New Refrigerant Molecules from Structure Optimization
Abstract
1. Introduction
2. Materials and Methods
2.1. Functional Groups and Structural Constraints
2.2. Property Targets
2.3. Modeling of Physical Properties
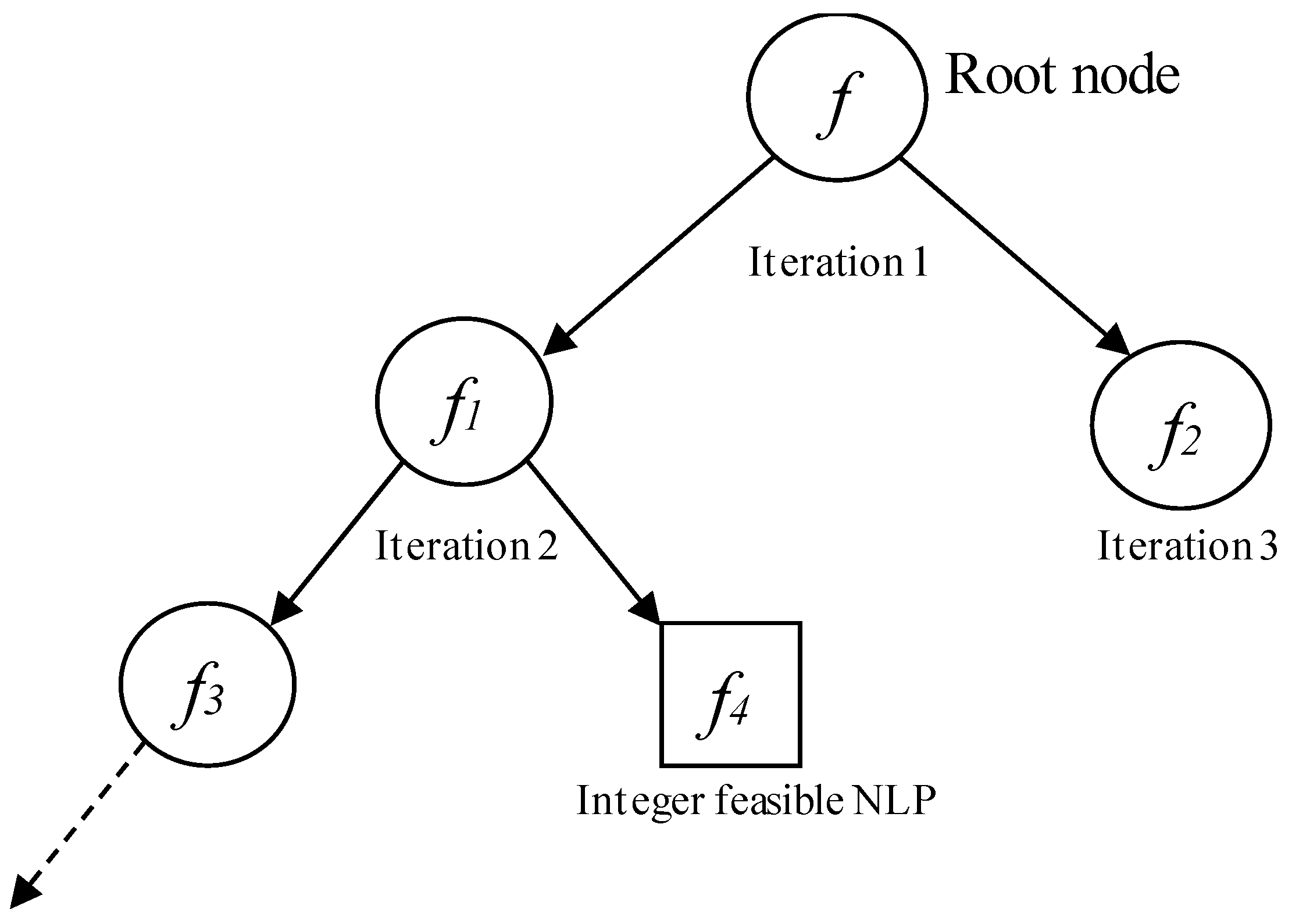
2.4. MINLP Solution
2.5. Environmental and Safety Evaluations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seider, W.D.; Seader, J.D.; Lewin, D.R.; Widagdo, S. Product and Process Design Principles, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Akintunde, M.A. Experimental Study of R134a, R406A and R600a Blends as Alternative To Freon 12. IOSR J. Mech. Civ. Eng. 2013, 7, 40–46. [Google Scholar] [CrossRef]
- Mondejar, M.E.; Haglind, F. The potential of halogenated olefins as working fluids for organic Rankine cycle technology. J. Mol. Liq. 2020, 310, 112971. [Google Scholar] [CrossRef]
- Calm, J.M. The next generation of refrigerants—Historical review, considerations, and outlook. Int. J. Refrig. 2008, 31, 1123–1133. [Google Scholar] [CrossRef]
- Booten, C.W.; Nicholson, S.R.; Mann, M.K.; Abdelaziz, O. Refrigerants: Market Trends and Supply Chain Assessment; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2020. [Google Scholar] [CrossRef]
- Markets and Markets. Refrigerants Market by Type (HCFC, HFC, HFO, Isobutane, Propane, Ammonia, Carbon Di-oxide, Air, Water, Propene, Isopentane), Application (Domestic, Industrial, Commercial, Chillers, Split AC, VRF, Window, MAC), and Region—Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/refrigerant-market-1082.html (accessed on 19 February 2021).
- Market Data Forecast. Latin America Refrigerant Market. Available online: https://www.marketdataforecast.com/market-reports/la-refrigerant-market (accessed on 19 February 2021).
- Ministerio de Producción Comercio Exterior Inversiones y Pesca. Resolución No. 031-2019. Available online: https://www.produccion.gob.ec/wp-content/uploads/2019/12/Resolución-COMEX-031-2019.pdf (accessed on 19 February 2021).
- Superintendencia de Control del Poder de Mercado. Informe del sector Lácteo en Ecuador. Available online: https://www.scpm.gob.ec/sitio/wp-content/uploads/2019/03/Version-publica-informe-sector-de-leche.pdf (accessed on 19 February 2021).
- Tomczyk, J.A.; Silberstein, E.; Whitman, W.C.; Johnson, W.M. Refrigeration and Air Conditioning Technology, 8th ed.; Cengage Learning: Boston, MA, USA, 2017. [Google Scholar]
- Mazur, V. Optimum Refrigerant Selection for Low Temperature Engineering. In Low Temperature and Cryogenic Re-frigeration; Kakaç, S., Smirnov, H.F., Avelino, M.R., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 101–118. [Google Scholar]
- Khetib, Y.; Meniai, A.-H.; Lallemand, A. Computer-aided design of CFC and HCFC substitutes using group contribution methods. Desalination 2009, 239, 82–91. [Google Scholar] [CrossRef]
- Joback, K.; Reid, R. Estimation of Pure-Component properties from group-contributions. Chem. Eng. Commun. 1987, 57, 233–243. [Google Scholar] [CrossRef]
- Churi, N.; Achenie, L.E.K. Novel Mathematical Programming Model for Computer Aided Molecular Design. Ind. Eng. Chem. Res. 1996, 35, 3788–3794. [Google Scholar] [CrossRef]
- Duvedi, A.P.; Achenie, L.E. Designing environmentally safe refrigerants using mathematical programming. Chem. Eng. Sci. 1996, 51, 3727–3739. [Google Scholar] [CrossRef]
- Sahinidis, N.; Tawarmalani, M.; Yu, M. Design of alternative refrigerants via global optimization. AIChE J. 2003, 49, 1761–1775. [Google Scholar] [CrossRef]
- Owen, M.S. ASHRAE Handbook; ASHRAE: Atlanta, GA, USA, 2009. [Google Scholar]
- Rabie, M.; Franck, C.M. Computational screening of new high voltage insulation gases with low global warming potential. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 296–302. [Google Scholar] [CrossRef]
- Gani, R.; Nielsen, B.; Fredenslund, A. A group contribution approach to computer-aided molecular design. AIChE J. 1991, 37, 1318–1332. [Google Scholar] [CrossRef]
- Apostolakou, A.; Adjiman, C.S. Refrigerant Design Case Study. In Computer Aided Molecular Design: Theory and Practice; Achenie, L.E.K., Gani, R., Venkatasubramania, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 289–301. [Google Scholar]
- Reid, R.C.; Prausnitz, J.M.; Poling, B.E. The Properties of Gases and Liquids, 4th ed.; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar]
- Edgar, T.F.; Himmelblau, D.M.; Lasdon, L.S. Optimization of Chemical Processes, 2nd ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Salhi, A.; Horst, R.; Tuy, H. Global Optimization: Deterministic Approaches (2nd Edition). J. Oper. Res. Soc. 1994, 45, 595. [Google Scholar] [CrossRef]
- Morrison, D.R.; Jacobson, S.H.; Sauppe, J.J.; Sewell, E.C. Branch-and-bound algorithms: A survey of recent advances in searching, branching, and pruning. Discret. Optim. 2016, 19, 79–102. [Google Scholar] [CrossRef]
- Calm, J.M.; Didion, D.A. Trade-offs in refrigerant selections: Past, present, and future. Int. J. Refrig. 1998, 21, 308–321. [Google Scholar] [CrossRef]
- Kondo, S.; Urano, Y.; Tokuhashi, K.; Takahashi, A.; Tanaka, K. Prediction of flammability of gases by using F-number analysis. J. Hazard. Mater. 2001, 82, 113–128. [Google Scholar] [CrossRef]
- Gao, C.; Govind, R.; Tabak, H.H. Application of the group contribution method for predicting the toxicity of organic chemicals. Environ. Toxicol. Chem. 1992, 11, 631–636. [Google Scholar] [CrossRef]
- Martin, T.M.; Young, D.M. Prediction of the Acute Toxicity (96-h LC50) of Organic Compounds to the Fathead Minnow (Pimephales promelas) Using a Group Contribution Method. Chem. Res. Toxicol. 2001, 14, 1378–1385. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.S.; Lee, S.D.; Lee, W.K.; Kim, H. Selective production of difluoromethyl methyl ether from chlorodifluoromethane using alkali metal carbonates. J. Fluor. Chem. 2001, 107, 133–136. [Google Scholar] [CrossRef]
- Abbas, L.T.; Fortman, G.C. Refrigerants with Reduced Flammability Profile. U.S. Patent 0,017,743 A1, 3 September 2019. [Google Scholar]
- Atwood, D.A. Fluorine: Inorganic Chemistry. In Encyclopedia of Inorganic Chemistry; King, R.B., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 1–25. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 24524, Fluorine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Fluorine (accessed on 6 March 2021).
| Alkane Groups | Alkene Groups | Oxygen Groups | Nitrogen Groups | Sulfur Groups | Halogen Groups |
|---|---|---|---|---|---|
| –CH3 | =CH2 | –OH | –NH2 | –S– | –F |
| –CH2– | =CH– | –O– | –NH– | –SH | –Cl |
| >CH– | =C< | –N< | |||
| >C< |
| Objective Function | Formula | Molecular Structure | kJ mol−1 | cal mol−1K−1 | Bar | Bar | P | Objective Function Value |
|---|---|---|---|---|---|---|---|---|
| CH3FO2 | CH3—O—O—F | 22.6606 | 29.5882 | 1.2528 | 5.6324 | 0.7659 | 4.4958 | |
| C2H4F2O | CH3—CH<(—O—F)(—F) | 22.3006 | 30.3052 | 1.2838 | 5.5870 | 0.7359 | 1.1777 | |
| CH2F2O2 | F—CH2—O—O—F | 22.2679 | 32.1216 | 1.2989 | 5.9995 | 0.6932 | 1.2147 | |
| CH2F2O | F—CH2—O—F | 18.9674 | 23.7566 | 3.1516 | 11.8755 | 0.7984 | 531.4080 | |
| C3H4F2 | F—CH=CH—CH2—F | 23.1438 | 25.5730 | 1.0451 | 4.5789 | 0.9050 | −0.8926 | |
| C2H2F2O | F—CH=CH—O—F | 23.0463 | 26.6724 | 1.0662 | 4.8336 | 0.8641 | 0.2206 |
| Formula | F | X |
|---|---|---|
| CH3FO2 | 0.5703 | 0.5422 |
| C2H4F2O | 0.5488 | 1.337 |
| CH2F2O2 | 0.5167 | 0.7748 |
| C3H4F2 | 0.5641 | 2.4373 |
| C2H2F2O | 0.5831 | 1.7517 |
| CH2F2O | 0.4522 | 1.014 |
| C2H2F4 | 0.4478 | 1.8739 |
| CCl2F2 | 0.1571 | 2.4147 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosero, C.R.; Espinel, P.S.; Tuza, P.V. New Refrigerant Molecules from Structure Optimization. Processes 2021, 9, 949. https://doi.org/10.3390/pr9060949
Rosero CR, Espinel PS, Tuza PV. New Refrigerant Molecules from Structure Optimization. Processes. 2021; 9(6):949. https://doi.org/10.3390/pr9060949
Chicago/Turabian StyleRosero, Cristhian R., P. Sebastián Espinel, and Pablo V. Tuza. 2021. "New Refrigerant Molecules from Structure Optimization" Processes 9, no. 6: 949. https://doi.org/10.3390/pr9060949
APA StyleRosero, C. R., Espinel, P. S., & Tuza, P. V. (2021). New Refrigerant Molecules from Structure Optimization. Processes, 9(6), 949. https://doi.org/10.3390/pr9060949







