Effect of Oxidative Stress on Physicochemical Quality of Taiwanese Seagrape (Caulerpa lentillifera) with the Application of Alternating Current Electric Field (ACEF) during Post-Harvest Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Packaging
2.3. Alternating Current Electric Field (ACEF) Treatment for Oxidative Stress Suppression on Seagrape
2.4. Water Content Assay
2.5. Malondialdehyde (MDA) Assay
2.6. Total Phenolic Content (TPC) Assay
2.7. Chlorophyll Content Assay
2.8. Kinetic Analysis
2.9. Principal Component Analysis (PCA)
2.10. Statistical Analysis
3. Results and Discussion
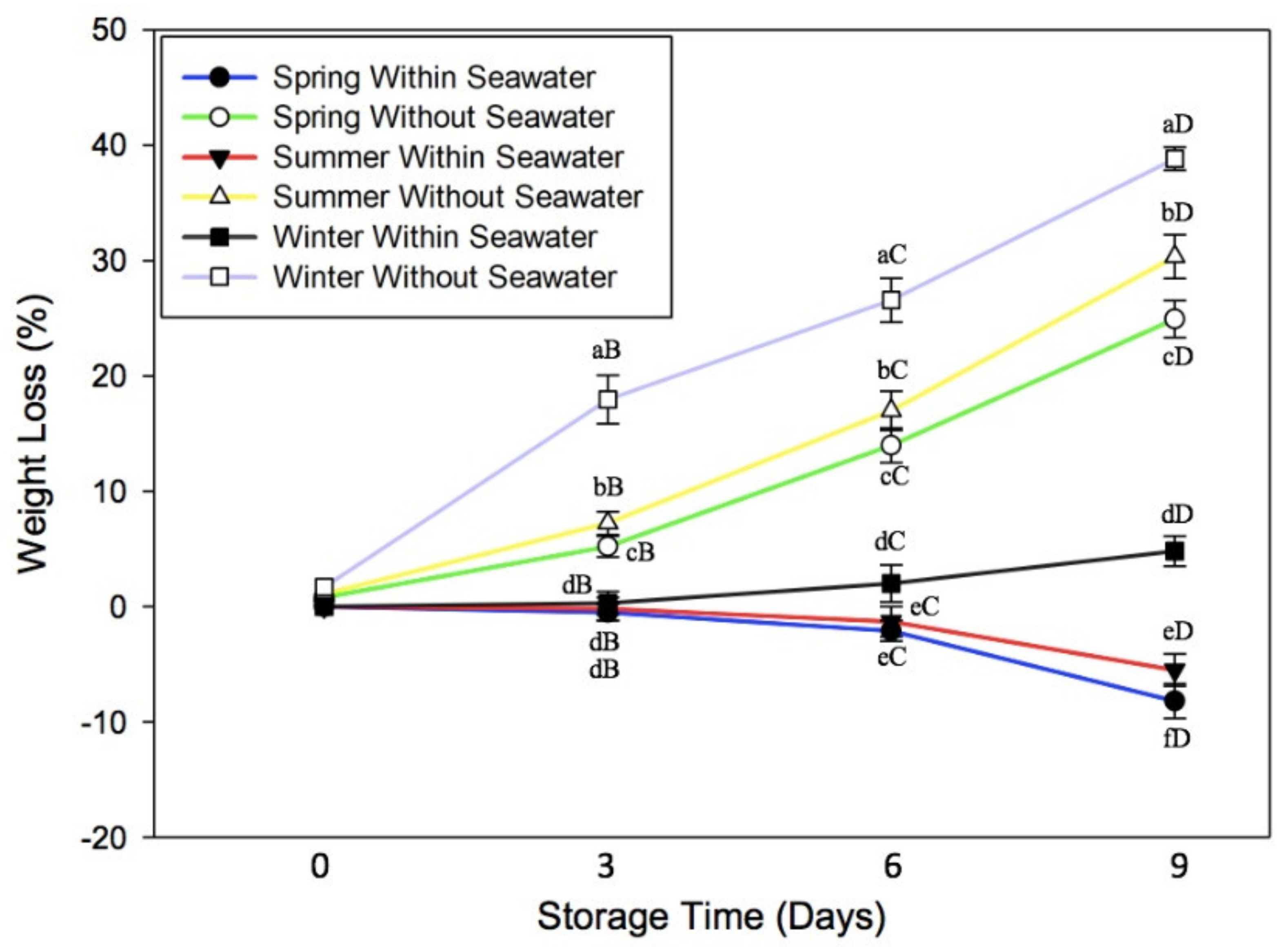
3.1. Effect of Oxidative Stress on Water Content of Seagrape
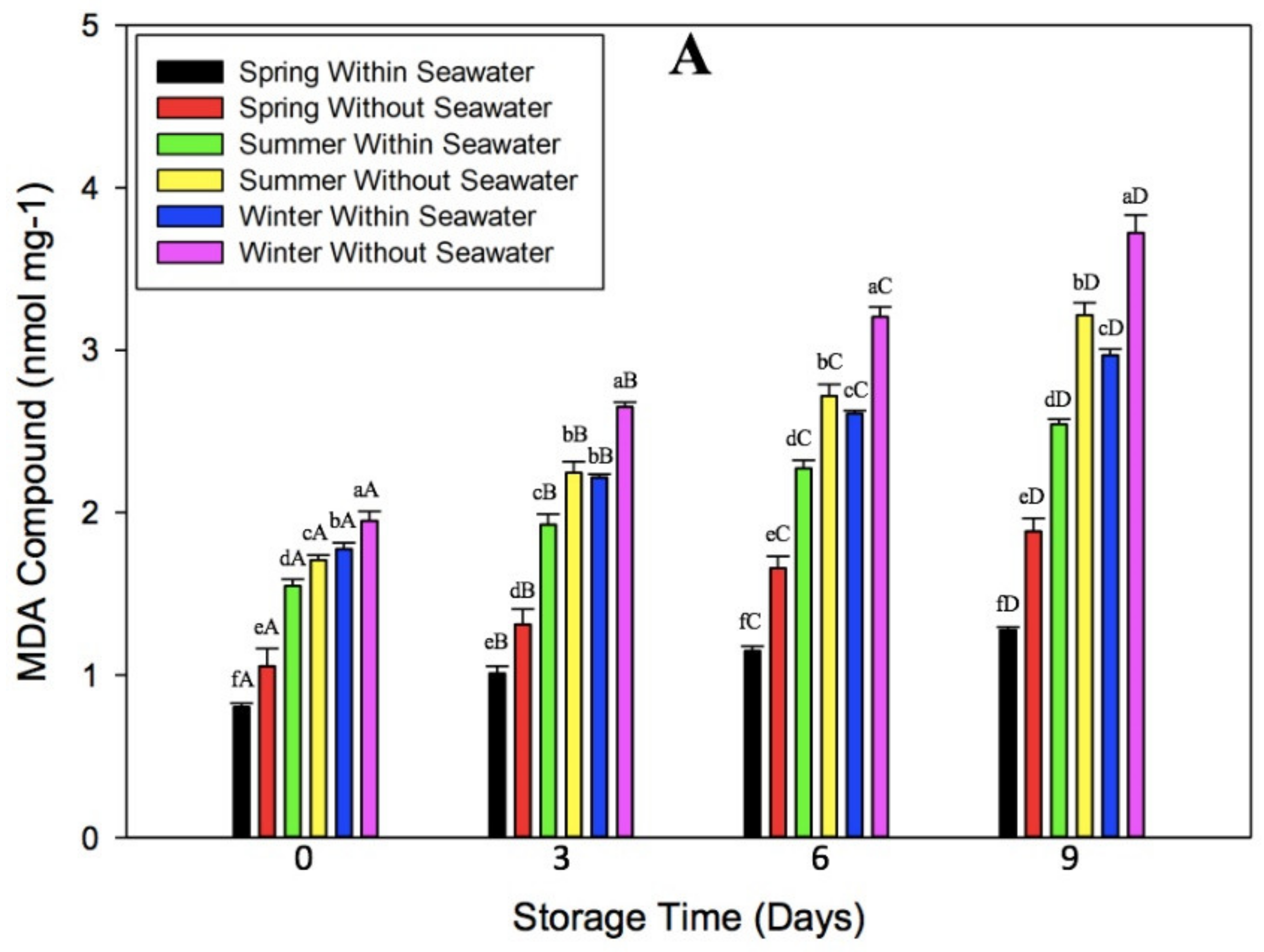
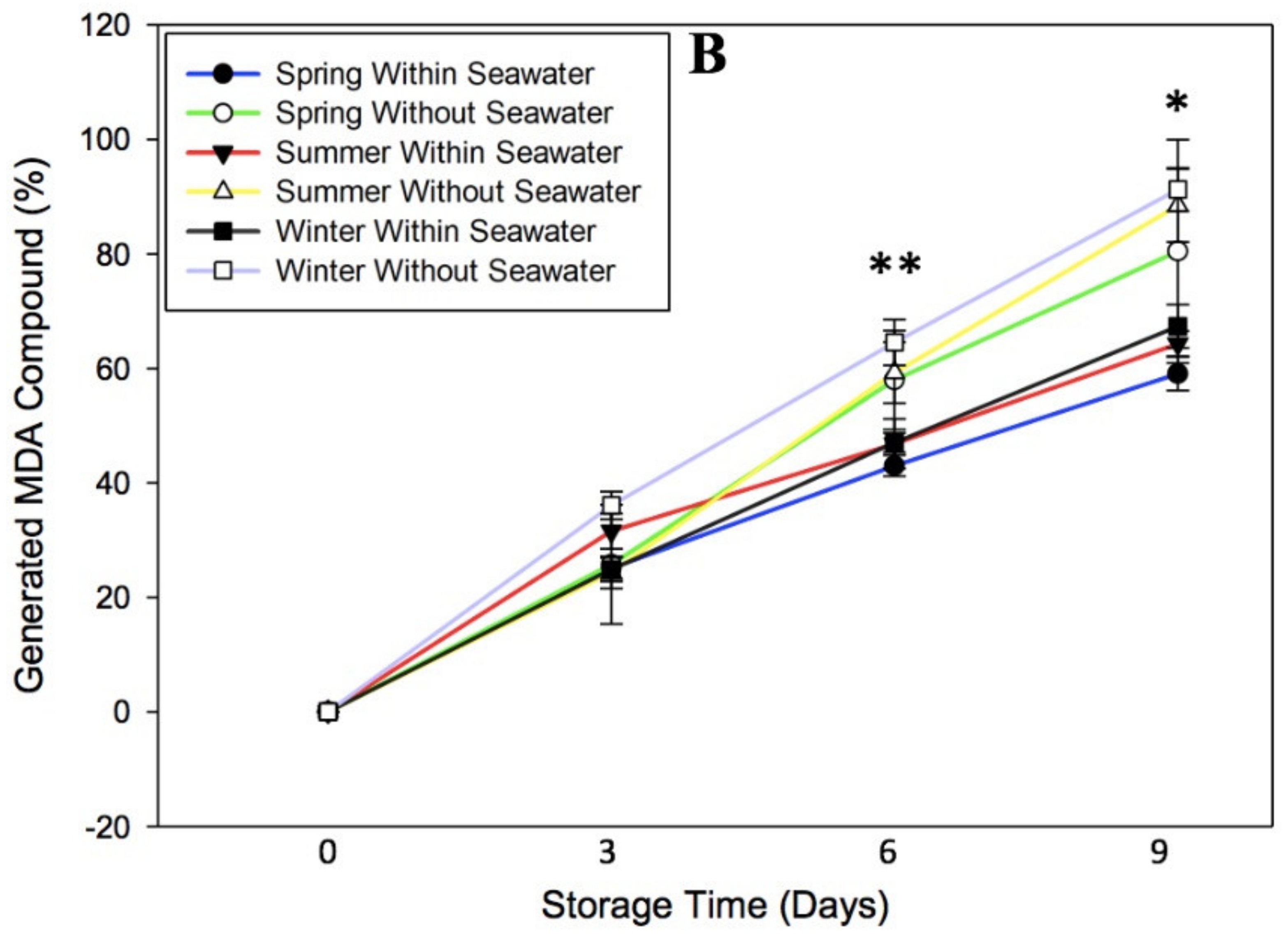
3.2. Effect of Oxidative Stress on Malondialdehyde Compound of Seagrape
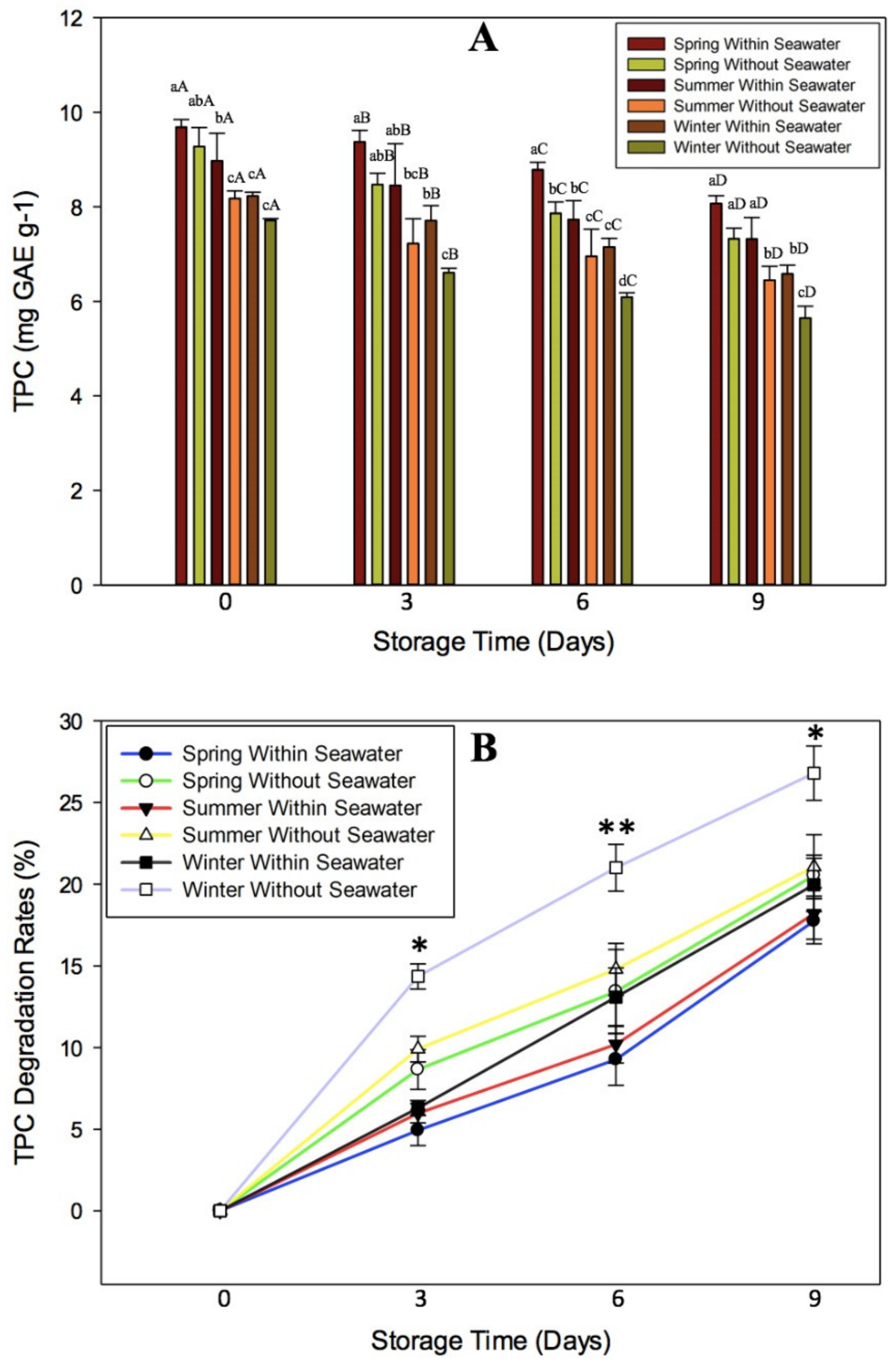
3.3. Effect of Oxidative Stress on TPC of Seagrape
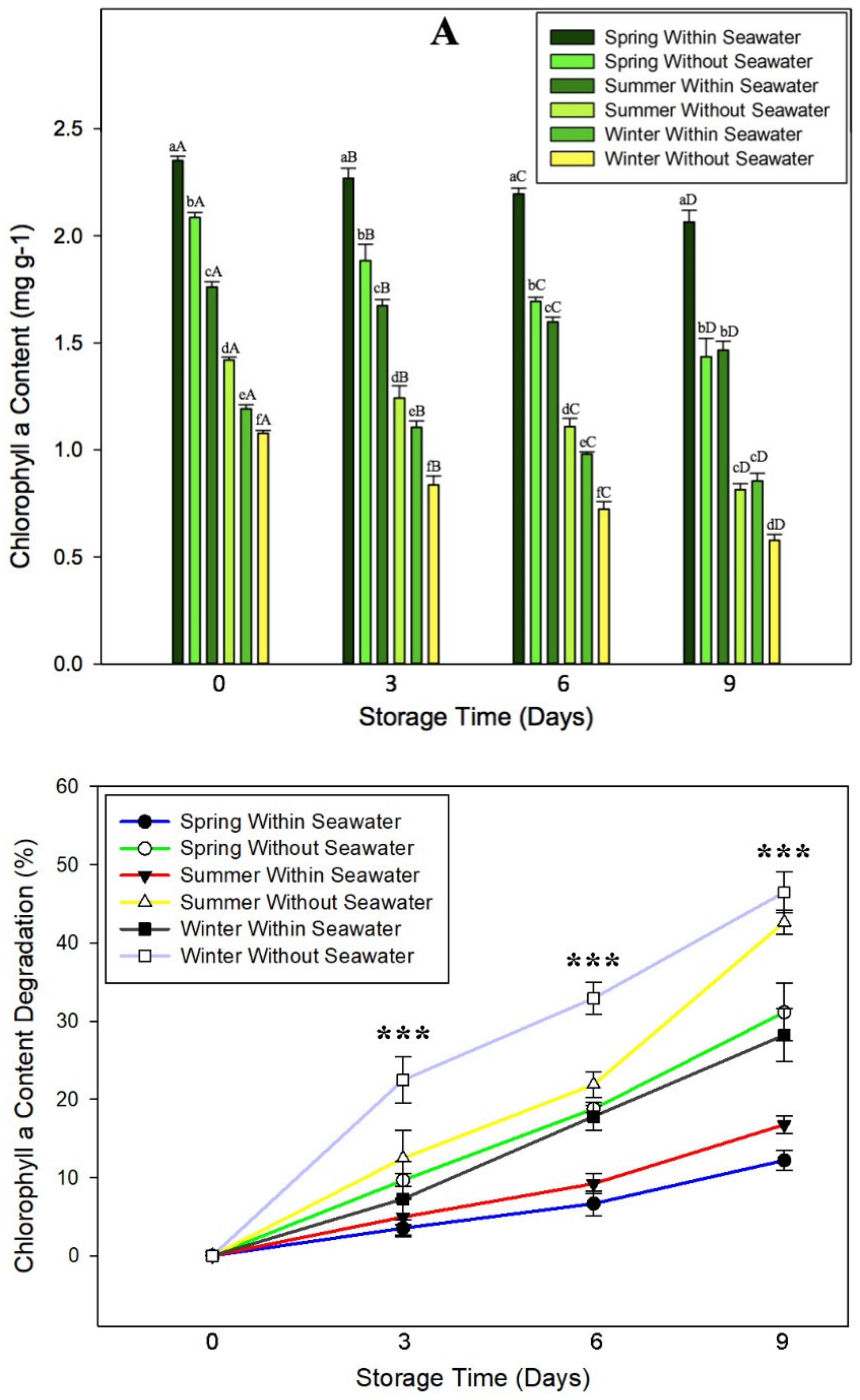
3.4. Effect of Oxidative Stress on Chlorophyll Content of Seagrape
3.5. Effect of Cultivation Season on Physicochemical Quality of Seagrape under Post-Harvest Storage
3.6. Effect of Oxidative Stress on Kinetics of Storage Time to the Chlorophyll Content of Seagrape in Different Cultivated Seasons
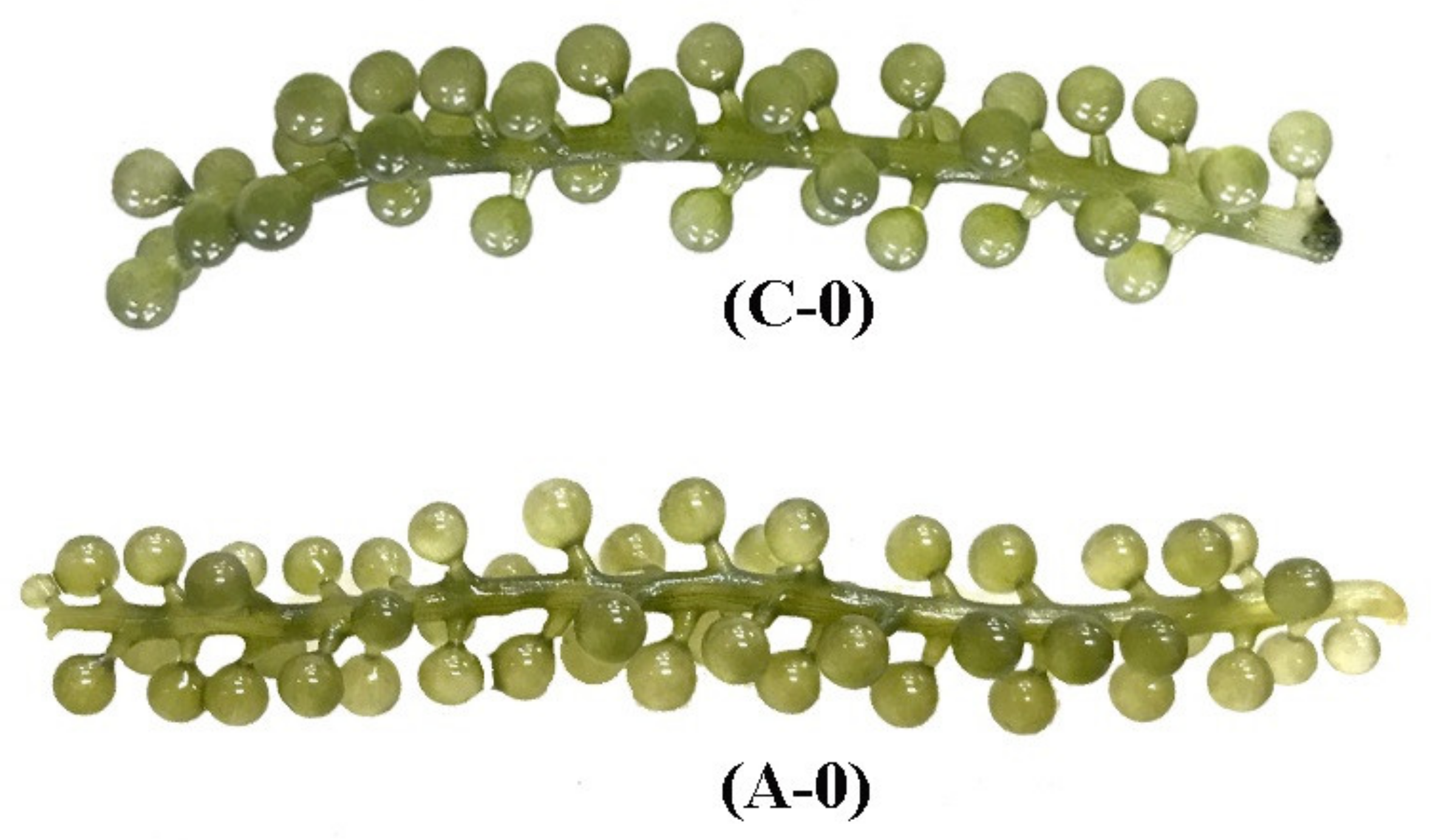
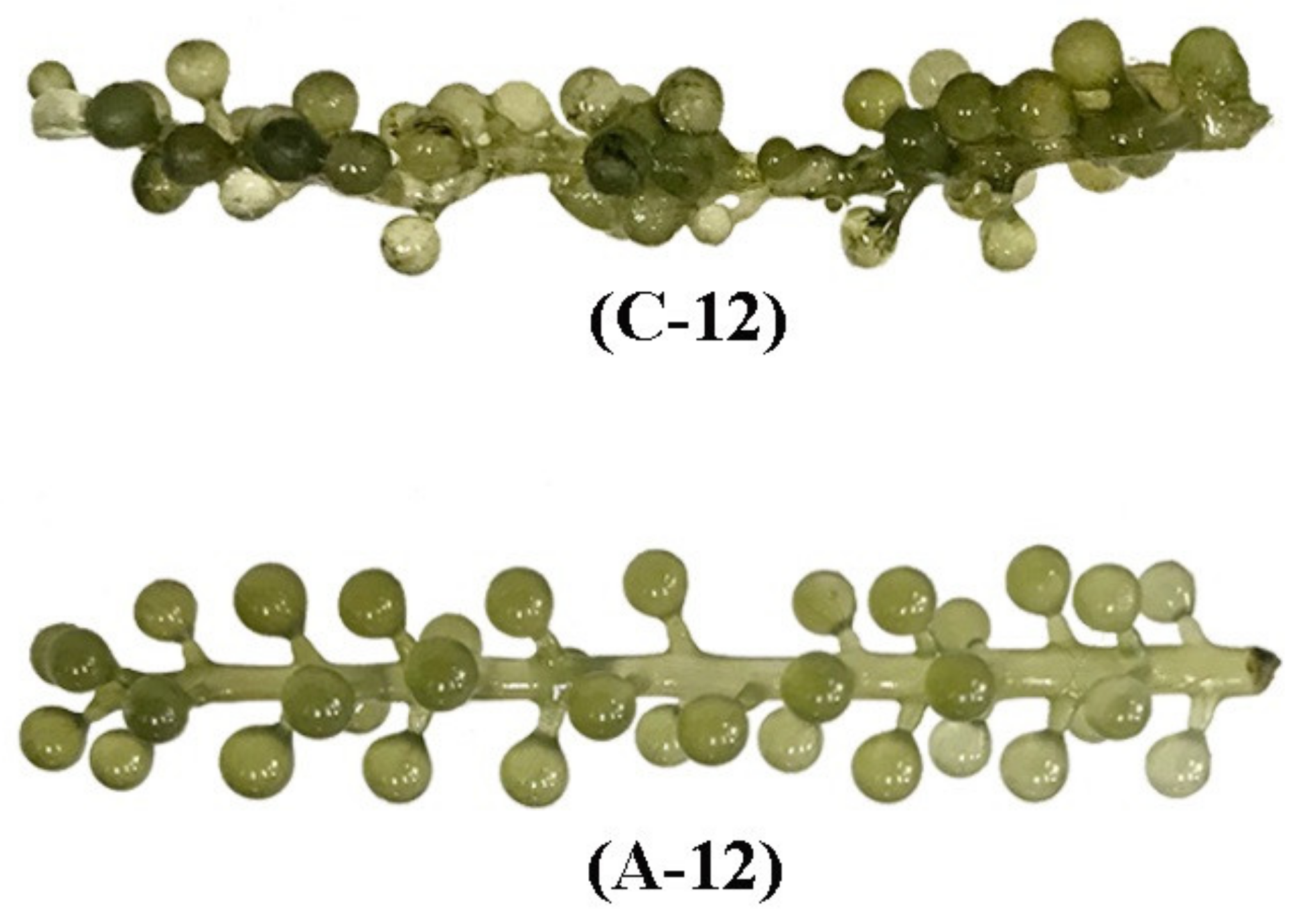
3.7. Effect of ACEF on Physicochemical Quality of Seagrape during Post-Harvest Storage
3.7.1. Effect of ACEF on Water Content of Seagrape
3.7.2. Effect of ACEF on MDA of Seagrape
3.7.3. Effect of ACEF on TPC of Seagrape
3.7.4. Effect of ACEF on Chlorophyll Content of Seagrape
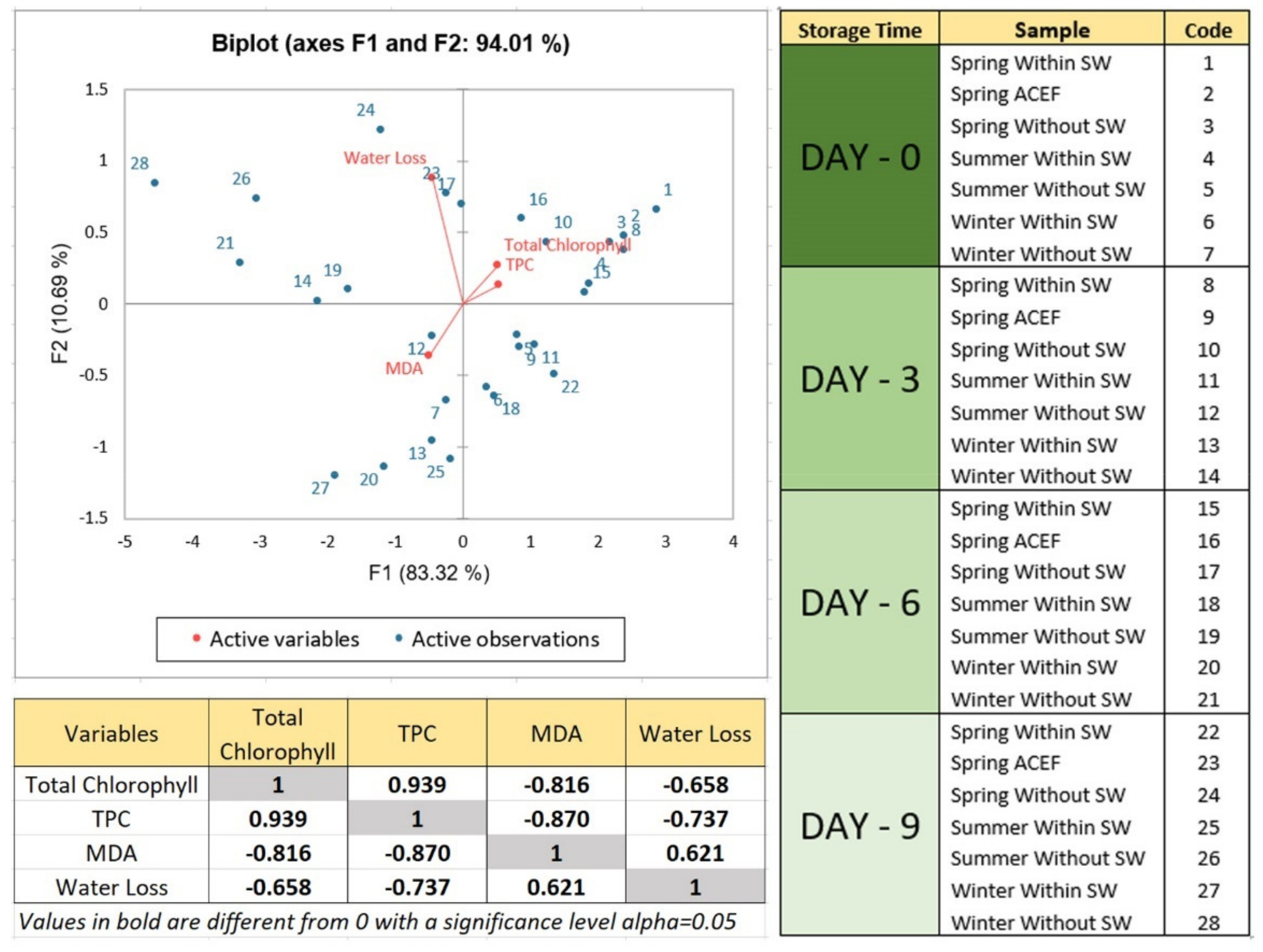
3.8. Principal Component Analysis (PCA) on Physicochemical Quality of Seagrape
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zubia, M.; Draisma, S.G.A.; Morrissey, K.L.; Varela-Álvarez, E.; De Clerck, O. Concise review of the genus Caulerpa J.V. Lamouroux. J. Appl. Phycol. 2020, 32, 23–39. [Google Scholar] [CrossRef]
- De Gaillande, C.; Payri, C.; Remoissenet, G.; Zubia, M. Caulerpa consumption, nutritional value and farming in the Indo-Pacific region. J. Appl. Phycol. 2017, 29, 2249–2266. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Srinorasing, T.; Chirasuwan, N.; Tamtin, M.; Bunnag, B. The potential of polysaccharide extracts from Caulerpa lentillifera waste. Int. J. Biol. Macromol. 2020, 161, 1021–1028. [Google Scholar] [CrossRef]
- Morris, C.; Bala, S.; South, G.R.; Lako, J.; Lober, M.; Simos, T. Supply chain and marketing of sea grapes, Caulerpa racemosa (Forsskål) J. Agardh (Chlorophyta: Caulerpaceae) in Fiji, Samoa and Tonga. J. Appl. Phycol. 2014, 26, 783–789. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holzinger, A.; Karsten, U. Desiccation stress and tolerance in green algae: Consequences for ultrastructure, physiological, and molecular mechanisms. Front. Plant. Sci. 2013, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, R.; Beck, E. Drought, Desiccation, and Oxidative Stress. In Plant Desiccation Tolerance; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2011; Volume 215, pp. 209–231. [Google Scholar]
- Terada, R.; Nakazaki, Y.; Borlongan, I.A.; Endo, H.; Nishihara, G.N. Desiccation effect on the PSII photochemical efficiency of cultivated Japanese Caulerpa lentillifera under the shipping package environment. J. Appl. Phycol. 2018, 30, 2533–2538. [Google Scholar] [CrossRef]
- Kudaka, J.; Itokazu, K.; Taira, K.; Nidaira, M.; Okano, S.; Nakamura, M.; Iwanaga, S.; Tominaga, M.; Ohno, A. Investigation and culture of microbial contaminants of Caulerpa lentillifera (Sea Grape). J. Food Hyg. Soc. Jpn. 2008, 49, 11–15. [Google Scholar] [CrossRef]
- Anantpinijwatna, A.; Nuntamongkol, S.; Tudkesorn, B.; Sukchoy, O.; Deetae, P. The kinetic model and temperature effect of Caulerpa lentillifera drying process. AIP Conf. Proc. 2018, 2026, 020036. [Google Scholar]
- Lapong, I.; Paul, N.; Reza, A. Evaluation of the brine preservation method of sea grapes (Caulerpa lentillifera) as a commercial product. Mar. Chim. Acta 2019, 20, 45–50. [Google Scholar]
- Djaeni, M.; Sari, D.A. Low temperature seaweed drying using dehumidified air. Procedia Environ. Sci. 2015, 23, 2–10. [Google Scholar] [CrossRef]
- Lapong, I.; Paul, N.; Reza, A. Characterization of sea grape (Caulerpa lentillifera) from Vietnamese company’s products. Mar. Chim. Acta 2019, 2, 51–57. [Google Scholar]
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A.; Xanthakis, E. The principles of high voltage electric field and its application in food processing: A review. Food Res. Int. 2016, 89, 48–62. [Google Scholar] [CrossRef]
- Muthukumaran, A.; Orsat, V.; Bajgai, T.R.; Raghavan, G.S.V. Effect of high electric field on food processing. In Novel Food Processing: Effects on Rheological and Functional Properties; Ahmed, J., Ramaswamy, H.S., Kasapis, S., Boye, J.I., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 35–46. [Google Scholar]
- Ko, W.C.; Yang, S.Y.; Chang, C.K.; Hsieh, C.W. Effects of adjustable parallel high voltage electrostatic field on the freshness of tilapia (Orechromis niloticus) during refrigeration. LWT-Food Sci. Technol. 2016, 66, 151–157. [Google Scholar] [CrossRef]
- Liu, C.E.; Chen, W.J.; Chang, C.K.; Li, P.H.; Lu, P.L.; Hsieh, C.W. Effect of a high voltage electrostatic field (HVEF) on the shelf life of persimmons (Diospyros kaki). LWT-Food Sci. Technol. 2017, 75, 236–242. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Chang, C.K.; Wong, L.W.; Hu, C.C.; Lin, J.A.; Hsieh, C.W. Alternating current electric field inhibits browning of Pleurotus ostreatus via inactivation of oxidative enzymes during postharvest storage. LWT-Food Sci. Technol. 2020, 134, 110212. [Google Scholar] [CrossRef]
- Hsieh, C.W.; Ko, W.C. Effect of high-voltage electrostatic field on quality of carrot juice during refrigeration. LWT-Food Sci. Technol. 2008, 41, 1752–1757. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Charrier, B.; Abreu, M.H.; Araujo, R.; Bruhn, A.; Coates, J.C.; De Clerck, O.; Katsaros, C.; Robaina, R.R.; Wichard, T. Furthering knowledge of seaweed growth and development to facilitate sustainable aquaculture. New Phytol. 2017, 216, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Paull, R.E.; Chen, N.J. Postharvest handling and storage of the edible red seaweed Gracilaria. Postharvest Biol. Technol. 2008, 48, 302–308. [Google Scholar] [CrossRef]
- Seremet, L.; Botez, E.; Nistor, O.V.; Andronoiu, D.G.; Mocanu, G.D. Effect of different drying methods on moisture ratio and rehydration of pumpkin slices. Food Chem. 2016, 195, 104–109. [Google Scholar] [CrossRef]
- Jaballi, I.; Sallem, I.; Feki, A.; Cherif, B.; Kallel, C.; Boudawara, O.; Jamoussi, K.; Mellouli, L.; Nasri, M.; Amara, I.B. Polysaccharide from a Tunisian red seaweed Chondrus canaliculatus: Structural characteristics, antioxidant activity and in vivo hemato-nephroprotective properties on maneb induced toxicity. Int. J. Biol. Macromol. 2019, 123, 1267–1277. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Korbee, N.; Pérez-Ruzafa, I.M.; Marcos, C.; Domínguez, B.; Figueroa, F.L.; Pérez-Ruzafa, Á. Physiological response and photoacclimation capacity of Caulerpa prolifera (Forsskål) J.V. Lamouroux and Cymodocea nodosa (Ucria) Ascherson meadows in the Mar Menor lagoon (SE Spain). Mar. Environ. Res. 2012, 79, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Nurjanah, N.; Jacoeb, A.M.; Asmara, D.A.; Hidayat, T. Phenol Component of Fresh and Boiled Sea Grapes (Caulerpa Sp.) From Tual, Maluku. Food Sci. J. 2019, 1, 31–39. [Google Scholar] [CrossRef]
- Guo, H.; Yao, J.; Sun, Z.; Duan, D. Effect of temperature, irradiance on the growth of the green alga Caulerpa lentillifera (Bryopsidophyceae, Chlorophyta). J. Appl. Phycol. 2015, 27, 879–885. [Google Scholar] [CrossRef]
- Molaveisi, M.; Beigbabaei, A.; Akbari, E.; Noghabi, M.S.; Mohamadi, M. Kinetics of temperature effect on antioxidant activity, phenolic compounds and color of Iranian jujube honey. Heliyon 2019, 5, e01129. [Google Scholar] [CrossRef]
- Vidal, N.P.; Manful, C.F.; Pham, T.H.; Stewart, P.; Keough, D.; Thomas, R. MethodsX The use of XLSTAT in conducting principal component analysis (PCA) when evaluating the relationships between sensory and quality attributes in grilled foods. MethodsX 2020, 7, 100835. [Google Scholar] [CrossRef]
- Kranner, I.; Beckett, R.; Hochman, A.; Nash, T.H. Desiccation-tolerance in lichens: A review. Bryologist 2008, 111, 576–593. [Google Scholar] [CrossRef]
- Watanabe, Y.; Morikawa, T.; Mine, T.; Kawamura, Y.; Nishihara, G.N.; Terada, R. Chronological change and the potential of recovery on the photosynthetic efficiency of Pyropia yezoensis f. narawaensis (Bangiales) during the sporelings frozen storage treatment in the Japanese Nori cultivation. Phycol. Res. 2017, 65, 265–271. [Google Scholar] [CrossRef]
- Guo, H.; Yao, J.; Sun, Z.; Duan, D. Effects of salinity and nutrients on the growth and chlorophyll fluorescence of Caulerpa lentillifera. Chin. J. Oceanol. Limnol. 2015, 33, 410–418. [Google Scholar] [CrossRef]
- Suriati, L.; Utama, I.M.S.; Harsojuwono, B.A.; Gunam, I.B.W. Incorporating additives for stability of Aloe gel potentially as an edible coating. AIMS Agric. Food. 2020, 5, 327–336. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, W.; Tian, X.; Hou, M.; Dai, R.; Li, X. Unraveling proteome changes of Holstein beef M. semitendinosus and its relationship to meat discoloration during post-mortem storage analyzed by label-free mass spectrometry. J. Proteom. 2017, 154, 85–93. [Google Scholar] [CrossRef]
- Hung, L.H.; Kimura, Y.; Adachi, S. Discoloration kinetics of L-ascorbyl 6-palmitate powders with various water contents. Food Sci. Technol. Res. 2007, 13, 7–12. [Google Scholar] [CrossRef]
- Shad, Z.M.; Atungulu, G.G. Post-harvest kernel discoloration and fungi activity in long-grain hybrid, pureline and medium-grain rice cultivars as influenced by storage environment and antifungal treatment. J. Stored Prod. Res. 2019, 81, 91–99. [Google Scholar] [CrossRef]
- Catalá, A.; Díaz, M. Editorial: Impact of lipid peroxidation on the physiology and pathophysiology of cell membranes. Front. Physiol. 2016, 7, 1–3. [Google Scholar] [CrossRef]
- Wang, W.J.; Wang, F.J.; Zhu, J.Y.; Sun, X.T.; Yao, C.Y.; Xu, P. Freezing tolerance of Porphyra yezoensis (Bangiales, Rhodophyta) gametophyte assessed by chlorophyll fluorescence. J. Appl. Phycol. 2011, 23, 1017–1022. [Google Scholar] [CrossRef]
- Saito, H.; Xue, C.; Yamashiro, R.; Moromizato, S.; Itabashi, Y. High polyunsaturated fatty acid levels in two subtropical macroalgae, cladosiphon okamuranus and caulerpa lentillifera. J. Phycol. 2010, 46, 665–673. [Google Scholar] [CrossRef]
- Nagappan, T.; Vairappan, C.S. Nutritional and bioactive properties of three edible species of green algae, genus Caulerpa (Caulerpaceae). J. Appl. Phycol. 2014, 26, 1019–1027. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Hao, H.; Fu, M.; Yan, R.; He, B.; Li, M.; Liu, Q.; Cai, Y.; Zhang, X.; Huang, R. Chemical composition and immunostimulatory properties of green alga Caulerpa racemosa var peltata. Food Agric. Immunol. 2019, 30, 937–954. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, S.; Dai, H.; Kong, X.; Hao, J.; Wang, S.; Zhou, X.; Zhao, Y.; Wei, B.; Cheng, S.; et al. Changes in membrane lipid metabolism accompany pitting in blueberry during refrigeration and subsequent storage at room temperature. Front. Plant Sci. 2019, 10, 829. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Kong, W.; Liu, F.; Zhang, C.; Zhang, J.; Feng, H. Non-destructive determination of Malondialdehyde (MDA) distribution in oilseed rape leaves by laboratory scale NIR hyperspectral imaging. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Kim, A.N.; Kim, H.J.; Chun, J.; Heo, H.J.; Kerr, W.L.; Choi, S.G. Degradation kinetics of phenolic content and antioxidant activity of hardy kiwifruit (Actinidia arguta) puree at different storage temperatures. LWT-Food Sci. Technol. 2018, 89, 535–541. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Ma, Z.; Luan, Z.; Wang, Y.; Wang, Z.; Wang, L. Removal of phenolic substances from wastewater by algae. A review. Environ. Chem. Lett. 2019, 8, 377–392. [Google Scholar] [CrossRef]
- Duan, A.W.; Meng, F.; Lin, Y. Toxicological effects of phenol on four marine microalgae. Environ. Toxicol. Pharmacol. 2017, 52, 170–176. [Google Scholar] [CrossRef]
- Aisyah, Y.; Rasdiansyah, M. Effect of heating on antioxidant activity in several types of vegetables. J. Teknol. Ind. Pertan. Indones. 2014, 6, 28–32. [Google Scholar]
- Cho, M.; Kang, I.J.; Won, M.H.; Lee, H.S.; You, S. The antioxidant properties of ethanol extracts and their solvent-partitioned fractions from various green seaweeds. J. Med. Food. 2010, 13, 1232–1239. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Ming, C.H. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J. Appl. Phycol. 2008, 20, 367–373. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Ueng, J.P.; Tsai, G.J. Proximate Composition, Total Phenolic Content, and Antioxidant Activity of Seagrape (Caulerpa lentillifera). J. Food Sci. 2011, 76, 950–958. [Google Scholar] [CrossRef]
- Mekinić, I.G.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Perković, Z.P. Phenolic content of brown algae (Pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar]
- Monteiro, M.; Santos, R.A.; Iglesias, P.; Couto, A.; Serra, C.R.; Gouvinhas, I.; Barros, A.; Oliva-Teles, A.; Enes, P.; Díaz-Rosales, P. Effect of extraction method and solvent system on the phenolic content and antioxidant activity of selected macro- and microalgae extracts. J. Appl. Phycol. 2020, 32, 349–362. [Google Scholar] [CrossRef]
- Paul, N.A.; Neveux, N.; Magnusson, M.; de Nys, R. Comparative production and nutritional value of “sea grapes”-the tropical green seaweeds Caulerpa lentillifera and C. racemosa. J. Appl. Phycol. 2014, 26, 1833–1844. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Cao, K.F. Gas exchange, chlorophyll fluorescence, and osmotic adjustment in two mango cultivars under drought stress. Acta Physiol. Plant. 2008, 30, 769–777. [Google Scholar] [CrossRef]
- Pongsri, R.; Aiamla-or, S.; Srilaong, V.; Uthairatanakij, A.; Jitareerat, P. Impact of electron-beam irradiation combined with shellac coating on the suppression of chlorophyll degradation and water loss of lime fruit during storage. Postharvest Biol. Technol. 2021, 172, 111364. [Google Scholar] [CrossRef]
- Aiamla-or, S.; Shigyo, M.; Yamauchi, N. UV-B treatment controls chlorophyll degradation and related gene expression in broccoli (Brassica oleracea L. Italica Group) florets during storage. Sci. Hortic. 2019, 243, 524–527. [Google Scholar] [CrossRef]
- Luo, F.; Cheng, S.C.; Cai, J.H.; Wei, B.D.; Zhou, X.; Zhou, Q.; Zhao, Y.B.; Ji, S.J. Chlorophyll degradation and carotenoid biosynthetic pathways: Gene expression and pigment content in broccoli during yellowing. Food Chem. 2019, 297, 124964. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V. Seaweed cultivation: Methods and problems. Russ. J. Mar. Biol. 2010, 36, 227–242. [Google Scholar] [CrossRef]
- Britton, D.; Schmid, M.; Revill, A.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L.; Mundy, C.N. Seasonal and site-specific variation in the nutritional quality of temperate seaweed assemblages: Implications for grazing invertebrates and the commercial exploitation of seaweeds. J. Appl. Phycol. 2021, 33, 603–616. [Google Scholar] [CrossRef]
- Fabrowska, J.; Messyasz, B.; Pankiewicz, R.; Wilińska, P.; Łęska, B. Seasonal differences in the content of phenols and pigments in thalli of freshwater Cladophora glomerata and its habitat. Water Res. 2018, 135, 66–74. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; da Costa, E.; Melo, T.; Sulpice, R.; Cardoso, S.M.; Pitarma, B.; Pereira, R.; Abreu, M.H.; Domingues, P.; Calado, R.; et al. Seasonal plasticity of the polar lipidome of Ulva rigida cultivated in a sustainable integrated multi-trophic aquaculture. Algal Res. 2020, 49, 101958. [Google Scholar] [CrossRef]
- Gosch, B.J.; Paul, N.A.; de Nys, R.; Magnusson, M. Spatial, seasonal, and within-plant variation in total fatty acid content and composition in the brown seaweeds Dictyota bartayresii and Dictyopteris australis (Dictyotales, Phaeophyceae). J. Appl. Phycol. 2015, 27, 1607–1622. [Google Scholar] [CrossRef]
- Fariman, G.A.; Shastan, S.J.; Zahedi, M.M. Seasonal variation of total lipid, fatty acids, fucoxanthin content, and antioxidant properties of two tropical brown algae (Nizamuddinia zanardinii and Cystoseira indica) from Iran. J. Appl. Phycol. 2016, 28, 1323–1331. [Google Scholar] [CrossRef]
- Choo, K.S.; Snoeijs, P.; Pedersén, M. Oxidative stress tolerance in the filamentous green algae Cladophora glomerata and Enteromorpha ahlneriana. J. Exp. Mar. Biol. Ecol. 2004, 298, 111–123. [Google Scholar] [CrossRef]
- Tưởng, L.T.; Trang, N.T.M. Optimization of early processing of grape seaweed (Caulerpa lentillifera J. Agardh, 1837) after harvested. Can. Tho Univ. J. Sci. 2016, 47, 54–61. [Google Scholar] [CrossRef]
- Marangoni, A.G. Chlorophyll degradation in green tissues: Olives, cabbage and pickles. In Kinetic Analysis of Food Systems; Springer: Cham, Switzerland, 2017; pp. 55–63. [Google Scholar]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Nofiani, R.; Hertanto, S.; Zaharah, T.A.; Gafur, S. Proximate Compositions and Biological Activities of Caulerpa lentillifera. Molekul 2018, 13, 141–147. [Google Scholar] [CrossRef]
- Jespersen, D.; Zhang, J.; Huang, B. Chlorophyll loss associated with heat-induced senescence in bentgrass. Plant Sci. 2016, 249, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Buvé, C.; Kebede, B.T.; De Batselier, C.; Carrillo, C.; Pham, H.T.T.; Hendrickx, M.; Grauwet, T.; Van Loey, A. Kinetics of colour changes in pasteurised strawberry juice during storage. J. Food Eng. 2018, 216, 42–51. [Google Scholar] [CrossRef]
- Sonar, C.R.; Rasco, B.; Tang, J.; Sablani, S.S. Natural color pigments: Oxidative stability and degradation kinetics during storage in thermally pasteurized vegetable purees. J. Sci. Food Agric. 2019, 99, 5934–5945. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, S.; Grauwet, T.; Santiago, J.S.; Tomic, J.; Vervoort, L.; Hendrickx, M.; Van Loey, A. Quality changes of pasteurised orange juice during storage: A kinetic study of specific parameters and their relation to colour instability. Food Chem. 2015, 187, 140–151. [Google Scholar] [CrossRef]
- Sun, Y.; Li, W. Effects the mechanism of micro-vacuum storage on broccoli chlorophyll degradation and builds prediction model of chlorophyll content based on the color parameter changes. Sci. Hortic. 2017, 224, 206–214. [Google Scholar] [CrossRef]
- Aryanti, N.; Nafiunisa, A. Extraction, characterization and degradation of chlorophyll from Suji Leaves (Pleomele angustifolia). Orient. J. Chem. 2017, 33, 3185–3190. [Google Scholar] [CrossRef]
- Nayak, P.K.; Mohan, C.C.; Radhakrishnan, K. Effect of microwave pretreatment on the color degradation kinetics in mustard greens (Brassica juncea). Chem. Eng. Commun. 2018, 205, 1261–1273. [Google Scholar] [CrossRef]
- Benlloch-Tinoco, M.; Kaulmann, A.; Corte-Real, J.; Rodrigo, D.; Martínez-Navarrete, N.; Bohn, T. Chlorophylls and carotenoids of kiwifruit puree are affected similarly or less by microwave than by conventional heat processing and storage. Food Chem. 2015, 187, 254–262. [Google Scholar] [CrossRef]
- Zhao, R.; Hao, J.; Xue, J.; Liu, H.; Li, L. Effect of high-voltage electrostatic field pretreatment on the antioxidant system in stored green mature tomatoes. J. Sci. Food Agric. 2011, 91, 1680–1686. [Google Scholar] [CrossRef]
- Contreras-Porcia, L.; Thomas, D.; Flores, V.; Correa, J.A. Tolerance to oxidative stress induced by desiccation in Porphyra columbina (Bangiales, Rhodophyta). J. Exp. Bot. 2011, 62, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Gao, Q. Effects of high-voltage electric field treatment on physicochemical properties of potato starch. J. Food Meas. Charact. 2019, 13, 3069–3076. [Google Scholar] [CrossRef]
- Hu, Y.H.; Chen, C.M.; Xu, L.; Cui, Y.; Yu, X.Y.; Gao, H.J.; Wang, Q.; Liu, K.; Shi, Y.; Chen, Q.X. Postharvest application of 4-methoxy cinnamic acid for extending the shelf life of mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2015, 104, 33–41. [Google Scholar] [CrossRef]
- Xu, D.; Xiao, Y.; Pan, H.; Mei, Y. Toxic effects of tetracycline and its degradation products on freshwater green algae. Ecotoxicol. Environ. Saf. 2019, 174, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yao, X.; Sun, S.; Wang, L.; Liu, W.; Jiang, X.; Wang, J. Effects of mesotrione on oxidative stress, subcellular structure, and membrane integrity in Chlorella vulgaris. Chemosphere 2020, 247, 125668. [Google Scholar] [CrossRef]
- Meng, D.M.; Zhang, Y.X.; Yang, R.; Wang, J.; Zhang, X.H.; Sheng, J.P.; Wang, J.P.; Fan, Z.C. Arginase participates in the methyl jasmonate-regulated quality maintenance of postharvest Agaricus bisporus fruit bodies. Postharvest Biol. Technol. 2017, 132, 7–14. [Google Scholar] [CrossRef]
- Tavakoli, A.; Sahari, M.A.; Barzegar, M.; Ahmadi Gavlighi, H. Optimization of high voltage electric field as a novel non-thermal method of sunflower oil neutralization. Sep. Purif. Technol. 2019, 211, 430–437. [Google Scholar] [CrossRef]
- Abedi, E.; Amiri, M.J.; Sahari, M.A. Kinetic, isotherm and thermodynamic investigations on adsorption of trace elements and pigments from soybean oil using high voltage electric field-assisted bleaching: A comparative study. Process Biochem. 2020, 91, 208–222. [Google Scholar] [CrossRef]
- Bensalem, S.; Pareau, D.; Cinquin, B.; Français, O.; Le Pioufle, B.; Lopes, F. Impact of pulsed electric fields and mechanical compressions on the permeability and structure of Chlamydomonas reinhardtii cells. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Yuan, B.; Xie, Y.; Cheng, S.; Huang, H.; Zhang, W. Postharvest Biology and Technology Improvement of postharvest quality, enzymes activity and polyphenoloxidase structure of postharvest Agaricus bisporus in response to high voltage electric field. Postharvest Biol. Technol. 2020, 166, 111230. [Google Scholar] [CrossRef]
- Yamauchi, N. Postharvest Chlorophyll Degradation and Oxidative Stress. In Abiotic Stress Biology in Horticultural Plants; Kanayama, Y., Kochetov, A., Eds.; Springer: Tokyo, Japan, 2015; pp. 101–113. [Google Scholar]
- Sairam, R.K.; Tyagi, A. Physiology and molecular biology of stress tolerance in plants. In Current Science; Current Science Association: Bangalore, India, 2004; Volume 86, pp. 407–421. [Google Scholar]
- Jiang, T.; Jahangir, M.M.; Jiang, Z.; Lu, X.; Ying, T. Influence of UV-C treatment on antioxidant capacity, antioxidant enzyme activity and texture of postharvest shiitake (Lentinus edodes) mushrooms during storage. Postharvest Biol. Technol. 2010, 56, 209–215. [Google Scholar] [CrossRef]
- Petriccione, M.; Pagano, L.; Forniti, R.; Zampella, L.; Mastrobuoni, F.; Scortichini, M.; Mencarelli, F. Postharvest treatment with chitosan affects the antioxidant metabolism and quality of wine grape during partial dehydration. Postharvest Biol. Technol. 2018, 137, 38–45. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Kudoh, H.; Sonoike, K. Irreversible damage to photosystem I by chilling in the light: Cause of the degradation of chlorophyll after returning to normal growth temperature. Planta 2002, 215, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Wang, L.H.; Zeng, X.A.; Han, Z.; Wang, M.S. Effect of pulsed electric fields (PEFs) on the pigments extracted from spinach (Spinacia oleracea L.). Innov. Food Sci. Emerg. Technol. 2017, 43, 26–34. [Google Scholar] [CrossRef]
- Sánchez-Vega, R.; Elez-Martínez, P.; Martín-Belloso, O. Effects of High-Intensity Pulsed Electric Fields Processing Parameters on the Chlorophyll Content and Its Degradation Compounds in Broccoli Juice. Food Bioprocess Technol. 2014, 7, 1137–1148. [Google Scholar] [CrossRef]
- Fang, H.; Luo, F.; Li, P.; Zhou, Q.; Zhou, X.; Wei, B.; Cheng, S.; Zhou, H.; Ji, S. Potential of jasmonic acid (JA) in accelerating postharvest yellowing of broccoli by promoting its chlorophyll degradation. Food Chem. 2019, 309, 125737. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.; Lv, X.; Cheng, N.; Peng, B.; Cao, W. Postharvest Biology and Technology Effect of 24-epibrassinolide on chilling injury of peach fruit in relation to phenolic and proline metabolisms. Postharvest Biol. Technol. 2016, 111, 390–397. [Google Scholar] [CrossRef]
- Sun, C.; Jin, L.; Cai, Y.; Huang, Y.; Zheng, X.; Yu, T. L-Glutamate Treatment enhances disease resistance of tomato fruit by inducing the expression of glutamate receptors and the accumulation of amino acids L -Glutamate. Food Chem. 2019, 293, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]






| Cultivation Season | Post-Harvest Storage | Zero-Order Reaction | First-Order Reaction | Second-Order Reaction | |||
|---|---|---|---|---|---|---|---|
| k | R2 | k | R2 | k | R2 | ||
| Spring | Within SW | −0.1228 | 0.9968 | −0.0283 | 0.749 | 0.0084 | 0.9999 |
| Without SW | −0.1923 | 0.9982 | −0.0598 | 0.9984 | 0.0191 | 0.9991 | |
| Summer | Within SW | −0.1606 | 0.9986 | −0.0455 | 0.9999 | 0.013 | 0.9995 |
| Without SW | −0.1642 | 0.9871 | −0.0703 | 0.9986 | 0.0287 | 0.9986 | |
| Winter | Within SW | −0.0371 | 0.9928 | −0.0349 | 0.9891 | 0.033 | 0.9846 |
| Without SW | −0.041 | 0.9393 | −0.0729 | 0.8417 | 0.0757 | 0.874 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulaimana, A.S.; Chang, C.-K.; Hou, C.-Y.; Yudhistira, B.; Punthi, F.; Lung, C.-T.; Cheng, K.-C.; Santoso, S.P.; Hsieh, C.-W. Effect of Oxidative Stress on Physicochemical Quality of Taiwanese Seagrape (Caulerpa lentillifera) with the Application of Alternating Current Electric Field (ACEF) during Post-Harvest Storage. Processes 2021, 9, 1011. https://doi.org/10.3390/pr9061011
Sulaimana AS, Chang C-K, Hou C-Y, Yudhistira B, Punthi F, Lung C-T, Cheng K-C, Santoso SP, Hsieh C-W. Effect of Oxidative Stress on Physicochemical Quality of Taiwanese Seagrape (Caulerpa lentillifera) with the Application of Alternating Current Electric Field (ACEF) during Post-Harvest Storage. Processes. 2021; 9(6):1011. https://doi.org/10.3390/pr9061011
Chicago/Turabian StyleSulaimana, Andi Syahrullah, Chao-Kai Chang, Chih-Yao Hou, Bara Yudhistira, Fuangfah Punthi, Chun-Ta Lung, Kuan-Chen Cheng, Shella Permatasari Santoso, and Chang-Wei Hsieh. 2021. "Effect of Oxidative Stress on Physicochemical Quality of Taiwanese Seagrape (Caulerpa lentillifera) with the Application of Alternating Current Electric Field (ACEF) during Post-Harvest Storage" Processes 9, no. 6: 1011. https://doi.org/10.3390/pr9061011
APA StyleSulaimana, A. S., Chang, C.-K., Hou, C.-Y., Yudhistira, B., Punthi, F., Lung, C.-T., Cheng, K.-C., Santoso, S. P., & Hsieh, C.-W. (2021). Effect of Oxidative Stress on Physicochemical Quality of Taiwanese Seagrape (Caulerpa lentillifera) with the Application of Alternating Current Electric Field (ACEF) during Post-Harvest Storage. Processes, 9(6), 1011. https://doi.org/10.3390/pr9061011









