, N.; Monser, L.; Bellakhal, N.; Belgaied, J.-E. Treatment of electroplating wastewater containing Cu2+, Zn2+ and Cr(VI) by electrocoagulation. J. Hazard. Mater. 2004, 112, 207–213. [Google Scholar] [CrossRef]
- Hunsom, M.; Pruksathorn, K.; Damronglerd, S.; Vergnes, H.; Duverneuil, P. Electrochemical treatment of heavy metals (Cu2+, Cr6+, Ni2+) from industrial effluent and modeling of copper reduction. Water Res. 2005, 39, 610–616. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: Review. Environ. Chem. Lett. 2021, 19, 1383–1393. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M. Ansarpour, Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar]
- Xin, H.; Xinhong, Q.; Jinyi, C. Preparation of Fe(II)–Al layered double hydroxides: Application to the adsorption/reduction of chromium. Colloids Surf. A Physicochem. Eng. Asp. 2017, 516, 362–374. [Google Scholar]
- Fu, R.; Zhang, X.; Xu, Z.; Guo, X.; Bi, D.; Zhang, W. Fast and highly efficient removal of chromium (VI) using humus-supported nanoscale zero-valent iron: Influencing factors, kinetics and mechanism. Sep. Purif. Technol. 2017, 174, 362–371. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J.; Li, B.; Liu, Z.; Tao, C. High-efficient recovery of chromium (VI) with lead sulfate. J. Taiwan Inst. Chem. Eng. 2018, 85, 149–154. [Google Scholar] [CrossRef]
- Zhao, Y. Removal of Chromium Using Electrochemical Approaches: A Review. Int. J. Electrochem. Sci. 2018, 13, 1250–1259. [Google Scholar] [CrossRef]
- Peng, H.; Leng, Y.; Guo, J. Electrochemical Removal of Chromium (VI) from Wastewater. Appl. Sci. 2019, 9, 1156. [Google Scholar] [CrossRef]
- Peng, H.; Leng, Y.; Cheng, Q.; Shang, Q.; Shu, J.; Guo, J. Efficient Removal of Hexavalent Chromium from Wastewater with Electro-Reduction. Processes 2019, 7, 41. [Google Scholar] [CrossRef]
- Zhao, Z.; He, A.; Lin, J.; Feng, M.; Murugadoss, V.; Ding, T.; Liu, H.; Shao, Q.; Mai, X.; Wang, N.; et al. Progress on the Photocatalytic Reduction Removal of Chromium Contamination. Chem. Rec. 2019, 19, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Kang, F.; Liu, X.; Peng, H.; JinYang, Z. Carbon-coated Mg–Al layered double oxide nanosheets with enhanced removal of hexavalent chromium. J. Ind. Eng. Chem. 2019, 80, 53–64. [Google Scholar] [CrossRef]
- Liu, X.Q.; Zhang, G.; Xing, H.Q.; Huang, P.; Zhang, X.L. Preparation of amphiphilic composite and removal of oil and hexavalent chromium from wastewater. Environ. Chem. Lett. 2011, 9, 127–132. [Google Scholar] [CrossRef]
- He, C.; Gu, L.; Xu, Z.; He, H.; Fu, G.; Han, F.; Huang, B.; Pan, X. Cleaning chromium pollution in aquatic environments by bioremediation, photocatalytic remediation, electrochemical remediation and coupled remediation systems. Environ. Chem. Lett. 2020, 18, 561–576. [Google Scholar] [CrossRef]
- Gallios, G.P.; Vaclavikova, M. Removal of chromium (VI) from water streams: A thermodynamic study. Environ. Chem. Lett. 2008, 6, 235–240. [Google Scholar] [CrossRef]
- Peng, H.; Shang, Q.; Chen, R.; Zhang, L.; Chen, Y.; Guo, J. Step-Adsorption of Vanadium (V) and Chromium (VI) in the Leaching Solution with Melamine. Sci. Rep. 2020, 10, 6326. [Google Scholar] [CrossRef]
- Guo, J.; Chen, R.; Zhang, L.; Shang, Q.; Chen, Y.; Peng, H. Adsorption of Chromium (III) on Melamine: Kinetic, Isotherm, Thermodynamics and Mechanism Analysis. IOP Conf. Ser. Earth Environ. Sci. 2020, 512, 012076. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Reduction behavior of chromium(VI) with oxalic acid in aqueous solution. Sci. Rep. 2020, 10, 17732. [Google Scholar] [CrossRef]
- Ashiq, A.; Sarkar, B.; Adassooriya, N.; Walpita, J.; Rajapaksha, A.U.; Ok, Y.S.; Vithanage, M. Sorption process of municipal solid waste biochar-montmorillonite composite for ciprofloxacin removal in aqueous media. Chemosphere 2019, 236, 124384. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-H.; Lee, D.-J.; Huang, C. Modification on biochars for applications: A research update. Bioresour. Technol. 2021, 319, 124100. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, Y.; Liu, X.; Fu, X.; Peng, H.; Lv, S. Enhanced adsorption capacity of MgO/N-doped active carbon derived from sugarcane bagasse. Bioresour. Technol. 2020, 297, 122413. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Belhaj, D. Competitive sorption affinity of sulfonamides and chloramphenicol antibiotics toward functionalized biochar for water and wastewater treatment. Bioresour. Technol. 2017, 238, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Karri, R.R.; Sahu, J.N.; Meikap, B.C. Improving efficacy of Cr (VI) adsorption process on sustainable adsorbent derived from waste biomass (sugarcane bagasse) with help of ant colony optimization. Ind. Crop. Prod. 2020, 143, 111927. [Google Scholar] [CrossRef]
- Zubair, M.; Ihsanullah, I.; Aziz, H.A.; Ahmad, M.A.; Al-Harthi, M.A. Sustainable wastewater treatment by biochar/layered double hydroxide composites: Progress, challenges, and outlook. Bioresour. Technol 2021, 319, 124128. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yang, L.; Wang, L.; Guo, J.; Li, B. Recovery of vanadium with urea in acidic medium. Environ. Chem. Lett. 2019, 17, 1867–1871. [Google Scholar] [CrossRef]
- Peng, H.; Shang, Q.; Chen, R.; Leng, Y.; Guo, J.; Liu, Z.; Tao, C. Oxidative Leaching Kinetics of Vanadium from the Vanadium-Chromium-Reducing Residue with K2Cr2O7. ACS Omega 2020, 5, 8777–8783. [Google Scholar] [CrossRef]
- Peng, H.; Wang, F.; Li, G.; Guo, J.; Li, B. Highly Efficient Recovery of Vanadium and Chromium: Optimized by Response Surface Methodology. ACS Omega 2019, 4, 904–910. [Google Scholar] [CrossRef]
- Peng, H.; Yang, L.; Chen, Y.; Guo, J.; Li, B. Recovery and Separation of Vanadium and Chromium by Two-Step Alkaline Leaching Enhanced with Electric Field and H2O2. ACS Omega 2020, 5, 5340–5345. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.P. Visual MINTEQ ver. 3.0. 2014. [Google Scholar]
- Chen, G.; Han, J.; Mu, Y.; Yu, H.; Qin, L. Two-stage chromium isotope fractionation during microbial Cr(VI) reduction. Water Res. 2019, 148, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Okello, V.A.; Mwilu, S.; Noah, N.; Zhou, A.; Chong, J.; Knipfing, M.T.; Doetschman, D.; Sadik, O.A. Reduction of hexavalent chromium using naturally-derived flavonoids. Environ. Sci. Technol. 2014, 46, 10743–10751. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Huang, S.; Liu, B.; Ge, Q.; Wang, M.; Wang, X. Separation and recovery of vanadium and chromium from acidic leach solution of V-Cr-bearing reducing slag. J. Environ. Chem. Eng. 2017, 5, 4702–4706. [Google Scholar] [CrossRef]


| Independent Variable | Unit | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| A:m(C)/m(Cr) | - | 0.5 | 1.75 | 3.0 |
| B: Reaction temperature | °C | 30 | 60 | 90 |
| C: Reaction time | min | 10 | 35 | 60 |
| D: Concentration of H2SO4 | g/L | 0 | 10 | 20 |
| Source | Sum of Squares | Z | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 11.76 | 14 | 0.84 | 14.47 | <0.0001 |
| A | 3.60 | 1 | 3.60 | 62.00 | <0.0001 |
| B | 1.76 | 1 | 1.76 | 30.29 | <0.0001 |
| C | 0.87 | 1 | 0.87 | 15.05 | 0.0017 |
| D | 3.31 | 1 | 3.31 | 56.94 | <0.0001 |
| AB | 0.12 | 1 | 0.12 | 2.10 | 0.1690 |
| AC | 0.19 | 1 | 0.19 | 3.36 | 0.0882 |
| AD | 0.24 | 1 | 0.24 | 4.22 | 0.0591 |
| BC | 0.00254 | 1 | 0.00254 | 0.044 | 0.8372 |
| BD | 0.26 | 1 | 0.26 | 4.39 | 0.0547 |
| CD | 0.16 | 1 | 0.16 | 2.67 | 0.1243 |
| A2 | 0.005787 | 1 | 0.005787 | 0.100 | 0.7569 |
| B2 | 0.41 | 1 | 0.41 | 7.05 | 0.0188 |
| C2 | 0.002533 | 1 | 0.002533 | 0.044 | 0.8375 |
| D2 | 0.60 | 1 | 0.60 | 10.32 | 0.0063 |
| Residual | 0.81 | 14 | 0.058 | - | - |
| Lack-of-fit | 0.81 | 10 | 0.081 | - | - |
| Pure error | 0.000 | 4 | 0.000 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Guo, J.; Qiu, H.; Wang, C.; Zhang, C.; Hao, Z.; Rao, Y.; Gong, Y. Efficient Removal of Cr (VI) with Biochar and Optimized Parameters by Response Surface Methodology. Processes 2021, 9, 889. https://doi.org/10.3390/pr9050889
Peng H, Guo J, Qiu H, Wang C, Zhang C, Hao Z, Rao Y, Gong Y. Efficient Removal of Cr (VI) with Biochar and Optimized Parameters by Response Surface Methodology. Processes. 2021; 9(5):889. https://doi.org/10.3390/pr9050889
Chicago/Turabian StylePeng, Hao, Jing Guo, Hongzhi Qiu, Caiqiong Wang, Chenyu Zhang, Zhihui Hao, Yating Rao, and Yanhong Gong. 2021. "Efficient Removal of Cr (VI) with Biochar and Optimized Parameters by Response Surface Methodology" Processes 9, no. 5: 889. https://doi.org/10.3390/pr9050889
APA StylePeng, H., Guo, J., Qiu, H., Wang, C., Zhang, C., Hao, Z., Rao, Y., & Gong, Y. (2021). Efficient Removal of Cr (VI) with Biochar and Optimized Parameters by Response Surface Methodology. Processes, 9(5), 889. https://doi.org/10.3390/pr9050889







