Cost-Efficacy of Antiretroviral Regimens Recommended in Treatment-Naive HIV-Infected Adults. A Single Center Experience
Abstract
1. Introduction
2. Methods
3. Results
3.1. Costs
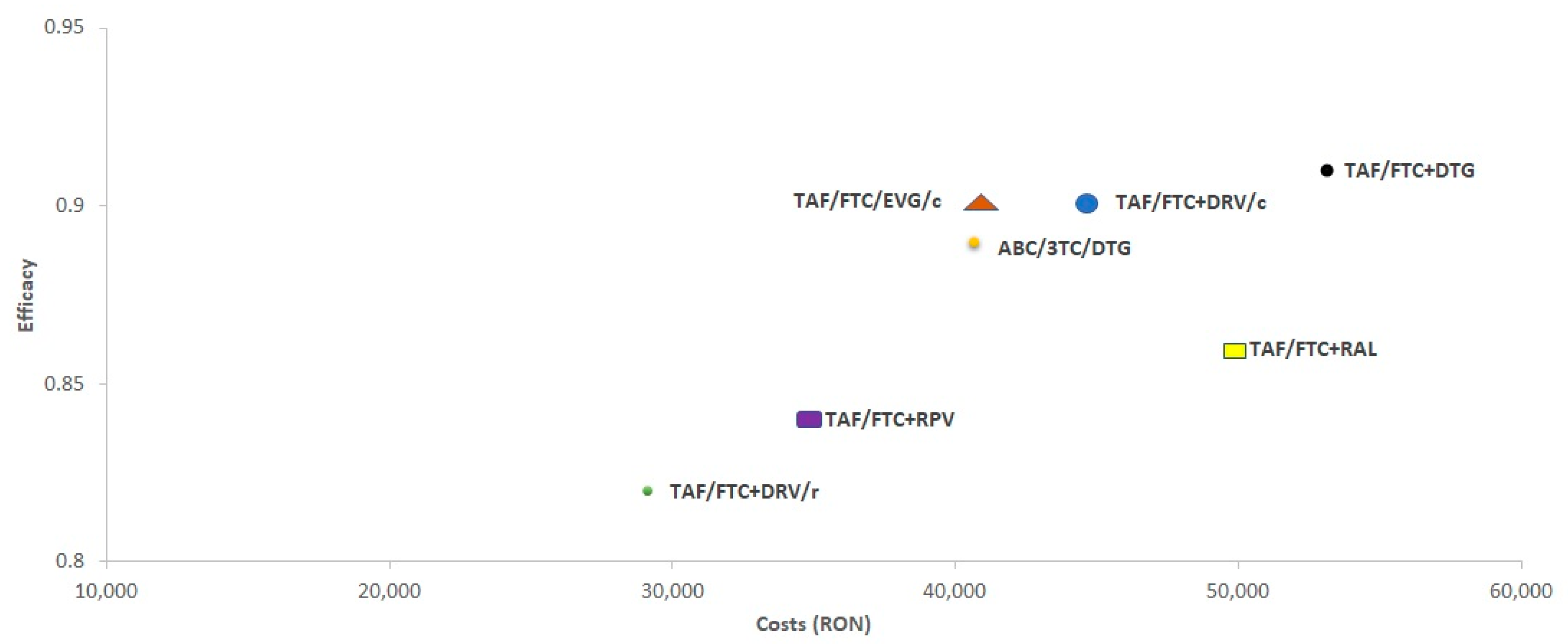
3.2. Cost/Efficacy (C/E)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EACS Guidelines 2018. (Version 9.1). Available online: http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html (accessed on 18 June 2020).
- The Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord; Lewden, C.; Bouteloup, V.; de Wit, S.; Sabin, C.; Mocroft, A.; Wasmuth, J.; van Sighem, A.; Kirk, O.; Obel, N.; et al. All-cause mortality in treated HIV-infected adults with CD4 ≥ 500/mm3 compared with the general population: Evidence from a large European observational cohort collaboration. Int. J. Epidemiol. 2012, 41, 433–445. [Google Scholar]
- Forsythe, S.S.; McGreevey, W.; Whiteside, A.; Shah, M.; Cohen, J.; Hecht, R.; Bollinger, L.A.; Kinghorn, A. Twenty years of antiretroviral therapy for people living with HIV: Global costs, health achievements, economic benefits. Health Aff. 2019, 38, 1163–1172. [Google Scholar] [CrossRef]
- Tran, B.X.; Nguyen, L.H.; Turner, H.C.; Nghiem, S.; Vu, G.T.; Nguyen, C.T.; Latkin, C.A.; Ho, C.S.H.; Ho, R.C.M. Economic evaluation studies in the field of HIV/AIDS: Bibliometric analysis on research development and scopes (GAPRESEARCH). BMC Health Serv. Res. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Tse, W.F.; Yang, W.; Huang, W. A narrative review of cost-effectiveness analysis of people living with HIV treated with HAART: From interventions to outcomes. Clin. Outcomes Res. 2015, 7, 431–439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freedberg, K.A.; Losina, E.; Weinstein, M.C.; Paltiel, A.D.; Cohen, C.J.; Seage, G.R.; Craven, D.E.; Zhang, H.; Kimmel, A.D.; Goldie, S.J. The Cost Effectiveness of Combination Antiretroviral Therapy for HIV Disease. N. Engl. J. Med. 2001, 344, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Holtgrave, D.R.; Qualls, N.L.; Graham, J.D. Economic evaluation of HIV prevention programs. Annu. Rev. Public Health 1996, 17, 467–488. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, O.; Colchero, M.A.; Wamai, R.G.; Bertozzi, S.M. HIV prevention costeffectiveness: A systematic review. BMC Public Health 2009, 9, S5. [Google Scholar] [CrossRef] [PubMed]
- Ruger, J.P.; Lazar, C.M. Economic evaluation of drug abuse treatment and HIV prevention programs in pregnant women: A systematic review. Addict. Behav. 2012, 37, 1–10. [Google Scholar] [CrossRef]
- Dibosa-Osadolor, O.; Roberts, T. Economic evaluation, human immunodeficiency virus infection and screening: A review and critical appraisal of economic studies. Int. J. Technol. Assess. Health Care 2010, 26, 301–308. [Google Scholar] [CrossRef]
- Streinu-Cercel, A.; Sandulescu, O.; Poiana, C.; Dorobantu, M.; Mircescu, G.; Lazureanu, V.E.; Dumitru, I.M.; Chirila, O.; Streinu-Cercel, A. Extended Consensus Group. Consensus statement on the assessment of comorbidities in people living with HIV in Romania. Germs 2019, 9, 198–210. [Google Scholar] [CrossRef]
- Perez-Molina, J.A.; Martinez, E.; Blasco, A.J.; Aribase, J.E.; Domingo, P.; Iribarren, J.A.; Knobel, H.; Lazaro, P.; López-Aldeguer, J.; Lozano, F.; et al. Analysis of the costs and cost-effectiveness of the guidelines recommended by the 2018 GESIDA/Spanish National AIDS Plan for initial antiretroviral therapy in HIV-infected adults. Enferm. Infecc. Microbiol. Clin. 2018, 37, 151–159. [Google Scholar] [CrossRef]
- Pitt, C.; Goodman, C.; Hanson, K. Economic evaluation in global perspective: A bibliometric analysis of the recent literature. Health Econ. 2016, 25 (Suppl. 1), 9–28. [Google Scholar] [CrossRef]
- Jacobsen, M.M.; Walensky, R.P. Modeling and Cost-Effectiveness in HIV Prevention. Curr. HIV/AIDS Rep. 2016, 13, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Cambiano, V.; Miners, A.; Dunn, D.; McCormack, S.; Ong, K.J.; Gill, O.N.; Nardone, A.; Desai, M.; Field, N.; Hart, G.; et al. Cost-effectiveness of pre-exposure prophylaxis for HIV prevention in men who have sex with men in the UK: A modelling study and health economic evaluation. Lancet Infect. Dis. 2018, 18, 85–94. [Google Scholar] [CrossRef]
- Huang, Y.L.; Lasry, A.; Hutchinson, A.B.; Sansom, S.L. A systematic review on cost effectiveness of HIV prevention interventions in the United States. Appl Health Econ. Health Policy 2015, 13, 149–156. [Google Scholar] [CrossRef]
- Werayingyong, P.; Phanuphak, N.; Chokephaibulkit, K.; Tantivess, S.; Kullert, N.; Tosanguan, K.; Butchon, R.; Voramongkol, N.; Boonsuk, S.; Pilasant, S.; et al. Economic evaluation of 3-drug antiretroviral regimens for the prevention of mother-to-child HIV transmission in Thailand. Asia Pac. J. Public Health 2015, 27, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Zaric, G.S.; Barnett, P.G.; Brandeau, M.L. HIV transmission and the cost-effectiveness of methadone maintenance. Am. J. Public Health 2000, 90, 1100–1111. [Google Scholar]
- Pho, M.T.; Swaminathan, S.; Kumarasamy, N.; Losina, E.; Ponnuraja, C.; Uhler, L.M.; Scott, C.A.; Mayer, K.H.; Freedberg, K.A.; Walensky, R.P. The Cost-Effectiveness of Tuberculosis Preventive Therapy for HIV-Infected Individuals in Southern India: A Trial-Based Analysis. PLoS ONE 2012, 7, e36001. [Google Scholar] [CrossRef]
- Li, X.; Stander, M.P.; Van Kriekinge, G.; Demarteau, N. Cost-effectiveness analysis of human papillomavirus vaccination in South Africa accounting for human immunodeficiency virus prevalence. BMC Infect. Dis. 2015, 15, 1–18. [Google Scholar] [CrossRef]
- Sweat, M.; Gregorich, S.; Sangiwa, G.; Furlonge, C.; Balmer, D.; Kamenga, C.; Grinstead, O.; Coates, T. Cost-effectiveness of voluntary HIV-1 counselling and testing in reducing sexual transmission of HIV-1 in Kenya and Tanzania. Lancet 2000, 356, 113–121. [Google Scholar] [CrossRef]
- Hove-Musekwa, S.D.; Nyabadza, F.; Mambili-Mamboundou, H.; Chiyaka, C.; Mukandavire, Z. Cost-Effectiveness Analysis of Hospitalization and Home-Based Care Strategies for People Living with HIV/AIDS: The Case of Zimbabwe. Int. Sch. Res. Not. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, K.A.; Sax, P.E. Improving on effective antiretroviral therapy: How good will a cure have to be? J. Med. Ethics 2017, 43, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Llibre, J.M.; De Lazzari, E.; Molina, J.-M.; Gallien, S.; Gonzalez-García, J.; Imaz, A.; Podzamczer, D.; Clotet, B.; Domingo, P.; Gatell, J.M. Cost–effectiveness of initial antiretroviral treatment administered as single vs. multiple tablet regimens with the same or different components. Enferm. Infecc. Microbiol. Clínica 2018, 36, 16–20. [Google Scholar] [CrossRef]
- Kockaya, G.; Zengin, T.E.; Yenilmez, F.B.; Dalgic, C.; Malhan, S.M.S.; Cerci, P.; Oksuz, E.; Ünal, S. Analysis of the treatment cost of HIV/AIDS in Turkey. Farmeconomia Health Econ. Ther. Pathways 2016, 17, 13–17. [Google Scholar] [CrossRef][Green Version]
- Beck, E.J.; Mandalia, S.; Sangha, R.; Sharott, P.; Youle, M.; Baily, G.; Brettle, R.; Gompels, M.; Johnson, M.; McCarron, B.; et al. The Cost-Effectiveness of Early Access to HIV Services and Starting cART in the UK 1996–2008. PLoS ONE 2011, 6, e27830. [Google Scholar] [CrossRef]
- Goldie, S.J.; Yazdanpanah, Y.; Losina, E.; Weinstein, M.C.; Anglaret, X.; Walensky, R.P.; Hsu, H.E.; Kimmel, A.; Holmes, C.; Kaplan, J.E.; et al. Cost-Effectiveness of HIV Treatment in Resource-Poor Settings—The Case of Côte d’Ivoire. N. Engl. J. Med. 2006, 355, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Scévola, S.; Tiraboschi, J.M.; Podzamczer, D. Nothing is perfect: The safety issues of integrase inhibitor regimens. Expert Opin. Drug Saf. 2020, 19, 683–694. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. HIV treatment and care. In Monitoring Implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2017 Progress Report Stockholm; ECDC: Stockholm, Sweden, 2017. [Google Scholar]
- The National Plan for HIV/AIDS. Available online: http://www.ms.ro/wp-content/uploads/2018/11/Anexa-la-HG-Plan-National-HIV-2019-2021.pdf (accessed on 18 June 2020). (In Romanian).
| Combination ART | Cost (RON) |
|---|---|
| ABC/3TC/DTG (PR) | 40,522 |
| TAF/FTC + DTG (PR) | 53,532 |
| TAF/FTC + RAL (PR) | 50,131 |
| TAF/FTC + RPV (PR) | 34,527 |
| TAF/FTC + DRV/r (PR) | 28,829 |
| TAF/FTC + DRV/c (PR) | 44,708 |
| TAF/FTC/EVGc (AR) | 40,720 |
| Resource | Cost (RON) |
|---|---|
| Drug-resistance testing | |
| NRTIs, NNRTIs, PIs | 290 |
| INSTIs | 290 |
| HLA-B 5701 | 150 |
| Specialist medical consultations | |
| First consultation | 200 |
| Control | 100 |
| Emergency department consultation | 200 |
| Day of hospitalization | 440 |
| Diagnostic testing | |
| Ultrasound | 120 |
| Complete blood count | 13 |
| Liver function (transaminases) | 10 |
| Coagulation testing | 15 |
| Stool culture | 187 |
| Initial Regimen | Base Case Scenario | Most Favorable Scenario | Least Favorable Scenario | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cost (RON) a | Efficacy | C/E b | Relative C/E c | Cost (RON) a | Efficacy | C/E b | Relative C/E c | Cost (RON) a | Efficacy | C/E b | Relative C/E c | |
| ABC/3TC/DTG | 40,640 | 0.89 | 45,481 | 1.000 | 40,605 | 0.91 | 44,479 | 1.000 | 40,681 | 0.87 | 46.534 | 1.000 |
| TAF/FTC+DTG | 53,122 | 0.91 | 58,402 | 1.284 | 53,208 | 0.93 | 57,189 | 1.286 | 53,040 | 0.89 | 59.677 | 1.282 |
| TAF/FTC+RAL | 49,801 | 0.86 | 58,047 | 1.276 | 49,830 | 0.87 | 57,003 | 1.282 | 49,775 | 0.84 | 59.134 | 1.271 |
| TAF/FTC/EVG/c | 40,759 | 0.90 | 45,190 | 0.994 | 40,738 | 0.91 | 44,614 | 1.003 | 40,784 | 0.89 | 45.785 | 0.984 |
| TAF/FTC+DRV/c | 44,666 | 0.90 | 49,667 | 1.092 | 44,667 | 0.92 | 48,487 | 1.090 | 44,671 | 0.88 | 50.912 | 1.094 |
| TAF/FTC+DRV/r | 29,142 | 0.82 | 35,508 | 0.781 | 29,095 | 0.84 | 34,633 | 0.779 | 29,193 | 0.80 | 36.430 | 0.783 |
| TAF/FTC+RPV | 34,819 | 0.84 | 41,226 | 0.906 | 34,759 | 0.87 | 39,920 | 0.898 | 34,885 | 0.82 | 42.621 | 0.916 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jipa, R.; Nedelcu, I.; Manea, E.; Damalan, A.; Hristea, A. Cost-Efficacy of Antiretroviral Regimens Recommended in Treatment-Naive HIV-Infected Adults. A Single Center Experience. Processes 2021, 9, 956. https://doi.org/10.3390/pr9060956
Jipa R, Nedelcu I, Manea E, Damalan A, Hristea A. Cost-Efficacy of Antiretroviral Regimens Recommended in Treatment-Naive HIV-Infected Adults. A Single Center Experience. Processes. 2021; 9(6):956. https://doi.org/10.3390/pr9060956
Chicago/Turabian StyleJipa, Raluca, Iulia Nedelcu, Eliza Manea, Anca Damalan, and Adriana Hristea. 2021. "Cost-Efficacy of Antiretroviral Regimens Recommended in Treatment-Naive HIV-Infected Adults. A Single Center Experience" Processes 9, no. 6: 956. https://doi.org/10.3390/pr9060956
APA StyleJipa, R., Nedelcu, I., Manea, E., Damalan, A., & Hristea, A. (2021). Cost-Efficacy of Antiretroviral Regimens Recommended in Treatment-Naive HIV-Infected Adults. A Single Center Experience. Processes, 9(6), 956. https://doi.org/10.3390/pr9060956






