Abstract
For the winemaking bioprocess of jujube wine, the selection of optimal starter cultures is one of the major concerns before fermentation. In this study, we investigated the effects of different winemaking yeasts on the composition of aroma-active compounds in the fermented jujube wine and identified the principal components that determine the flavor quality. It showed that the starter winemaking yeasts produced a total of 43 aroma-active compounds, of which esters (e.g., ethyl caprylate, ethyl decanoate, ethyl hexanoate, and phenethyl acetate) contribute more to the wine quality attributes, especially for the improvement of the aroma. Moreover, the composition of aroma-active compounds, for example, the ratio of the content of esters and alcohols, exerts a great impact on the flavor quality of jujube wine. Different starter winemaking yeasts resulted in significant differences in the composition (both species and content) of aroma-active compounds, and thus formed different flavors in the jujube wine. Thus, we propose that screening of a desirable starter winemaking yeast is essential before the fermentation of jujube wine at a large scale, and more considerations should be taken into the resulting composition of aroma-active compounds.
1. Introduction
Jujube (Zizyphus jujuba Mill.) widely grows in the temperate, subtropical, and tropical regions of mountains, hills, or plains, especially in the Northwest of China, such as in Xinjiang, Gansu, and the Shaanxi provinces [1,2,3]. The ripe jujube turns red and is usually dried for a longer shelf life in order to maintain the completeness of nutrients [2,4]. In China, this kind of fruit has been used as traditional food or medicinal materials for thousands of years, due to its abundance of bioactives characterized by immunomodulatory, antioxidant, antitumor, hepatoprotective, and gastrointestinal–protective activities [2,5,6]. Because of the preferable geographical properties in the Northwest of China, the production of jujube in China accounts for a great share of jujube production in the world. Despite the fact that jujube production is one of the pillar industries, particularly in Xinjiang and Gansu, jujube in the market has mainly been consumed as an unprocessed product, and highly value-added jujube products are very rare [7]. Currently, a lack of deep processing technologies has largely restricted the development of the jujube industry, resulting in a great waste of natural resources, and influencing the local economy to a large extent [3,8]. Thus, exploitation of more value-added jujube products is essential for the development of this industry.
Jujube wine, which is a kind of fermented drink made from ripe jujubes, not only has a unique flavor of the aroma and a pleasant taste of wine, but also possesses high values of nutrition and healthcare [9]. Definitely, there might be some differences in the definition of ‘wine’ between Europe and China. According to the Chinese legislation GB/T 17204-2008, the fermented beverage (with alcohol content larger than 0.5% vol) from fruits or fruit juices is defined as ‘fruit wine’. Jujube wine has alcohol content larger than 10% vol and, thus, should be called ‘wine’ rather than ‘drink’ or ‘beverage’. The current winemaking processes of jujube wine generally include selection of raw materials, leaching of juice, fermentation, and aging [10,11]. Although the winemaking technologies of jujube wine have a long history in China, they have been inherited mainly by ancestors’ experience without a standard or recognized guidance, therefore largely limiting the production at an industrial scale [11]. Moreover, both demanding consumers and competing products pose much bigger challenges to the flavor and quality of fruit wine. Therefore, improving the flavor and quality of jujube wine is necessary.
In recent years, many studies have been carried out to optimize the brewing process of jujube wine. Most of them focused mainly on the fermentation conditions and pretreatment of raw materials and suggested that they have great impacts on the quality stability of jujube wine [1,10,12]. Other studies on the dynamic changes of jujube wine quality during fermentation have also greatly helped the improvement of the quality [13]. However, the fermentation of jujube wine is a process of the growth and metabolism of winemaking yeasts [9,11]. During this process, these microorganisms convert the jujube substrate into a series of volatile compounds that are vital in the formation of flavor, including aroma and taste, which are important determinants of the quality of jujube wine [8,9,14]. Thus, choosing or obtaining a desirable starter culture is one of the most important preconditions for the production of jujube wine.
In this study, we attempted to use six different winemaking yeasts (i.e., Saccharomyces cerevisiae) as the starter culture to explore how they impact on the composition of aroma-active compounds and flavor of the fermented jujube wine. To achieve this aim, gas chromatography-mass spectrometry (GC-MS) analysis was employed to quantify and identify the aroma-active compounds produced by the six winemaking yeasts. Moreover, some statistical analyses based on the composition of aroma-active compounds and different winemaking yeasts were carried out to determine the principal aroma-active components shaping the flavor of the jujube wine and whether or not there are significant differences in the flavor produced by the six winemaking yeasts. Additionally, a conventional sensory analysis was performed to further evaluate the differences in the flavor of the six types of jujube wine.
2. Methods and Materials
2.1. Raw Materials and Winemaking Yeasts
The raw materials used for wine fermentation were fully mature and undamaged jujube (Z. jujuba Mill.), which were supplied by the TIANKUNGUOYE Co., Ltd., Xinjiang, China. All the winemaking yeasts used in this study were S. cerevisiae, including two strains, BV818 and CEC01, purchased from the Angel Yeast Co., Ltd., Wuhan, China, and four other strains, F33, RMS2, BAYANVS, and SPARK, from the Laffort Co., Ltd., Bordeaux, France.
2.2. Preparation of Jujube Juice
The raw jujube was weighed and washed thoroughly in tap water three times. After cleaning, the jujube was mixed with distilled water in a weight ratio of 1/3 (jujube/water) and boiled for 15 min. Then, the stone of jujube was removed and the stone-removed jujube was blended into pulp in a high-speed blender (L18-Y928, Joyoung Co., Ltd., Jinan, China). Once the pulp was cooled to 50 °C, it was subjected to pectinase treatment (Novozymes Co., Ltd., Copenhagen, Denmark, 0.35 g/L, enzyme activity: 10,000 U/g) for 90 min.
2.3. Fermentation
Before fermentation, the jujube juice was adjusted to 24 °Brix with sucrose and pH 4 with citric acid, followed by the addition of sulfur dioxide (50 mg/L). The ameliorated juice was individually inoculated with 0.04% (wt/wt) of commercial active winemaking dry yeasts of the six strains, and static fermentation was performed at 25 °C throughout the fermentation process until the residual sugar in the broth dropped to 0.5%. Meanwhile, the un-inoculated jujube juice was preserved and used as the control. Prior to the downstream analysis, all the resulting jujube wine samples were filtrated to remove jujube flesh and centrifuged (6000× g) at 4 °C for 15 min to terminate the fermentation. All the experiments were carried out in triplicate.
2.4. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
Prior to GC-MS analysis, 5 mL of sample were collected and mixed with 1 g of NaCl in a sampling vial sealed with a polytetrafluoroethene cap. Subsequently, the sampling vials were pre-heated at 40 °C for 30 min and the volatiles were adsorbed with the extraction needle for 30 min, followed by thermal desorption for 5 min before detection. GC-MS analysis was performed on an ISQ 7000 Single Quadrupole GC-MS System (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a CP-Wax 57 CB column (50 m × 0.25 mm × 0.20 μm, Agilent, Santa Clara, CA, USA). The carrier gas was helium at a constant flow rate (1.0 mL/min for TR-5 ms, and 1.5 mL/min for TRwax). The temperature programs were proceeded with as follows: maintaining at 35 °C for 5 min, increasing from 35 °C to 200 °C at 3.5 °C/min, maintaining at 200 °C for 5 min, and finally increasing from 200 °C to 250 °C at 15 °C/min). The samples were injected by split technique (split ratio 1:50) at 250 °C, and the injection volume was 1 μL. The ionization energy (EI) was 70 eV, the scan range was 35–450 m/z, the scan rate was 0.2 scans/s, and the ion source temperature was 280 °C.
2.5. Sensory Analysis
All the samples were assessed by ten experts (five females and five males, aged between 30 and 40, from Jiangnan University, Jiangsu, China), who all had more than eight years’ experience in the sensory evaluation of various wines. The description terms of six attributes (i.e., jujube, wine, fruit, flower, lemon, and sweet) were scored from 0 to 9 points (0 = weak and 9 = strong). To avoid sensory fatigue, each session was performed for one hour, all the panelists evaluated each sample twice, separated by a 25 min break, and each panelist individually scored for each attribute in the order mentioned above. Moreover, mineral water was provided for panelists as a palate cleanser after each evaluation. The mean score for each attribute of each sample was obtained from the ten experts.
2.6. Statistical Analysis
The results were expressed as mean ± standard deviation (SD) of the three replicates. Multivariate statistical analysis was conducted with the R software package (version 3.5.1,R Foundation for Statistical Computing, Murray Hill, NJ, USA). The figures were plotted and formed using the R software and the Origin 2021, OriginLab Corporation, Northampton, MA, USA). Moreover, univariate statistical analysis (one-way ANOVA) was performed to evaluate significant differences between the samples at a level of 0.001, 0.01, or 0.05 (p value). The contents of aroma-active compounds were compared using a paired two-sided Student’s t-test. The mean values of sensory data were compared by Tukey’s test using the SPSS Statistics 22.0 (SPSS Inc., International Business Machines Corporation, Chicago, IL, USA).
Principal component analysis (PCA) on the basis of the correlation matrix and Partial Least Squares (PLS) were carried out with the FactoMineR package [15]. Canonical correlation analysis (CCA) combined with the Multivariate Analysis of Variance (MANOVA) was conducted with a CCA package for the aroma profiles of the jujube wine fermented by the six yeasts [16].
3. Results and Discussion
3.1. Comparison of the Composition of Aroma-Active Compounds
Generally, aroma-active components in the jujube wine fermented by the six yeasts were mainly identified to be composed of 24 esters, 8 alcohols, 7 acids, and 4 other volatiles (Table 1). Among these 43 aroma-active compounds, esters, in particular, ethyl hexanoate (pineapple flavor), ethyl decanoate (pear flavor), and ethyl caprylate (apple flavor), were the predominant components, and thus contributed more to the aroma of the jujube wine, compared with alcohols and acids. Although the content of isoamyl alcohol was relatively higher, it could not significantly affect the aroma, due to its higher sensory threshold [17,18]. On the contrary, some components with a low sensory threshold, such as phenethyl acetate and ethyl 3-phenylpropionate, might have great contribution to the aroma, though their contents were also lower. Moreover, they had a higher response value in the sensory test (data not shown). For other components, they might not have been important contributors to the aroma because they had either a low content or high sensory threshold. Despite their low contribution to the aroma, they can exert a synergetic influence on the entire aroma of the fermented jujube wine and are the essential aroma-active compounds that shape the flavor characteristics [8,9,14,17,18].

Table 1.
The contents of aroma-active compounds of the jujube wine fermented by the six different winemaking yeasts.
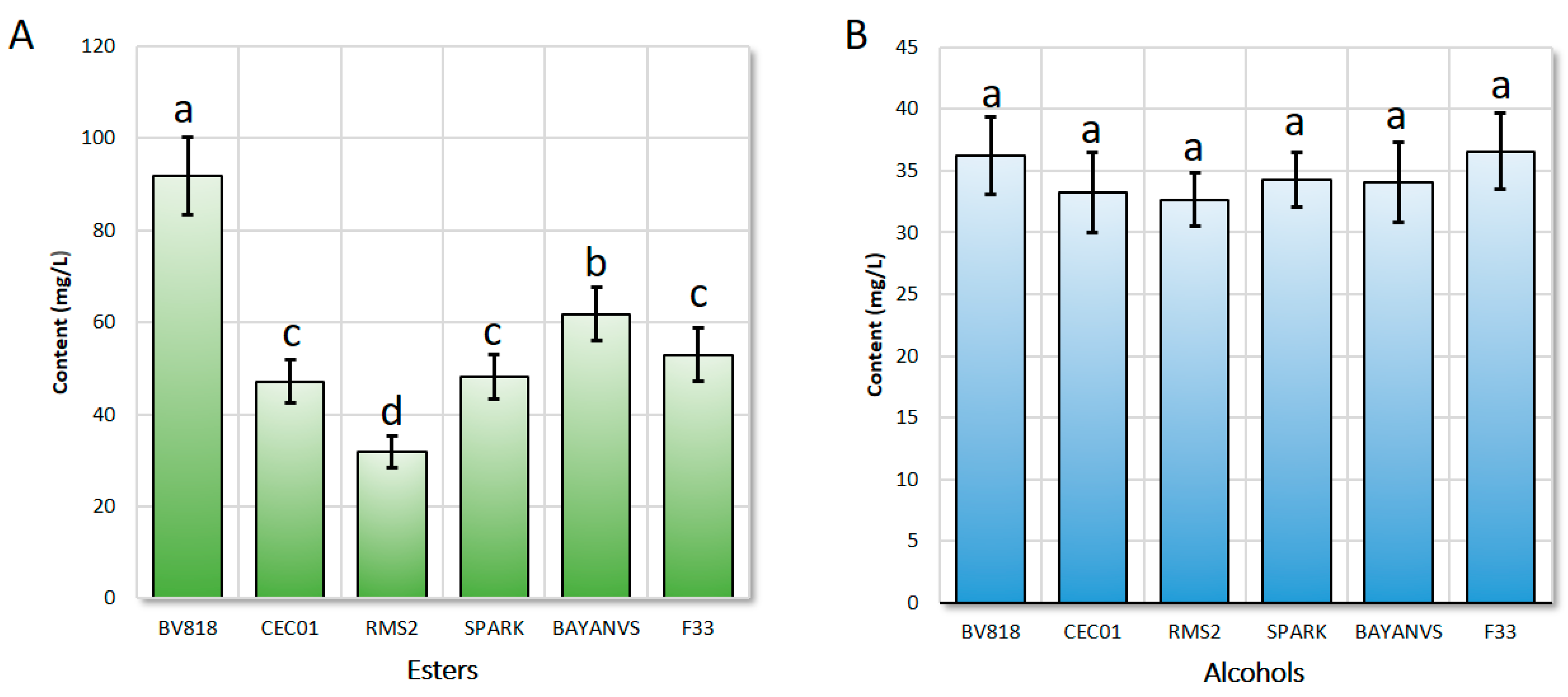
By comparison, the total content of esters in the wine fermented by the yeast BV818 was the highest, reaching up to 91.82 mg/L, significantly higher than that in the wine fermented by the other six yeasts (Figure 1A, p < 0.001). Meanwhile, the content of alcohols produced by the yeast BV818 was similar to that produced by the other yeasts (Figure 1B, p > 0.05). It has been reported that a high content of higher alcohols (e.g., isoamyl alcohol) results in a poor flavor of the jujube wine [19,20,21]. Thus, the flavor of the wine produced by the yeast BV818 would be the best, compared with that of the other six. Although the total content of esters (61.76 mg/L) produced by the yeast BAYANVS was significantly lower than that of esters produced by the yeast BV818, the composition of aroma-active compounds in the wine fermented by the yeast BAYANVS was considerably complete. Therefore, its flavor was just ranked only second to that of the wine produced by the yeast BV818, which is consistent with the result of the sensory test (data not shown). However, both the total content of esters and composition of aroma-active compounds produced by the yeast RMS2 were the lowest and, therefore, the flavor of its wine was the poorest. This might also result from the relatively lower ratio of the contents of esters and isoamyl alcohol [1,9,18,20]. For the wine fermented by the other three yeasts, the flavors tested similarly because there were no significant differences either in the composition or in the content of the aroma-active compounds.
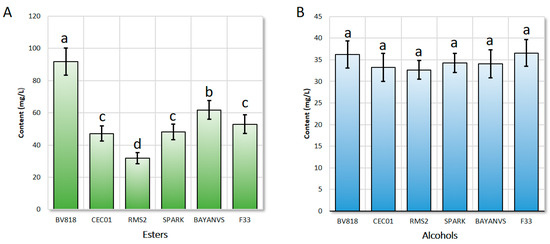
Figure 1.
Comparison of the contents of esters (A) and alcohols (B) of the jujube wine produced by the six winemaking yeasts. Bars with different letters on the top (from a to d) indicate that they have a significant difference from each other. The significance of difference between a and b, c, or d is at a level of 0.001 (p value); the significance of difference between b and c or between c and d is at a level of 0.05 (p value); and the significance of difference between b and d is at a level of 0.01 (p value).
3.2. Principle Component Analysis (PCA)
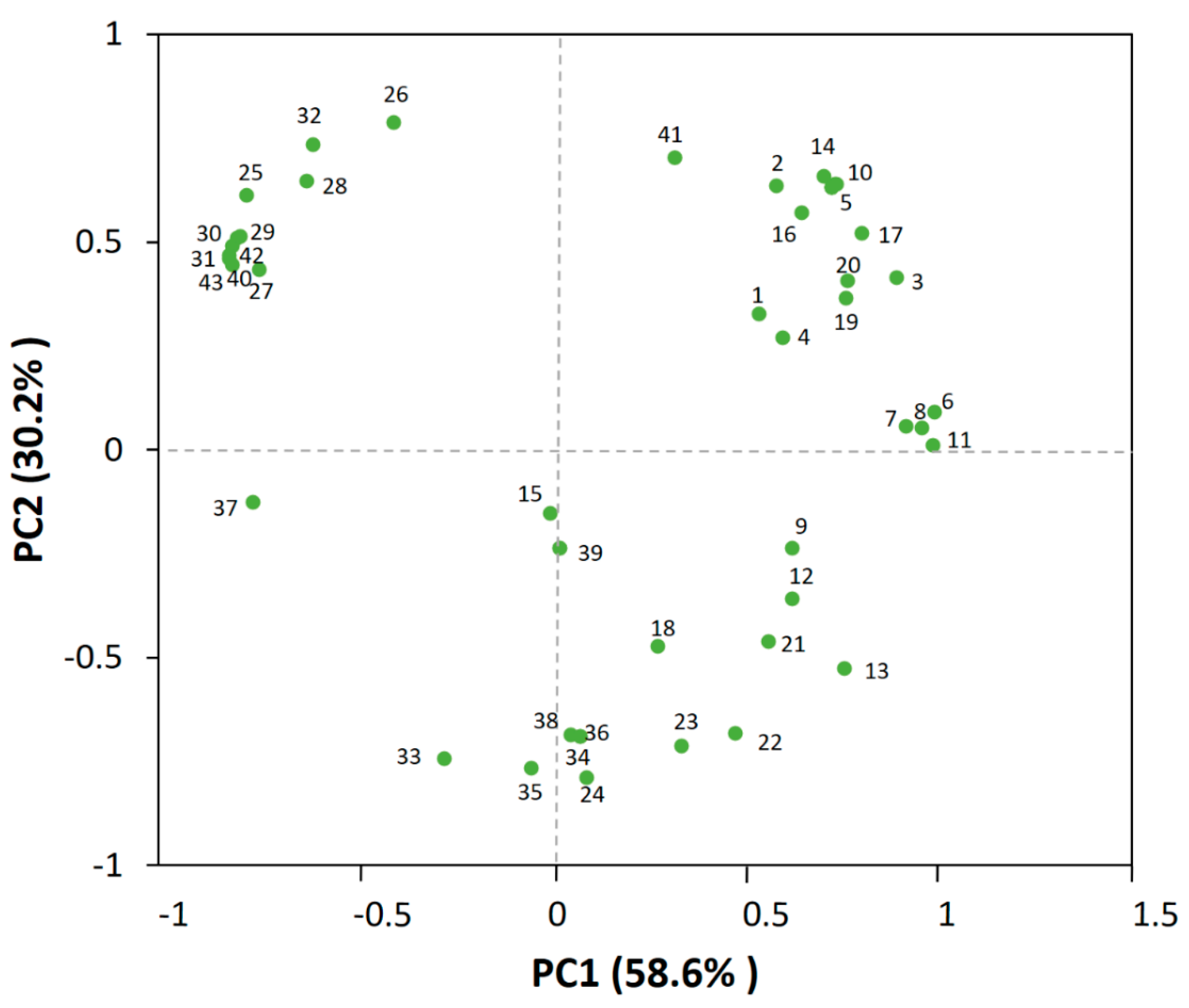
In order to discriminate the volatile profiles, 43 different aroma-active compounds numbered in Table 1 were separated in the biplot (Figure 2), among which the first principal component (PC1) and second principal component (PC2) individually accounted for 58.6% and 30.2% of all variance.
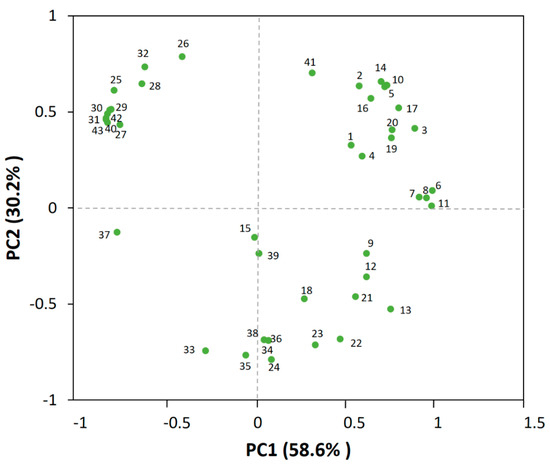
Figure 2.
Principal component analysis (PCA) of the 51 aroma-active compounds in the jujube wine. Numbers close to each dot correspond to the code number in Table 1.
The PCA plot showed that the majority of esters, such as ethyl caprylate (10), ethyl decanoate (14), ethyl hexanoate (5), phenethyl acetate (17), ethyl benzoate (16), ethyl butyrate (2), and ethyl 3-phenylpropionate (20), had been separated distinctly, which might be attributed to their higher contents or lower sensory thresholds that contributed more to the aroma of the wine [1,14,17], as discussed above. Moreover, some alcohols, such as phenylethanol (32) and isoamyl alcohol (26), also exerted a greater impact on the volatile profiles. For the volatile acids, they also clustered together but had no significant influences on the volatile profiles, suggesting that they might be not the principal components that determine the flavor of the jujube wine.
Our findings have coincided with some current observations on the fermentation of jujube wine. For example, a variety of volatile aroma-active components were quantified and identified as the main contributors of the flavor of jujube wine [8,14,17]. Other studies discovered that some volatile compounds, such as isoamyl octanoate, isoamyl decanoate, ethyl laurate, ethyl myristate, 2-phenethyl acetate, and benzaldehyde, appear to mainly contribute to the jujube-like flavor of jujube wine [14,18].
3.3. Canonical Correlation Analysis (CCA)
The PCA has discriminated the principal components that influence the aroma of the jujube wine. However, the composition of these principal components was different in the jujube wine when fermented by different yeasts. Therefore, CCA was employed to further confirm whether or not differences exist in the aroma among the jujube wine when fermented by different yeasts.
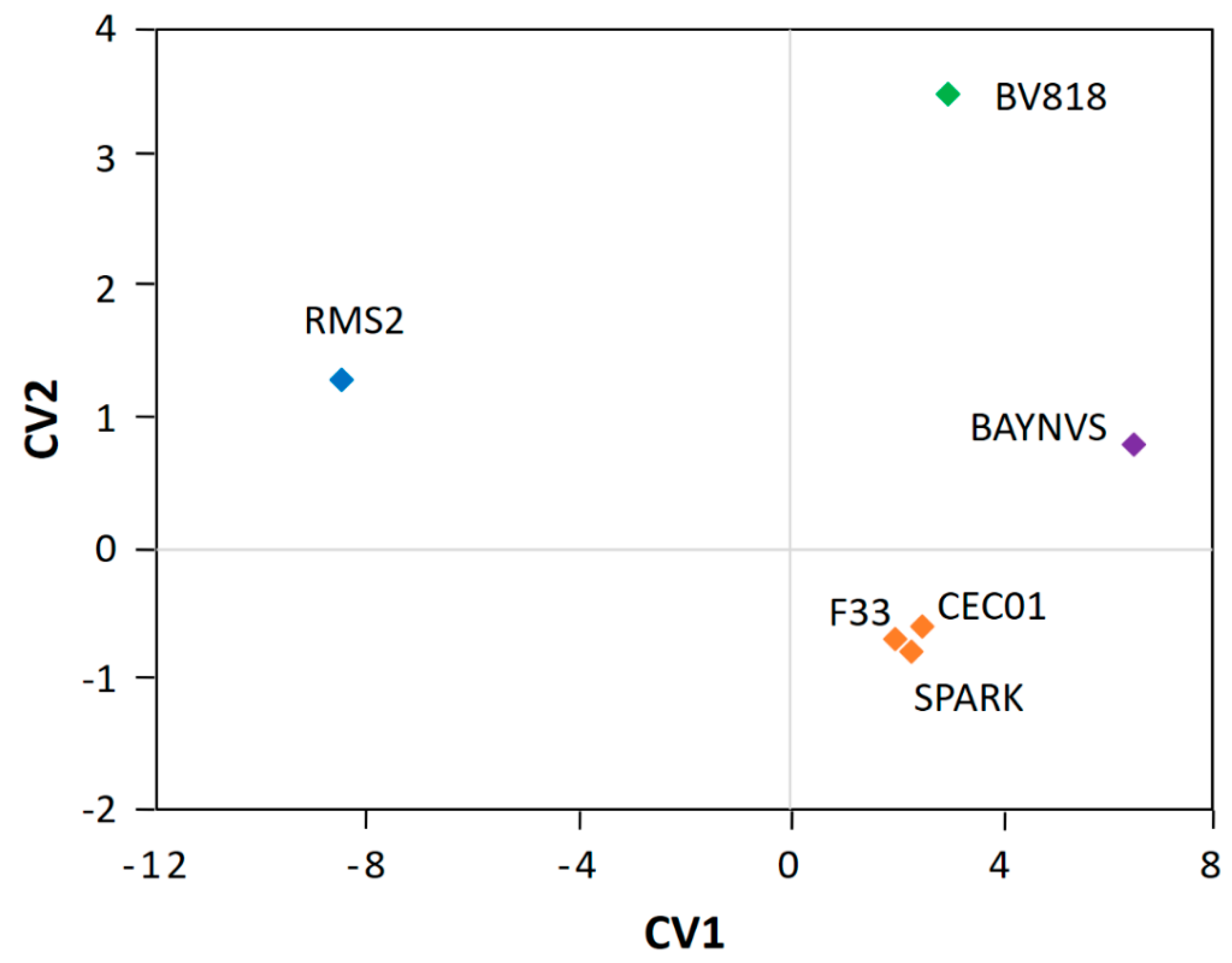
As observed in the CCA plot of aroma (Figure 3), the six samples were separated into four independent clusters that were situated in three quadrants, excluding the third one. The jujube wine fermented by the yeasts CEC01, SPARK, and F33 formed a single cluster that fell in the fourth quadrant of the plot, suggesting that these three yeasts produce wine with a similar aroma profile. The jujube wine samples of BV818 and BAYNVS were distributed in the first quadrant, while the jujube wine by RMS2 was positioned in the second quadrant. These four clusters were far away from each other in the plot, indicating that different yeasts might have great influences on the aroma of the jujube wine [8]. Furthermore, the MANOVA analysis also supported the above observations that there are significant differences in the aroma of the jujube wine fermented by the four clusters of yeasts (p < 0.05), but no significant differences within the cluster of the yeasts CEC01, SPARK, and F33 (p > 0.05). Thus, it is necessary to select the optimal starter culture before the production of jujube wine at a larger scale [22,23].
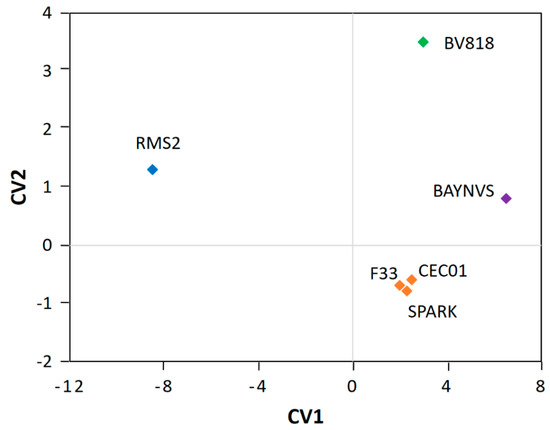
Figure 3.
Canonical correlation analysis (CCA) of similarity of the jujube wine produced by the six winemaking yeasts. The plot was defined by canonical variates 1 and 2.
3.4. Evaluation of the Overall Flavor Quality
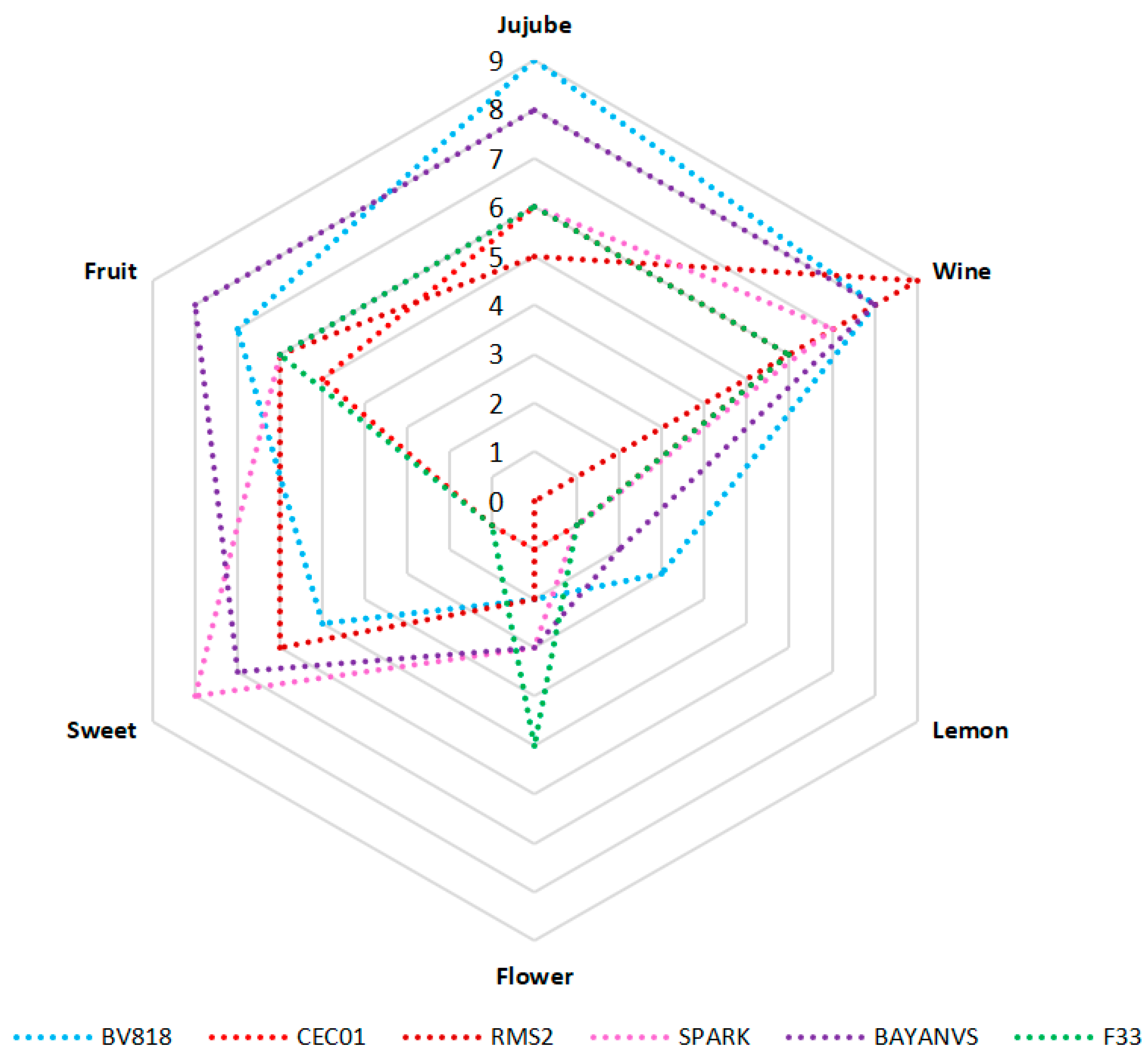
To provide a more visual comparison of the aroma of the jujube wine fermented by the six yeasts, a radar plot was generated on the basis of sensory profiles (Figure 4). Generally, all the six types of jujube wine had higher scores of jujube, wine, sweet, and fruit flavors; but had lower scores of flower or lemon flavor. Among the six types of jujube wine, the wine produced by BV818 exhibited the strongest jujube flavor and had the best sensory quality. In contrast, the wine of BAYNAVS was characterized by the fruit flavor and also showed better scores of other flavors. Therefore, its overall flavor quality was just next to that of the wine produced by BV818. Besides, the yeast SPARK produced the jujube wine characterized by the sweet flavor. However, the wine produced by RMS2 had the strongest wine flavor due to the highest ratio of the contents of total alcohols and esters [21,24,25]. Additionally, it had relatively lower scores for other flavors, resulting in the poorest flavor quality compared with other types of jujube wine.
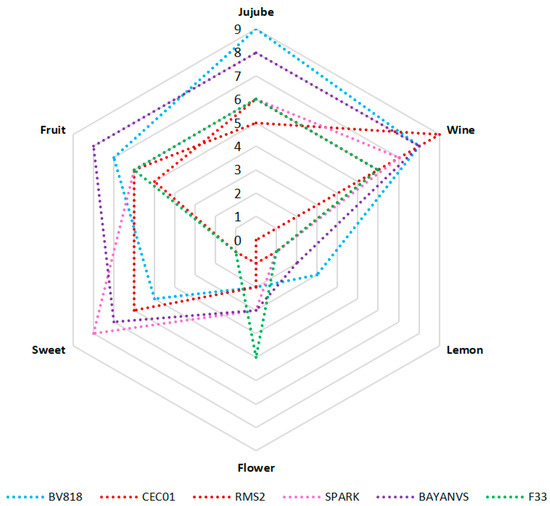
Figure 4.
Radar plot of sensory profiles for the jujube wine produced by the six winemaking yeasts.
Taken together, the overall flavor quality of the jujube wine produced by the six yeasts was generally consistent with the patterns observed above, which further confirmed the effects of different winemaking yeasts on the composition of aroma-active compounds in the fermented jujube wine. Thus, we propose that selection of suitable starter cultures for the jujube wine fermentation is of great importance.
4. Conclusions
In this study, we evaluated the effects of different winemaking yeasts on the composition of aroma-active compounds in the fermented jujube wine and determined the principal components that affect the overall flavor quality. It showed that the starter winemaking yeasts determine the composition of aroma-active compounds, of which esters (e.g., ethyl caprylate, ethyl decanoate, ethyl hexanoate, and phenethyl acetate) contribute more to the wine quality attributes, especially for improvement of the aroma. Moreover, it is also evident that the composition of aroma-active compounds, for example, the ratio of the content of esters and alcohols, exerts a great impact on the overall flavor quality of jujube wine. Consequently, selection of a desirable starter winemaking yeast is of great importance to the fermentation of jujube wine.
Author Contributions
Y.Z. (Yan Zhao), X.Y. (Xiaobin Yu), and F.Z. designed the study and acquired funding; Y.Z. (Yan Zhao), G.L. (Guangpeng Liu), L.C., X.Y. (Xinhuan Yan), Y.M., F.H., Y.Z. (Ying Zhang), M.T., Y.L. and G.L. (Gen Li) performed the experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Taishan Industry Leader (LJNY202001) and the National key R & D plan of the 14th five year plan (2017YFD0400104).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
This paper does not contain any studies with human participants or animals performed by any of the authors.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
This work was supported by the Research Project of the Taishan Industry Leader (Grant No. LJNY202001), the national first-class discipline program of Light Industry Technology and Engineering (Grant No. LITE2018-11), and the Open Project of Key Laboratory of Carbohydrate Chemistry and Biotechnology Ministry of Education (Grant No. KLCCB-KF201808).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cai, W.; Tang, F.; Shan, C.; Hou, Q.; Zhang, Z.; Dong, Y.; Guo, Z. Pretreatment methods affecting the color, flavor, bioactive compounds, and antioxidant activity of jujube wine. Food Sci. Nutr. 2020, 8, 4965–4975. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Peng, Q.; Yuan, Y.; Shen, J.; Xie, X.; Wang, M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): A review. Food Chem. 2017, 227, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Shi, W.; Wang, X.; Wang, Y. Non-rainfall water contributions to dryland jujube plantation evapotranspiration in the Hilly Loess Region of China. J. Hydrol. 2020, 583, 124604. [Google Scholar] [CrossRef]
- Liao, C.; Liu, X.; Gao, A.; Zhao, A.; Hu, J.; Li, B. Maintaining postharvest qualities of three leaf vegetables to enhance their shelf lives by multiple ultraviolet-C treatment. LWT 2016, 73, 1–5. [Google Scholar] [CrossRef]
- Kuşçu, A.; Bulantekin, Ö. Determination of phenolics, organic acids, minerals and volatile compounds of jujube (Ziziphus jujuba miller) jam produced by under vacuum evaporation compared with open pan method. J. Food Meas. Charact. 2021, 15, 1127–1138. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Wang, Z.; Liu, Z.; Zhao, Z.; Zhou, G.; Liu, M.; Liu, P. Variations of the nutritional composition of jujube fruit (Ziziphus jujuba Mill.) during maturation stages. Int. J. Food Prop. 2020, 23, 1066–1081. [Google Scholar] [CrossRef]
- Hu, D.; Fan, Y.; Tan, Y.; Tian, Y.; Liu, N.; Wang, L.; Zhao, D.; Wang, C.; Wu, A. Metabolic profiling on Alternaria toxins and components of Xinjiang jujubes incubated with pathogenic Alternaria alternata and Alternaria tenuissima via orbitrap high-resolution mass spectrometry. J. Agric. Food Chem. 2017, 65, 8466–8474. [Google Scholar] [CrossRef]
- Cai, W.; Tang, F.; Shan, C.; Yang, L.; Zhan, Q.; Zhao, X.; Ning, M. Changes in volatile compounds of fermented mixed drink using commercial yeast strain. Przem. Chem. 2018, 97, 1398–1405. [Google Scholar]
- Kang, T.; Woo, K.; Lee, J.; Jeong, H. Fermentation characteristics of wine using fresh jujube. Food Eng. Prog. 2006, 10, 164–171. [Google Scholar]
- Yuan, L.; Li, G.; Yan, N.; Wu, J.; Due, J. Optimization of fermentation conditions for fermented green jujube wine and its quality analysis during winemaking. J. Food Sci. Technol. 2021, 58, 1–12. [Google Scholar]
- Zhang, R.; Song, Z.; Liu, Y.; Gao, X. Fermentation technology of red jujube wine. Food Res. Dev. 2017, 38, 138–142. [Google Scholar]
- Xu, L.F.; Tang, Z.S.; Wen, Q.H.; Zeng, X.A.; Brennan, C.; Niu, D. Effects of pulsed electric fields pretreatment on the quality of jujube wine. Int. J. Food Sci. Technol. 2019, 54, 3109–3117. [Google Scholar] [CrossRef]
- Tang, F.; Cai, W.; Shan, C.; Guo, Z.; Hou, Q.; Zhang, Z.; Dong, Y. Dynamic changes in quality of jujube wine during fermentation. J. Food Process. Preserv. 2020, 44, e14704. [Google Scholar] [CrossRef]
- Lee, J.-E.; Yun, J.H.; Lee, A.R.; Kim, S.S. Volatile components and sensory properties of jujube wine as affected by material preprocessing. Int. J. Food Prop. 2018, 21, 2052–2061. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- González, I.; Déjean, S.; Martin, P.; Baccini, A. CCA: An R package to extend canonical correlation analysis. J. Stat. Softw. 2008, 23, 1–14. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, Y.; Wang, J.; Shuang, Q. Assessment of key aroma compounds in fresh jujube brandy by GC-O-MS and odor activity value. J. Food Process. Preserv. 2020, 44, e14494. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, P.; Xiao, Z.; Zhu, J.; Sun, X.; Wang, R. Evaluation of the perceptual interaction among ester aroma compounds in cherry wines by GC–MS, GC–O, odor threshold and sensory analysis: An insight at the molecular level. Food Chem. 2019, 275, 143–153. [Google Scholar] [CrossRef]
- Guo, J.; Yan, Y.; Wang, M.; Wu, Y.; Liu, S.-Q.; Chen, D.; Lu, Y. Effects of enzymatic hydrolysis on the chemical constituents in jujube alcoholic beverage fermented with Torulaspora delbrueckii. LWT Food Sci. Technol. 2018, 97, 617–623. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Yuan, F.; Deng, B.; Yu, X. Biocatalysis of Heterogenously-Expressed Chitosanase for the Preparation of Desirable Chitosan Oligosaccharides Applied against Phytopathogenic Fungi. ACS Sustain. Chem. Eng. 2020, 8, 4781–4791. [Google Scholar] [CrossRef]
- Liguori, L.; Albanese, D.; Crescitelli, A.; Di Matteo, M.; Russo, P. Impact of dealcoholization on quality properties in white wine at various alcohol content levels. J. Food Sci. Technol. 2019, 56, 3707–3720. [Google Scholar] [CrossRef]
- Liao, C.; Liu, X.; Shan, L. Optimization of liquid media and biosafety assessment for algae-lysing bacterium NP23. Can. J. Microbiol. 2014, 60, 593–597. [Google Scholar] [CrossRef]
- Liao, C.; Liu, X. High-cell-density cultivation and algicidal activity assays of a novel algicidal bacterium to control algal bloom caused by water eutrophication. Water Air Soil Pollut. 2014, 225, 1–8. [Google Scholar] [CrossRef]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef]
- Shi, W.-K.; Wang, J.; Chen, F.-S.; Zhang, X.-Y. Effect of Issatchenkia terricola and Pichia kudriavzevii on wine flavor and quality through simultaneous and sequential co-fermentation with Saccharomyces cerevisiae. LWT Food Sci. Technol. 2019, 116, 108477. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).