Plasma Enhanced Chemical Vapor Deposition of Organic Polymers
Abstract
1. Introduction
1.1. Vapor Deposition Techniques

Polymerization in the Volume or at the Surface?
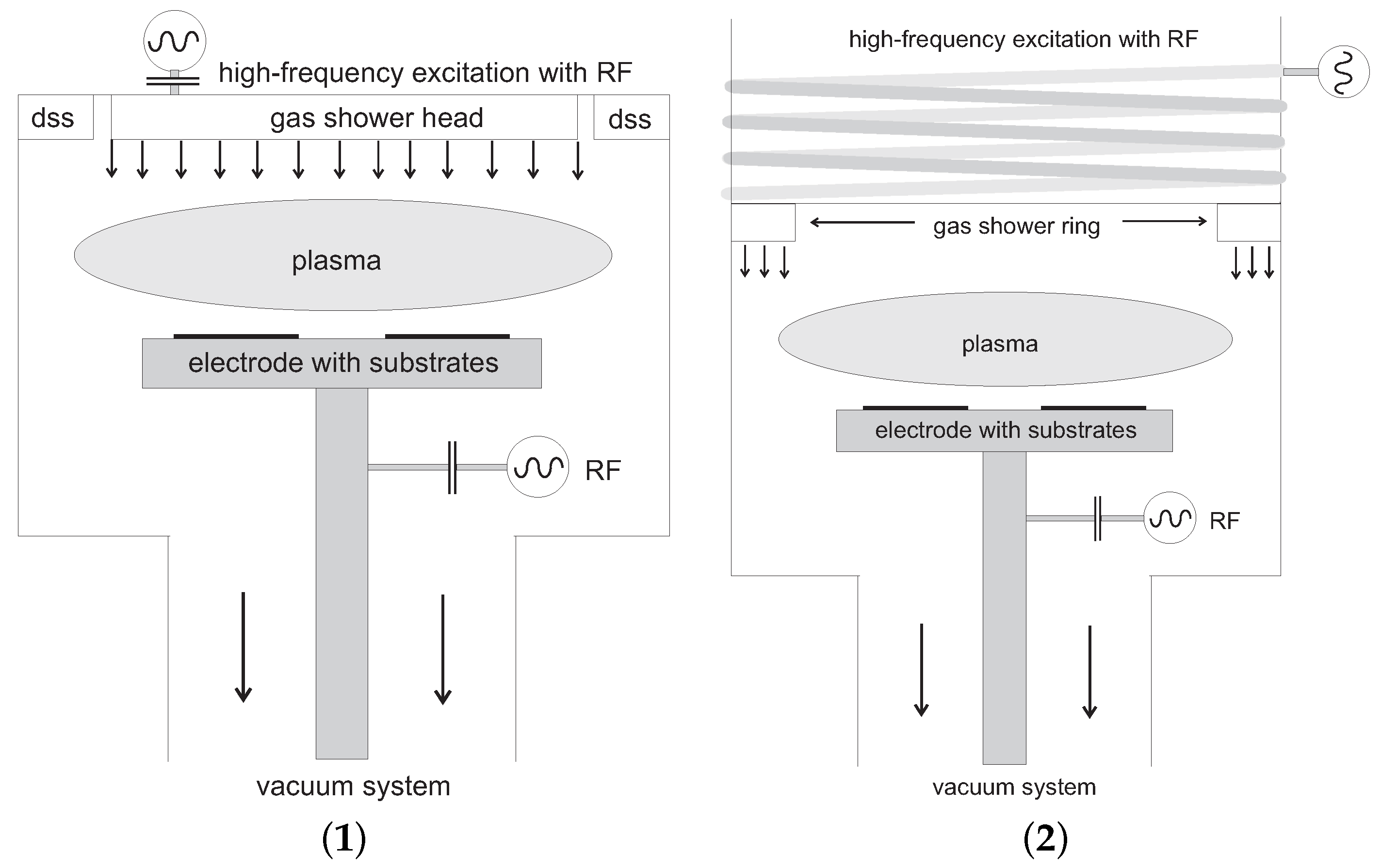
1.2. Plasma-Assisted Coating Techniques
- For transport in the interplanetary space, ion thrusters had been developed after the sputnik shock in the late 1950s. As spin-off, the first ion beam systems appeared, operated with a Kaufman-type plasma source and inert gases as Ar, N, and O in a pressure range below 1 mTorr [13]. Ions of these gases were directed to dielectric or metallic targets which caused sputtering. The removed species could be deposited on substrates in various compositions.
- As spin-off of RADAR, microwave-driven (MW) reactors were invented which operated in the optimum range of some Torrs (some hundreds of Pa). In the high-frequency regime of some GHz—the FCC frequency of 2.45 GHz was fixed in the late 1940s—the optimum pressure is some Torrs (for effectiveness of power transfer, the frequency of elastic collision should match the operating frequency [14]).
- In the 1960s, also the RF range came into focus. Parallel-plate reactors were operated at the FCC frequency of 13.56 MHz at pressures of some tens of mTorr. The first layers were deposited on lenses of silica for dielectric coating, by adding the two reactive gases SiH and NH or NO in traces to an inert carrier gas. The sample was placed on the “cold” electrode, i.e., the electrode which was opposed that electrode which was driven with the HF voltage. This caused a small voltage drop in front of the sample across the RF sheath of approx. 10–20 V from plasma potential to ground. This was the birth of real chemical vapor deposition with plasma triggering, which was coined later as Plasma-Enhanced Chemical Vapor Deposition (PECVD). The excitation mode was denoted capacitive coupling [14], abbreviated CCP for Capacitively Coupled Plasma.
1.3. Plasma-Assisted Etching Techniques
1.4. PECVD vs. RIE
1.5. Disadvantages and Advantages of Plasma Processing
2. Mechanisms
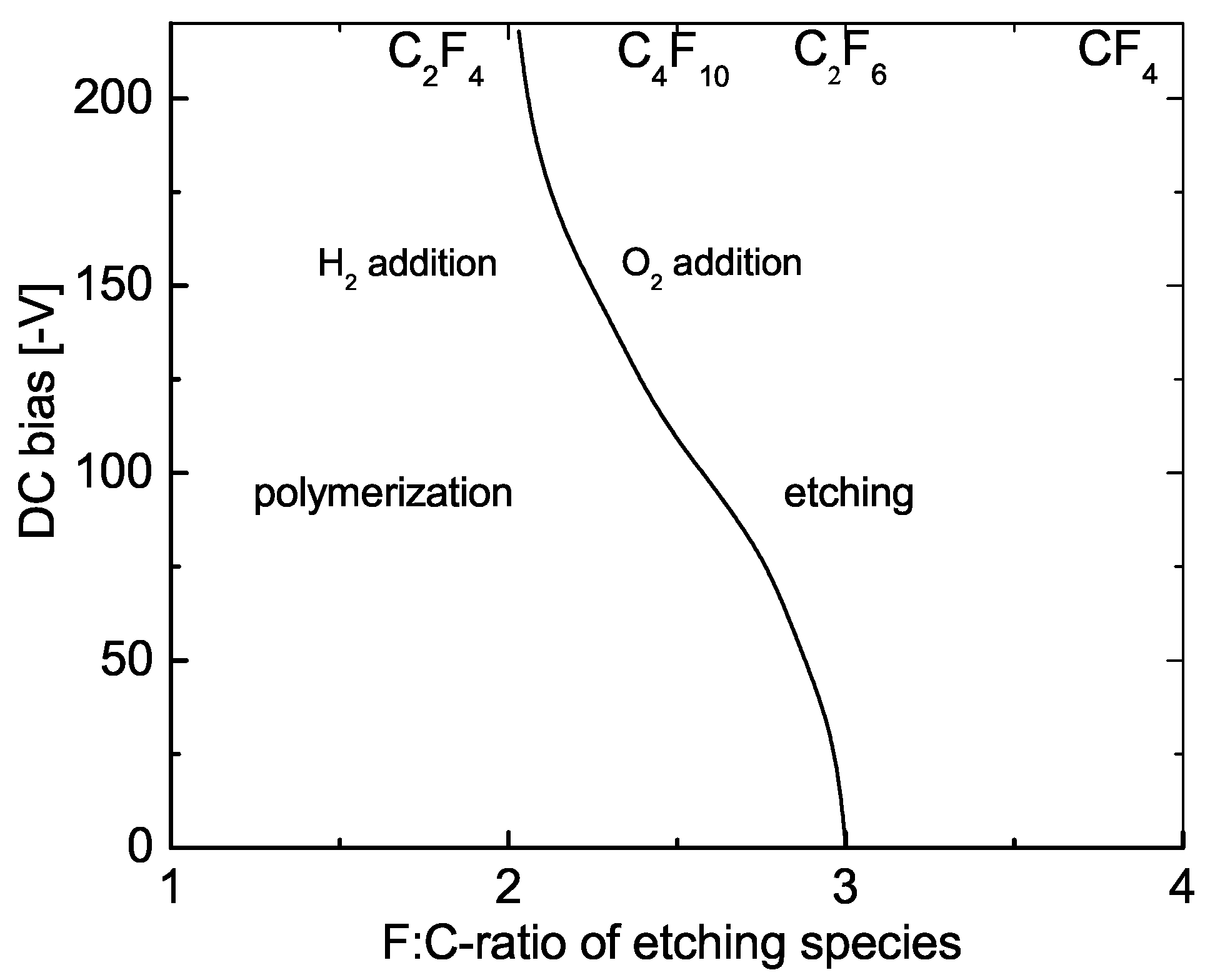
2.1. What Causes Ion Etching in Reality?
2.2. What Causes Plasma-Enhanced Chemical Vapor Deposition in Reality?
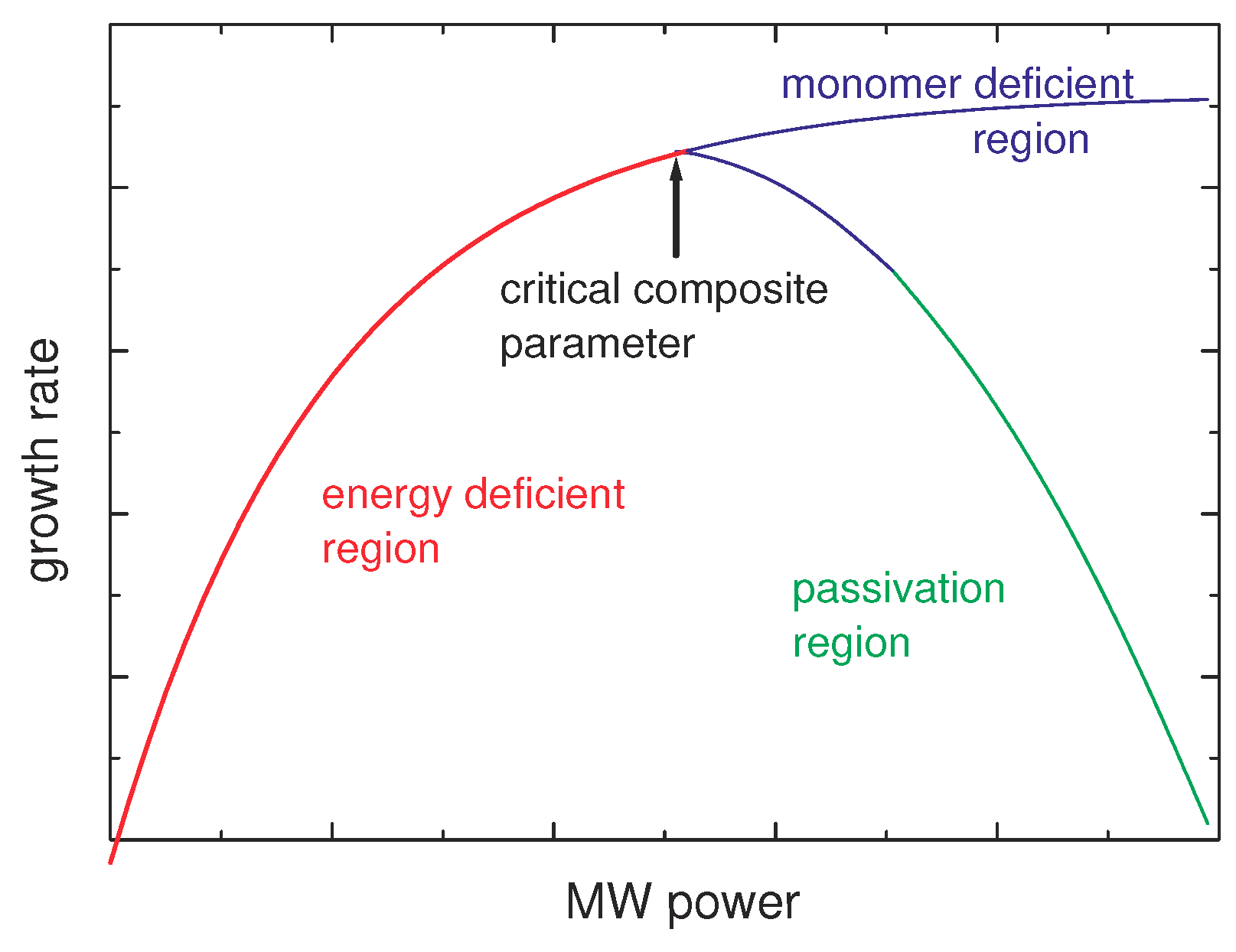
2.2.1. Growth Rate
- the equality of the density of the two products M and M· (), and that
- scales with the partial pressure of MM
2.2.2. Fragmentation in the Plasma
- a C atom is able to form bonds to other C atoms, and
- the length of the resulting polymers is not fixed but scatters around a certain average. The degree of this distribution is denoted dispersity. Even when only one type of chain is formed, the length cannot be fixed to a sharp value. In principle, the chain length can be steered by a catalyst which is simply impossible for a heterogeneous reaction which is discussed here (see the discussion which was started at ETH in Zurich [27]).
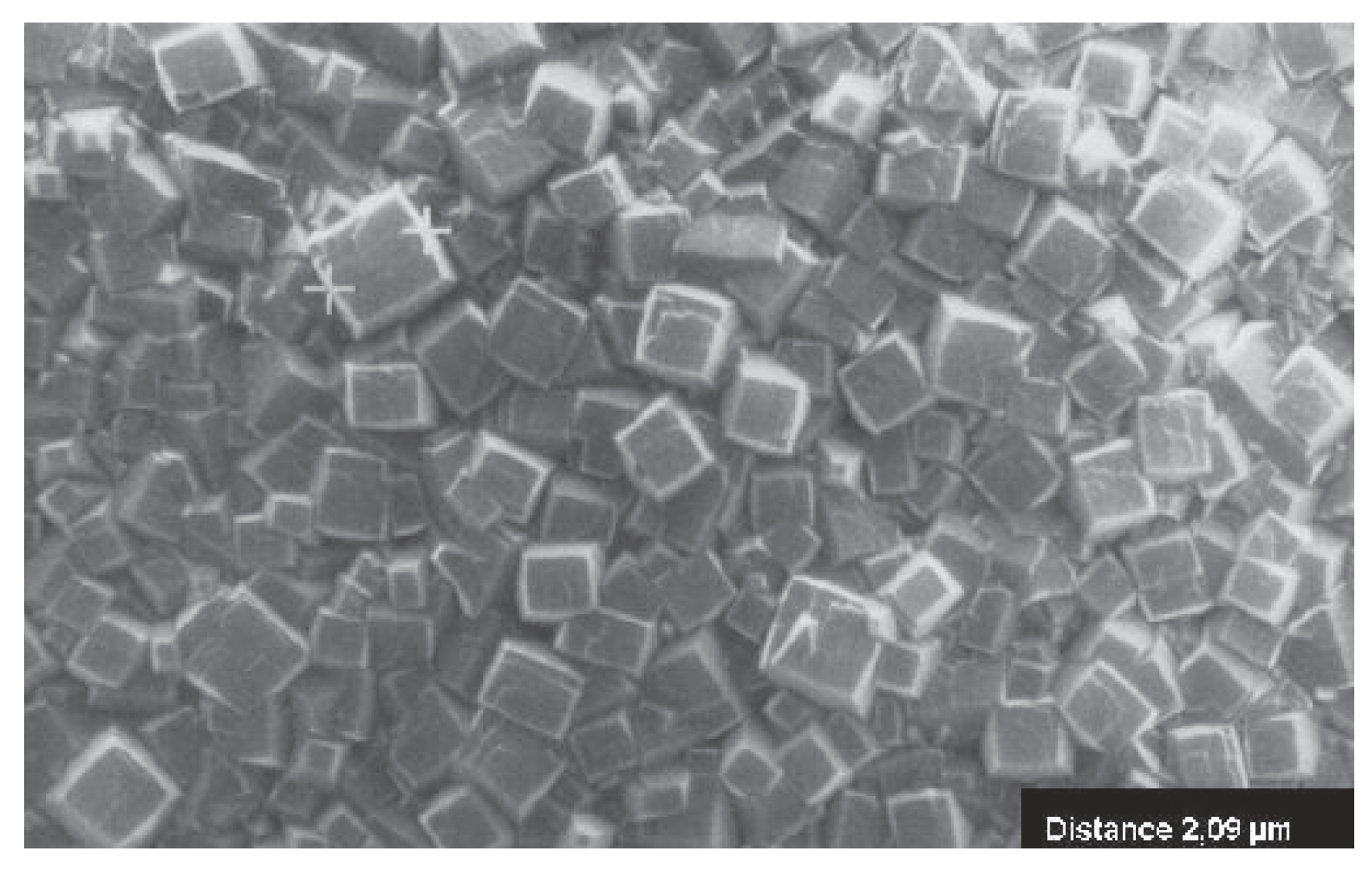
2.2.3. Diamonds and Diamond-Like Coatings
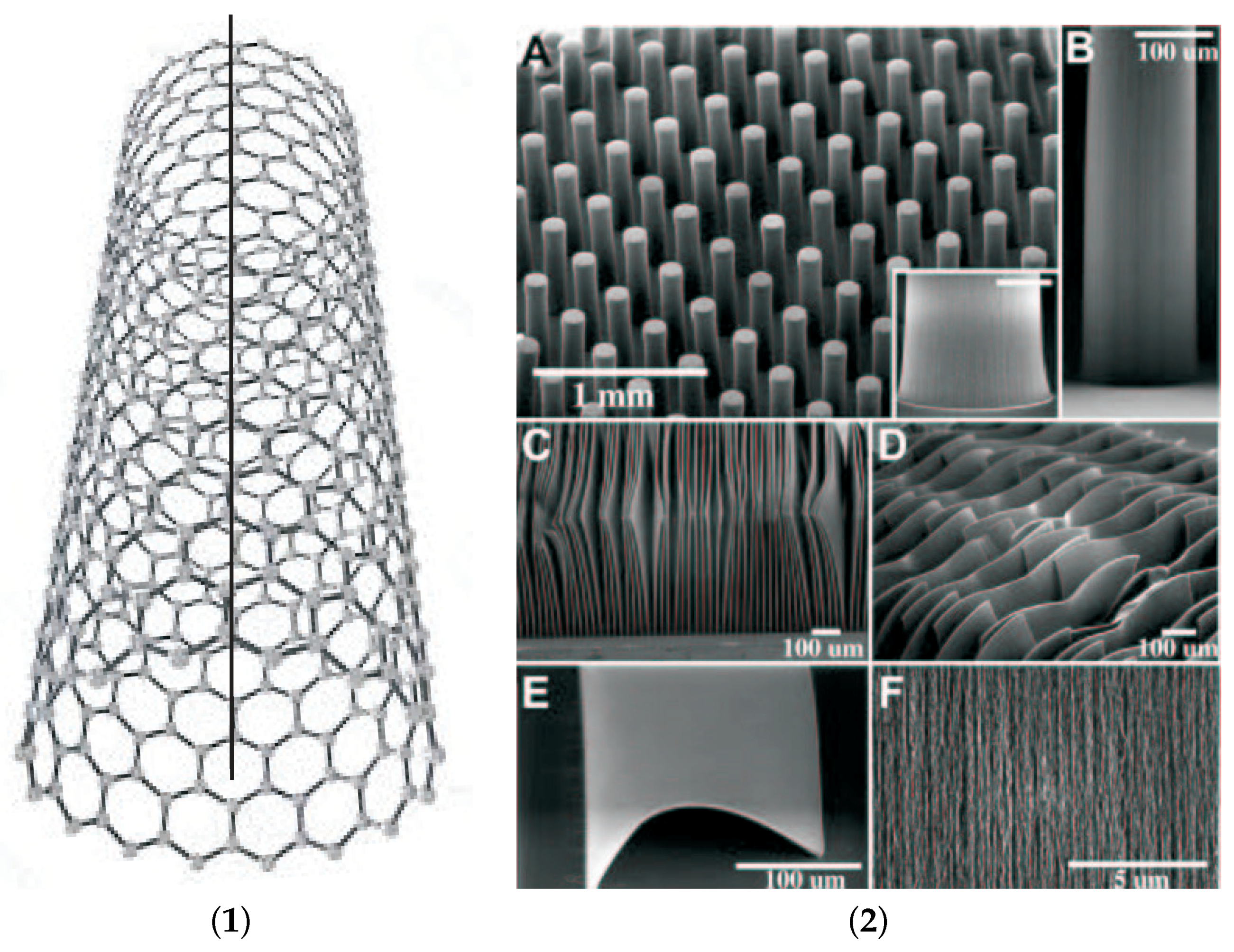
2.2.4. Carbon Nano Tubes
2.2.5. Analysis of the Deposited Layers
- In polymers, the unsaturated double bond of or character of a monomer will be changed to a saturated bond with character. This is revealed by vibration spectra (IR or Raman). However, it is not all quantitative even in high dilution. Moreover, IR spectroscopy is very sensitive to impurities but lacks for matrix effects. Therefore, the intensity does not scale with concentration (of impurities). Since the intensity is proportional to the square of with the dipole moment and x a length, it is very insensitive for bonds with low dipole character. But the C-H bond is unpolar which causes very weak IR intensities! Therefore, Raman spectroscopy is the method of choice [40].
- Residual electrons in radicals can be detected by electron spin resonance (ESR) [41]; this is a very sensitive method. However, it gives only information of the number (or density) of the unsaturated ends of the polymeric chains. But it does not discriminate between these intended radicals and other unintended radicals which might have been generated during plasma excitation.
- For diamond, Raman spectroscopy is second to none as quality probe. Only one absorption line, the symmetric valence vibration at cm must appear to prove the spectroscopic purity of diamond [42,43]. That is far beyond the demands on purity which have been used over centuries (from the four Cs cut, clarity, color and carat, the clarity is content with a flawless view through a microscopy with just 10-fold magnification!).
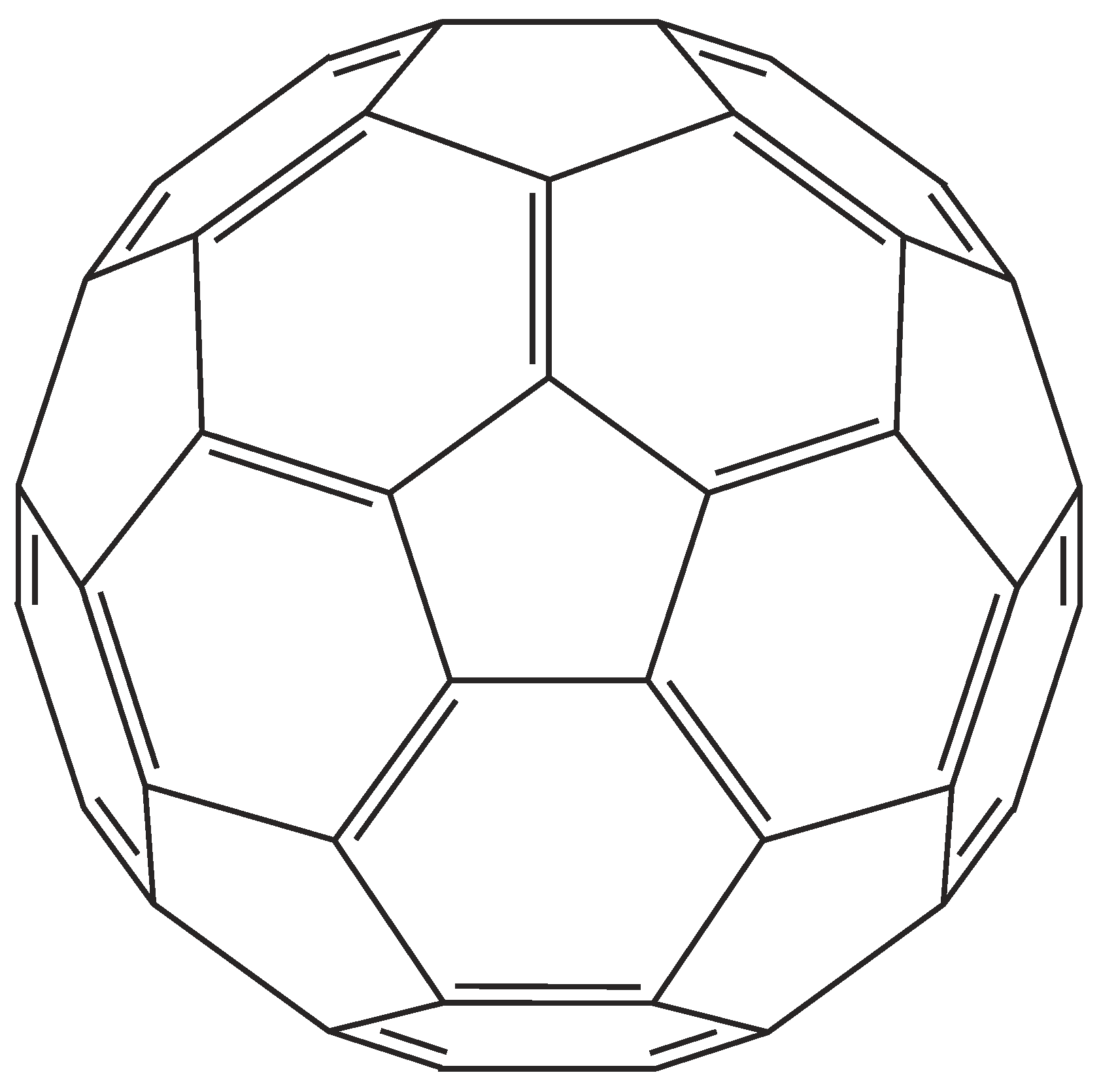
- From their exploration by Iijima in 1991 [35], CNTs have attracted extreme interest. CNTs are often prepared in hot plasmas. Laser ablation and arc discharge are the most common techniques. Simple CVD requires a catalytic guidance. Often, Fe is applied as catalyst which can easily be proven by optical emission spectroscopy in inductively-coupled plasmas (ICP-OES) [37]. The benzene rings consist of only carbon atoms.In fact, single-walled carbon nanotubes are cylinders consisting of hexagonal base units of benzene which form a single sheet of graphene. In contrast to a single sheet of graphene which is extended to ∞, the plain sheet is rolled up into the cylinder whose diameter is very small against its length [44]. Therefore, it can be regarded as one-dimensional hollow wire with a finite number of benzene units around the perimeter of the wire. Since the radius of curvature is very small, CNTs exhibit a sharp curvature around an imaginary one-dimensional thread which changes the optical qualities dramatically [40].Electrons have eigenstates quantized with the circumference of the nanotube. The electronic band structure can be developed from the benzene unit which is folded with the perimeter of the tube [40]. Also, photons and other quasiparticles are expected to exhibit a discrete wavelength spectrum quantized with this perimeter. In the direction of the nanotube, the states are continouos.But as in graphite, the distinct graphene structure with hybridized carbon atoms in a two-dimensional radial structure is loosely held together by weak van-der-Waals bonds in the third dimension. Therefore, the multi-walled nanotubes exhibit just a smaller radial bonding force, compared with the stronger tangential component which defines the structure.Since a pure CNT is composed only of hybridized carbon atoms, the absence of IR bands which are caused by single bonds prove their purity. As Caccamo et al. have shown, the FTIR spectra of CNTs lack any contour [45]. In the same study, they reported also Raman data. As it is explicitely analyzed by Thomsen and Reich [40], two prominent band systems can be distinguished,
- -
- the G band at cm which is caused by the valence vibrations of hybridized graphene C atoms—they form the concentric cylinders of the various types of CNTs [single-wall (SWCNT), double-wall (DWCNT), or multi-wall (MWCNT)], and are described as tangential vibrations along the nanotube walls [46,47], and
- -
- the D band at cm is due to hybridized C atoms with dangling bonds which terminate the graphitic structure.
- -
- Additionally, the first overtone of the G band occurs at approx. cm.
2.3. Mechanisms of Polymerization
Vacuum and Residual Gas
2.4. Comparison
3. Deposition of Parylene
3.1. Monomer Formation and Polymerization

- One of the most prominent issues which is easily available is the deposition rate. This property mainly depends on the rate of evaporation and the extent of monomerization, in the case of CVD, also on the dilution with inert gases (this dilution is difficult to investigate for PECVD).
- Is the polymerization controlled by diffusion?
- In contrast to the surface polymerization, which is supposed to be a 1st order reaction at low monomeric densities, is the volume polymerization at high monomeric densities a reaction of 2nd order?
- Organic reactions are always equilibria. Therefore, the yield is an important property for production purposes.
- Do the two different excitation modes generate coatings with different qualities?
- What happens to the dimers when they are exposed to microwaves?
- What are the morphological changes of the coatings between these two different methods of excitation?
3.2. CVD
3.2.1. Vapor Pressure
3.2.2. Equilibrium of Dissociation
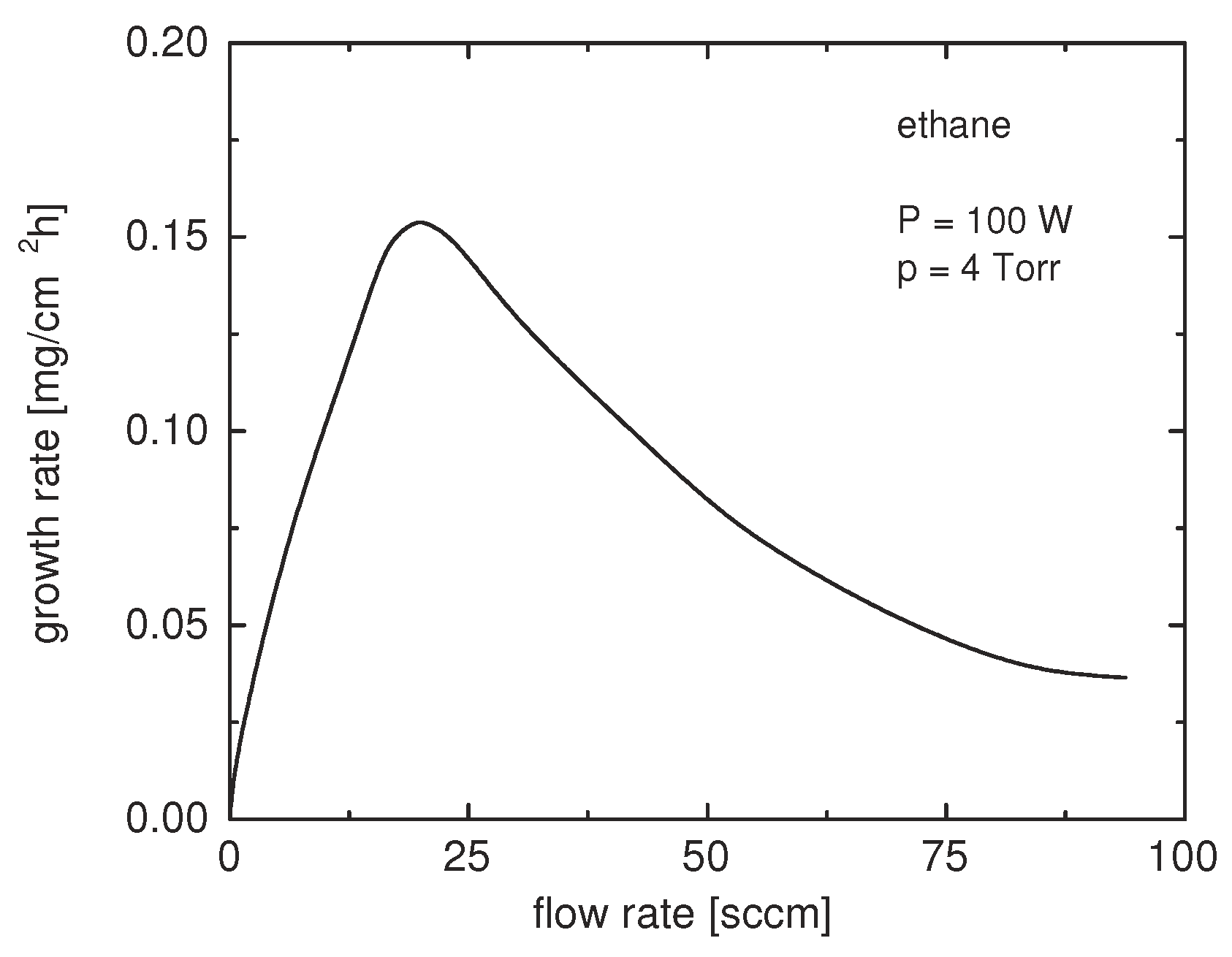
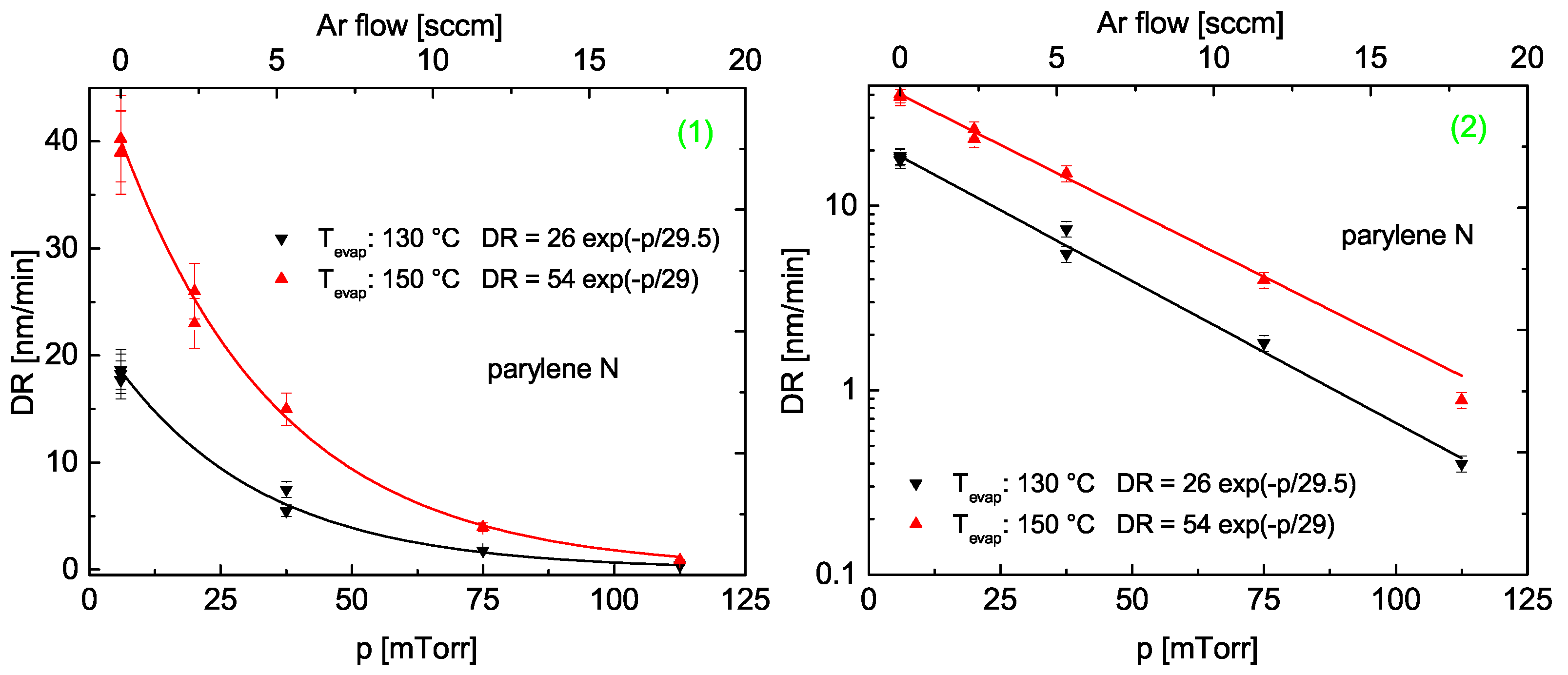
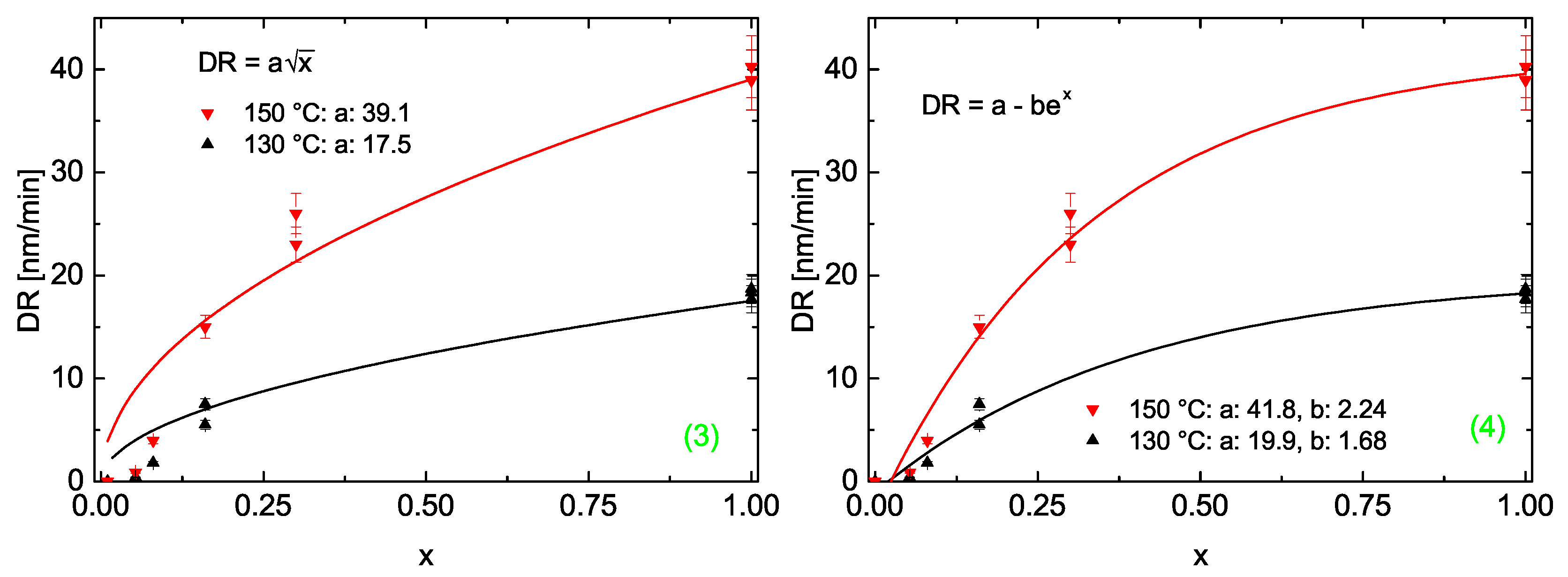
3.2.3. Deposition Rate
Models for the Deposition Rate as Function of Pressure
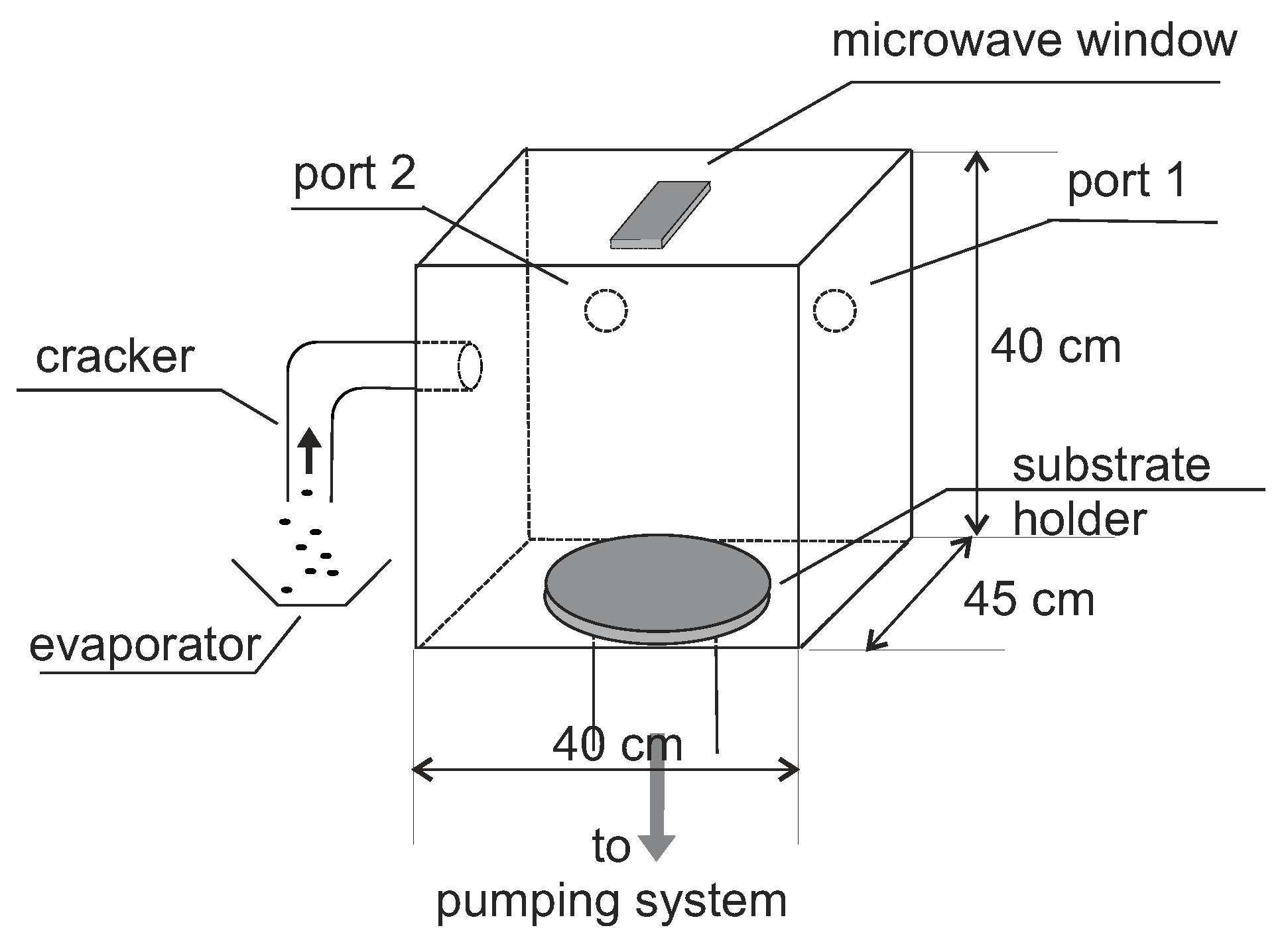
3.3. Plasma Enhanced Chemical Vapor Deposition
3.3.1. Plasma Activation
3.3.2. Discussion
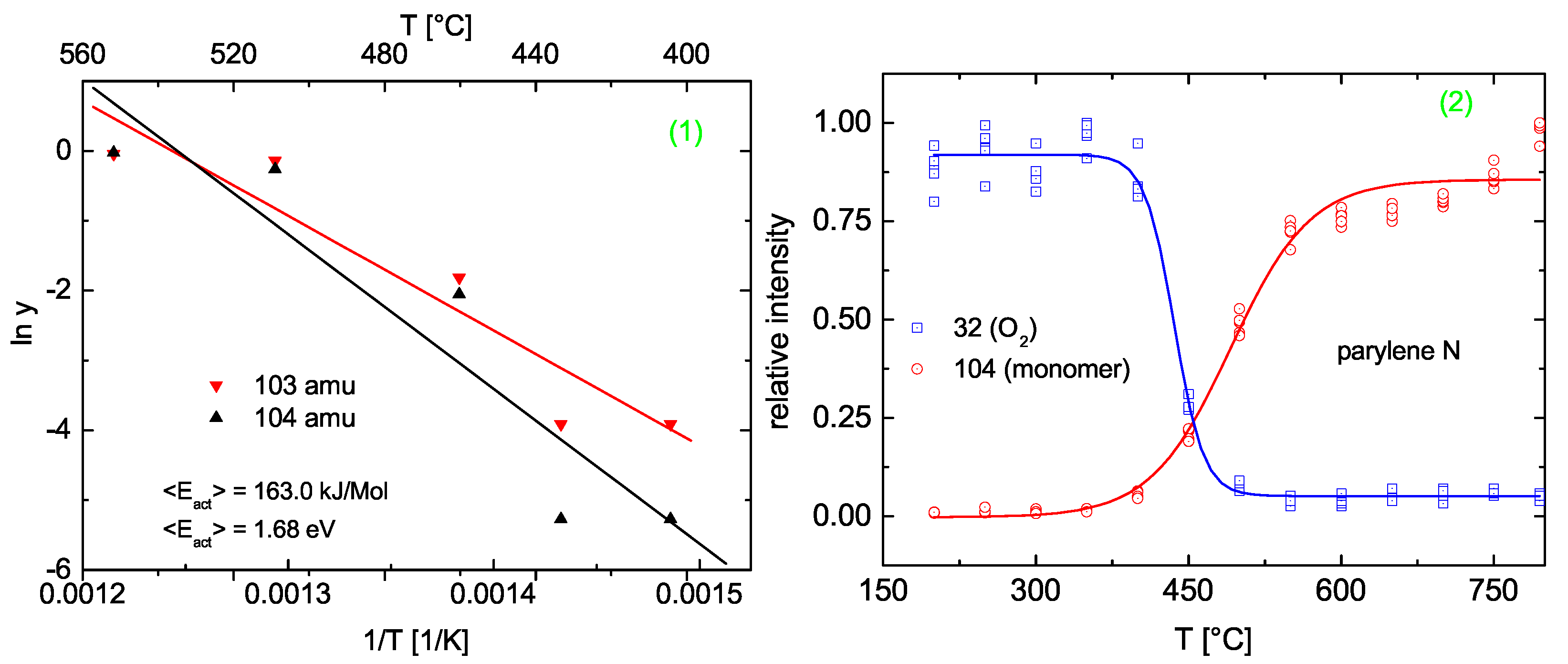
Efficiency of Thermal Cleavage
- entering velocity (K): 480 m/s, Å, 10 Pa (cm: mm;
- entering velocity (K): 480 m/s, Å, p = 40 Pa (cm: mm.
Efficiency of Plasma Cleavage
Surface Polymerization vs. Volume Polymerization
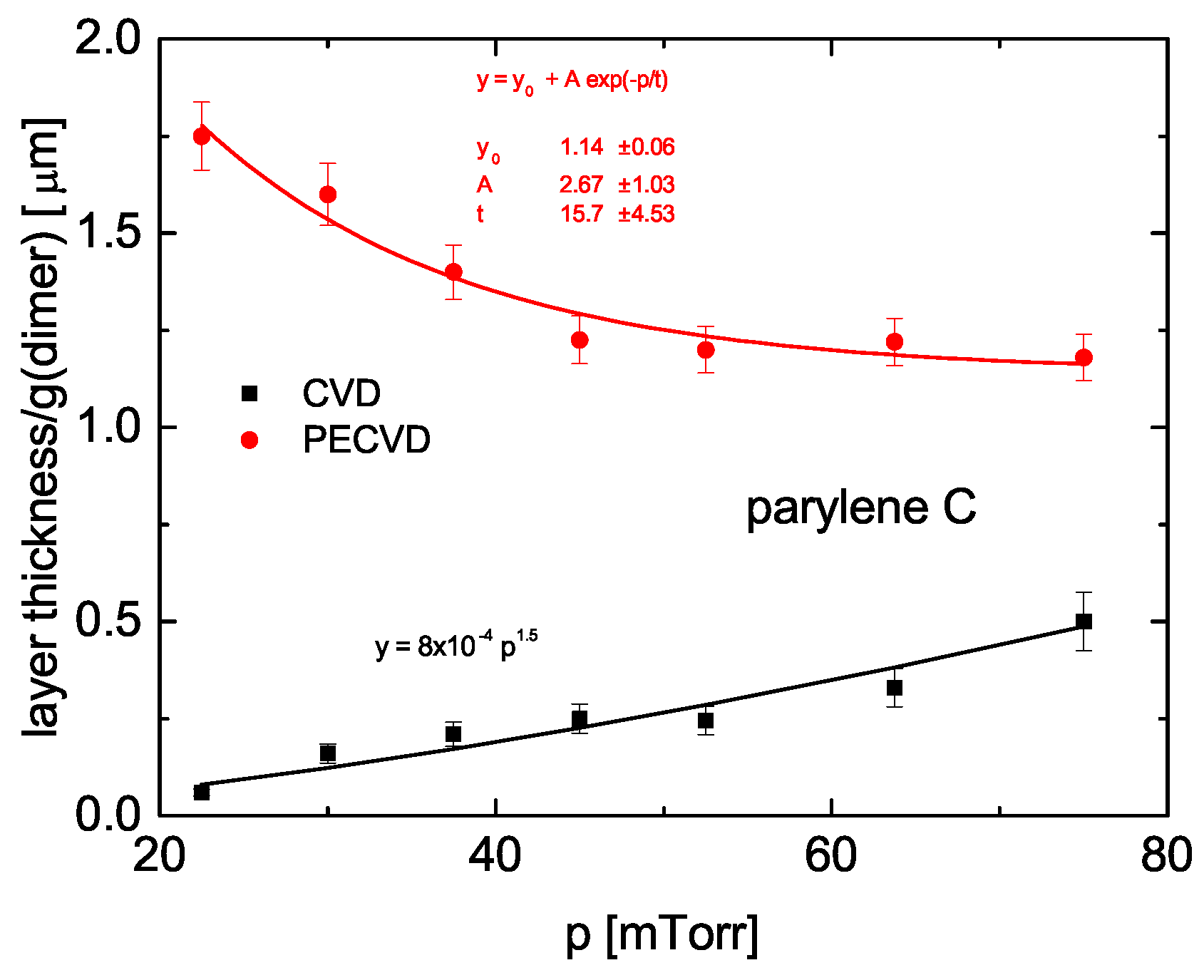
3.4. Comparison of Properties
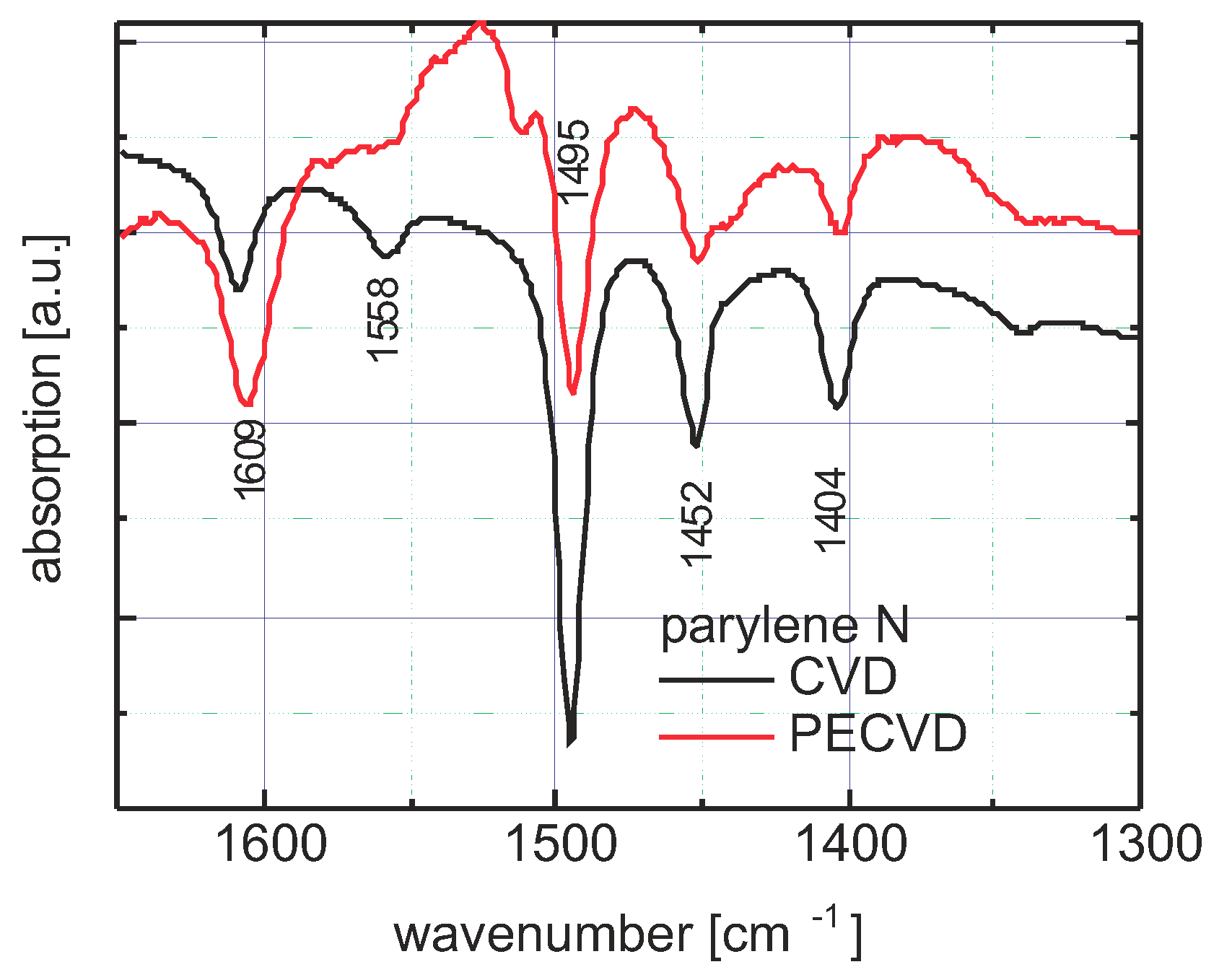
3.4.1. Spectral Identification
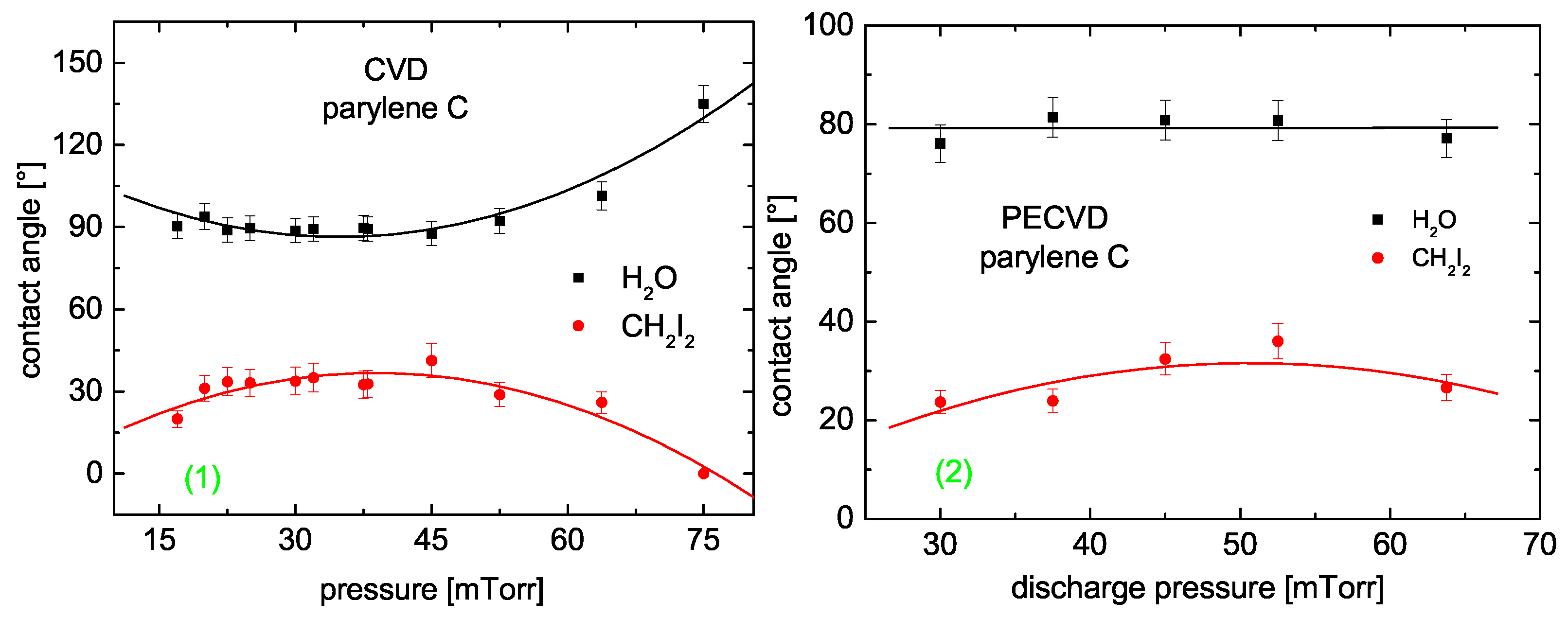
3.4.2. Surface Properties
- parylene N: CVD and pure PECVD (W if not specified differently),
- parylene C: CVD and thermally assisted PECVD ().
3.4.3. Parylene N
Contact Angle and Surface Energy
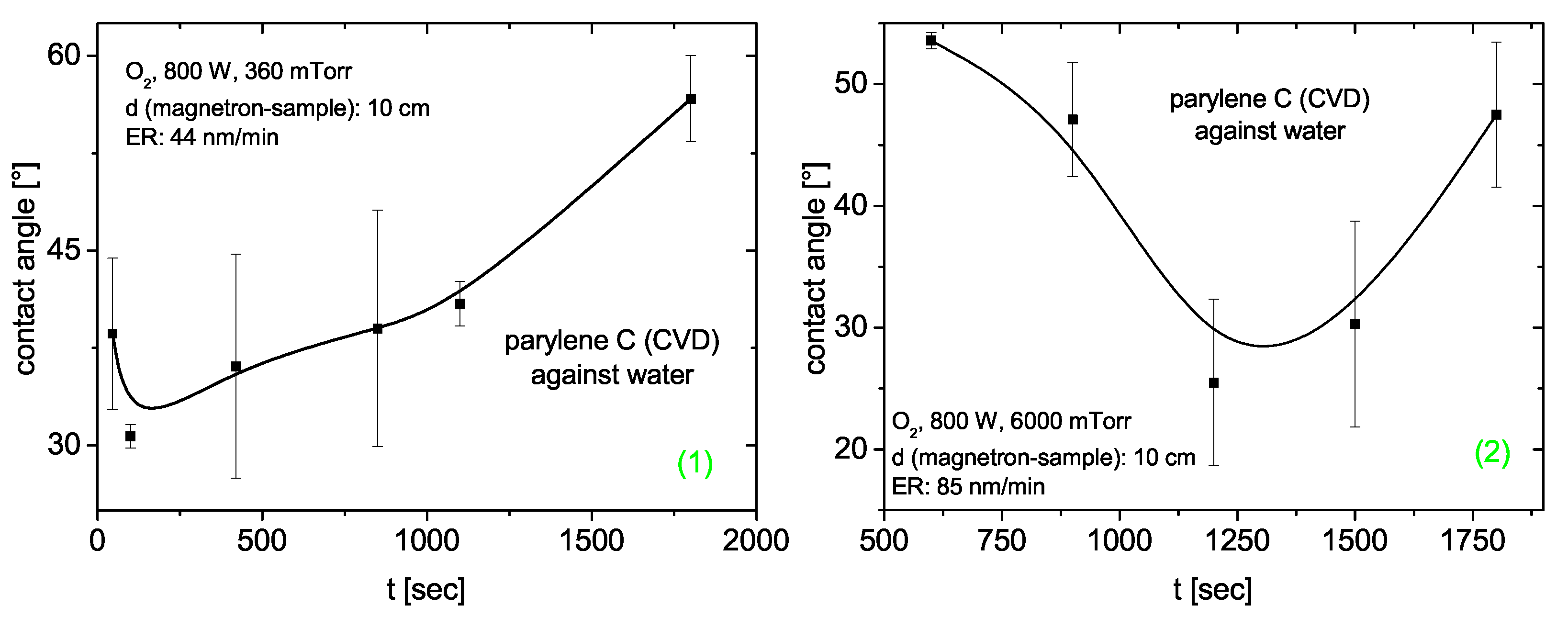
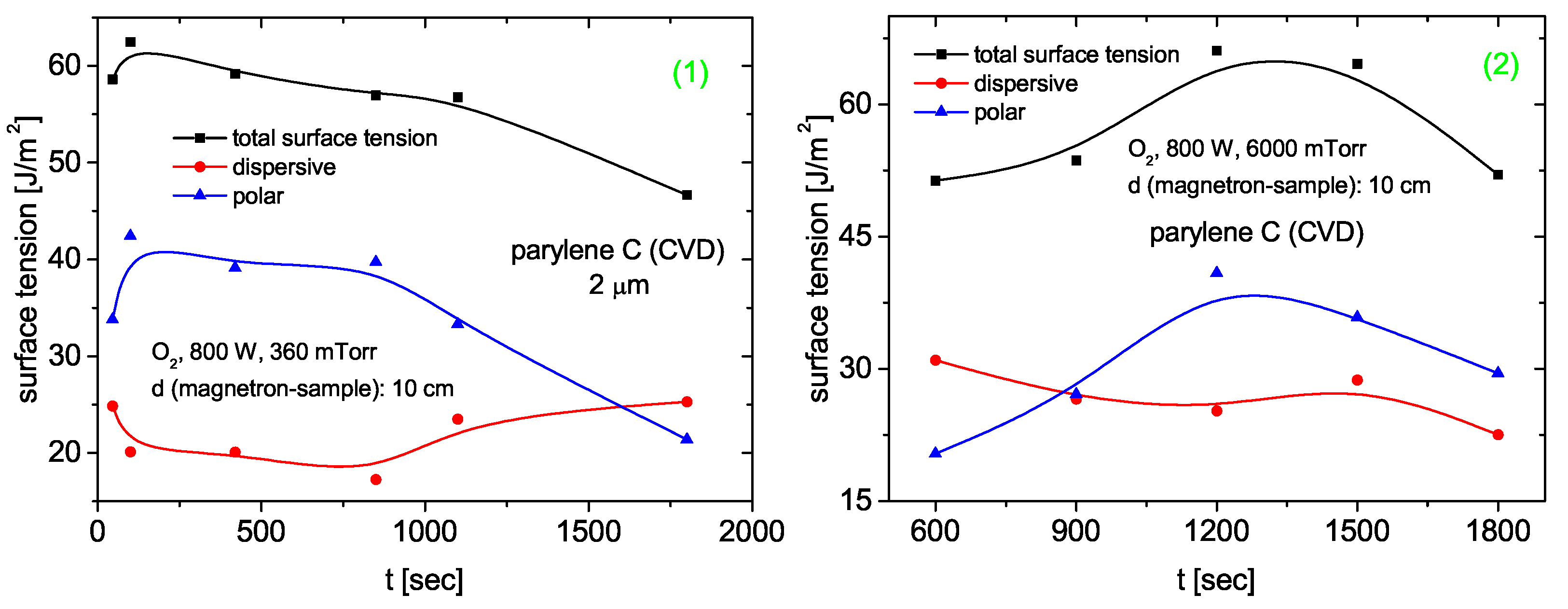
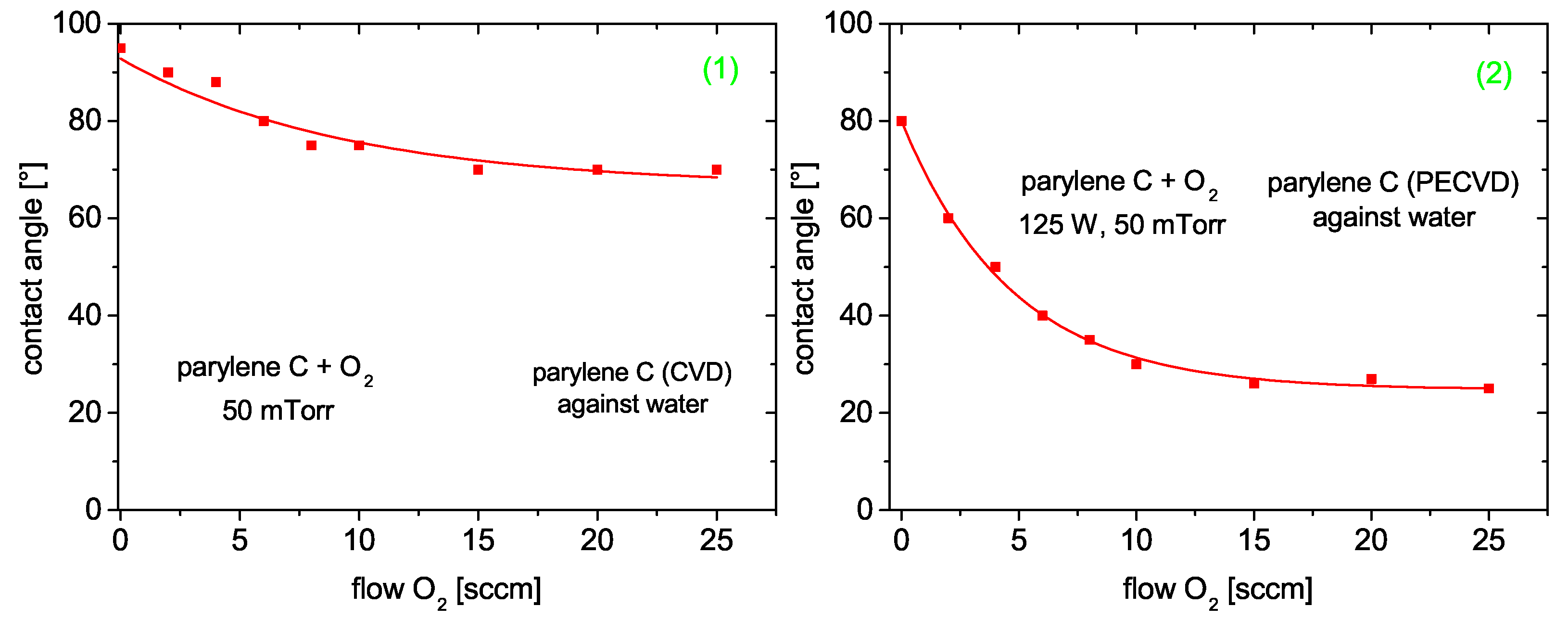
3.4.4. Parylene C
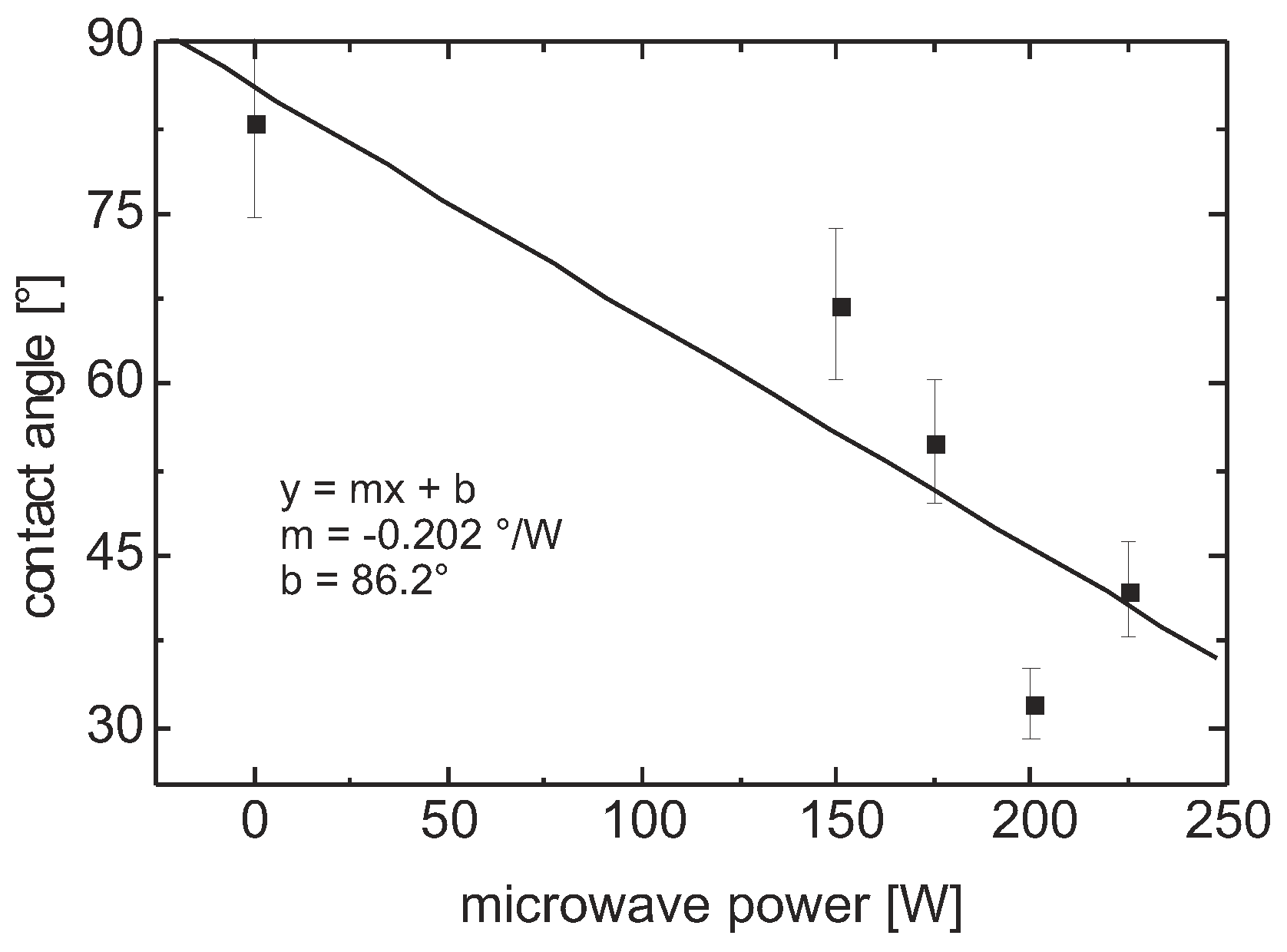
Contact Angle
Surface Tension
3.5. Surface Behavior
3.5.1. Incorporation of Hydrophilic Groups

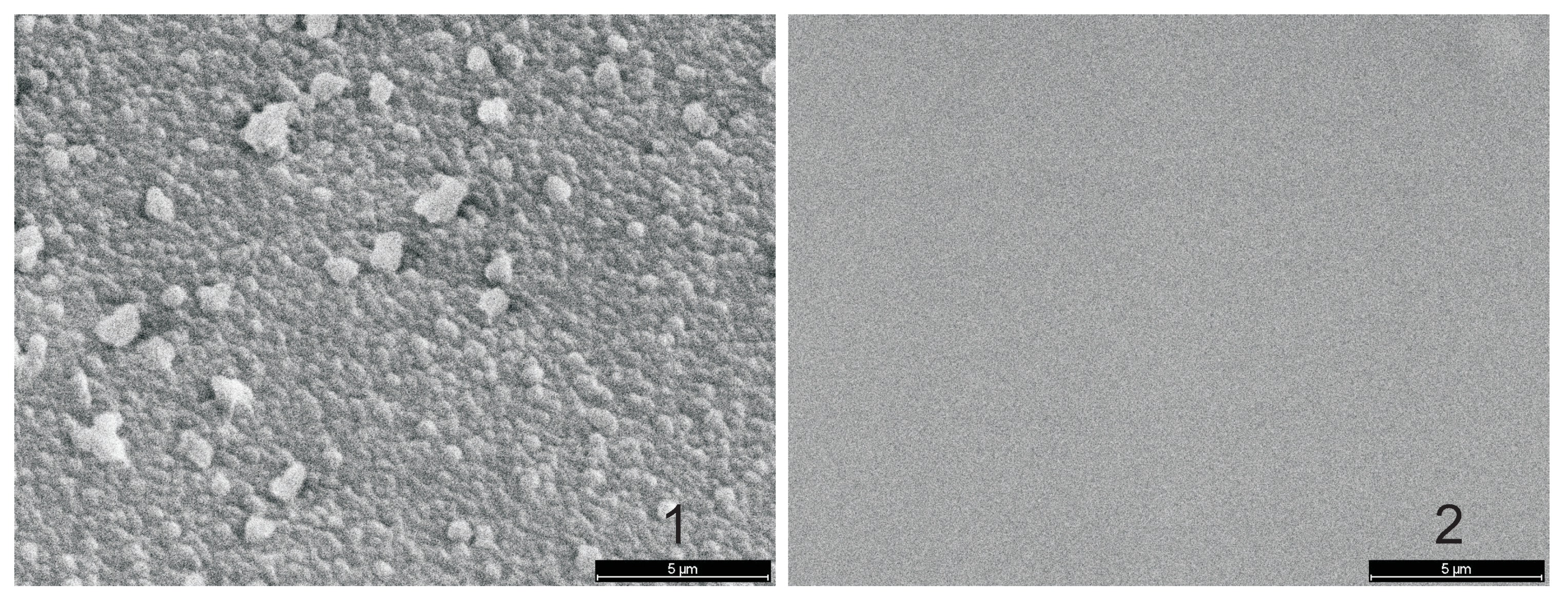
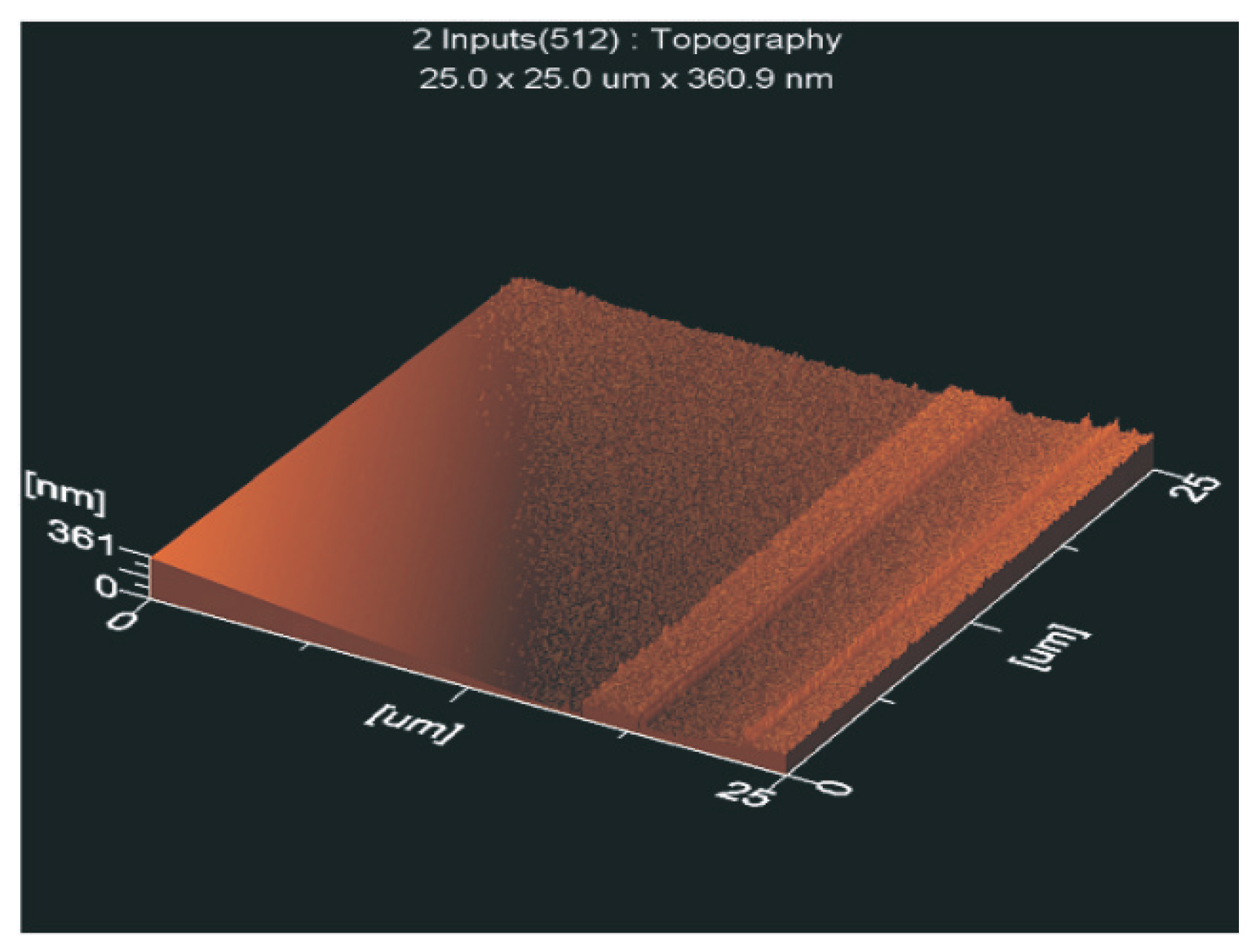
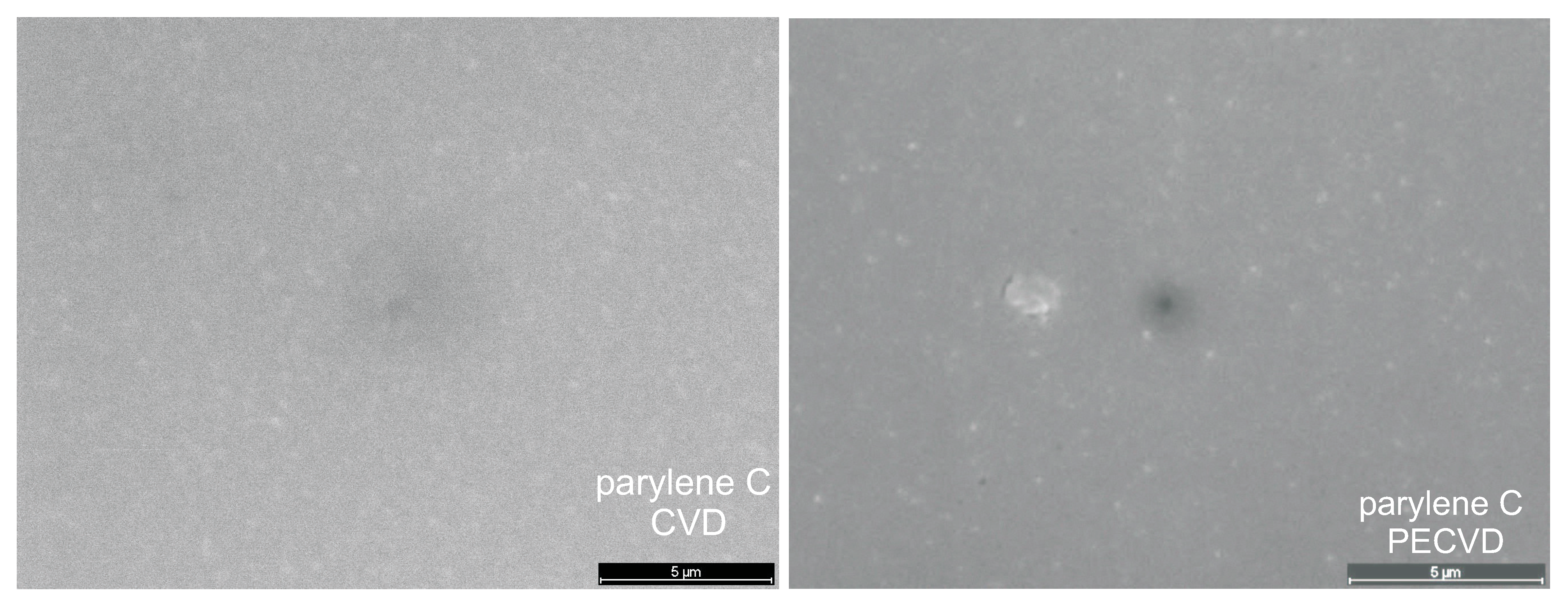
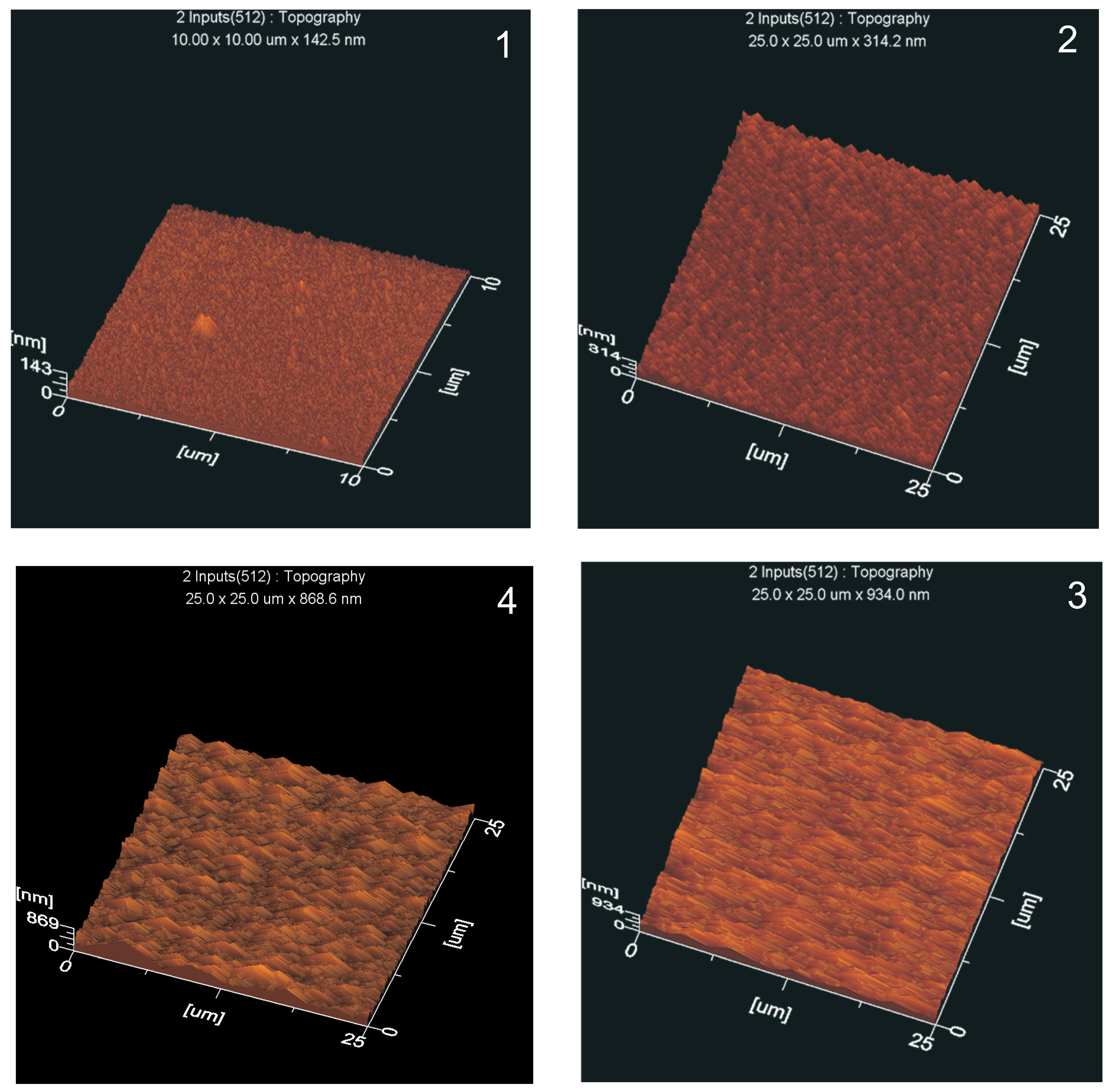
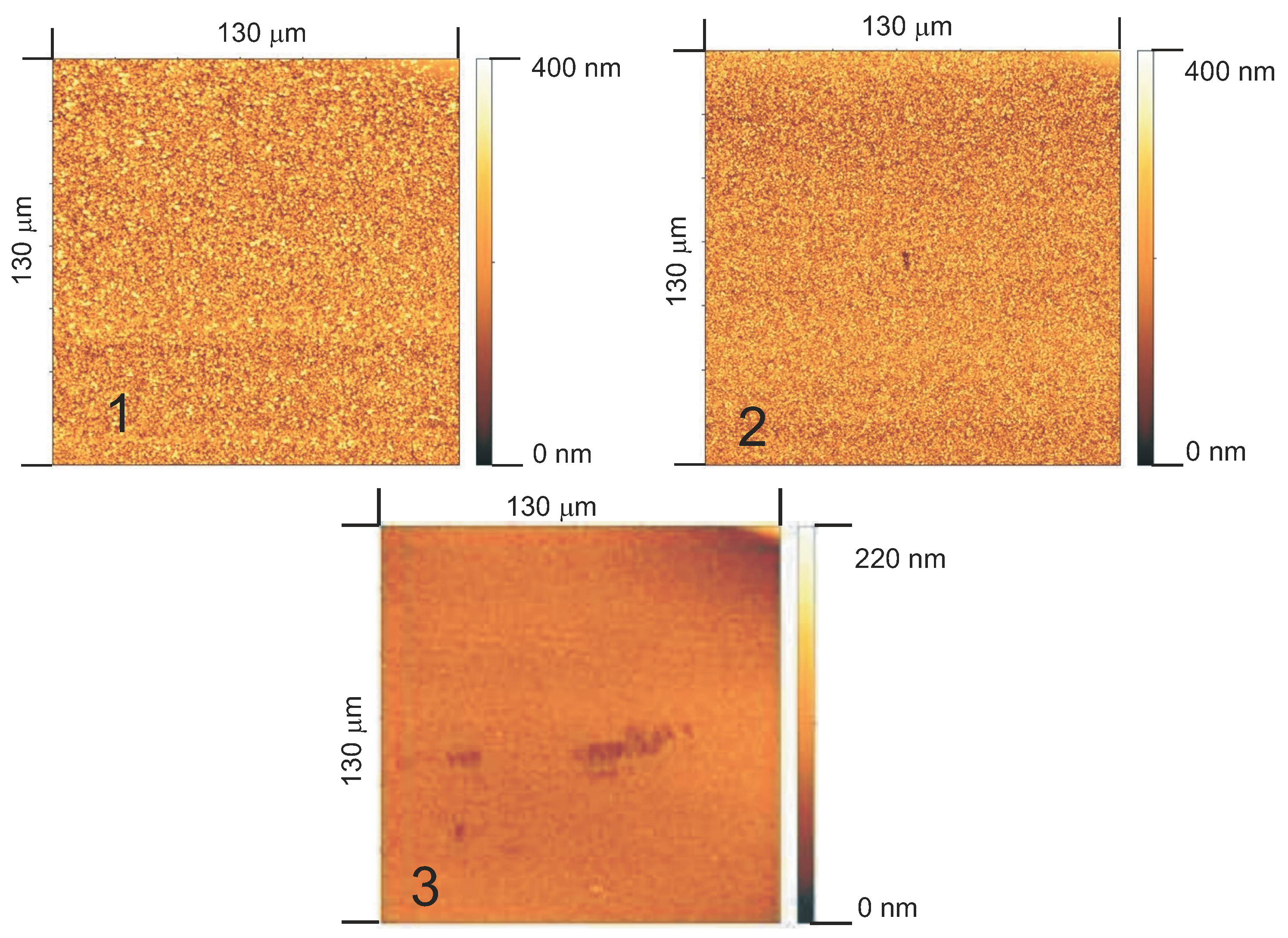
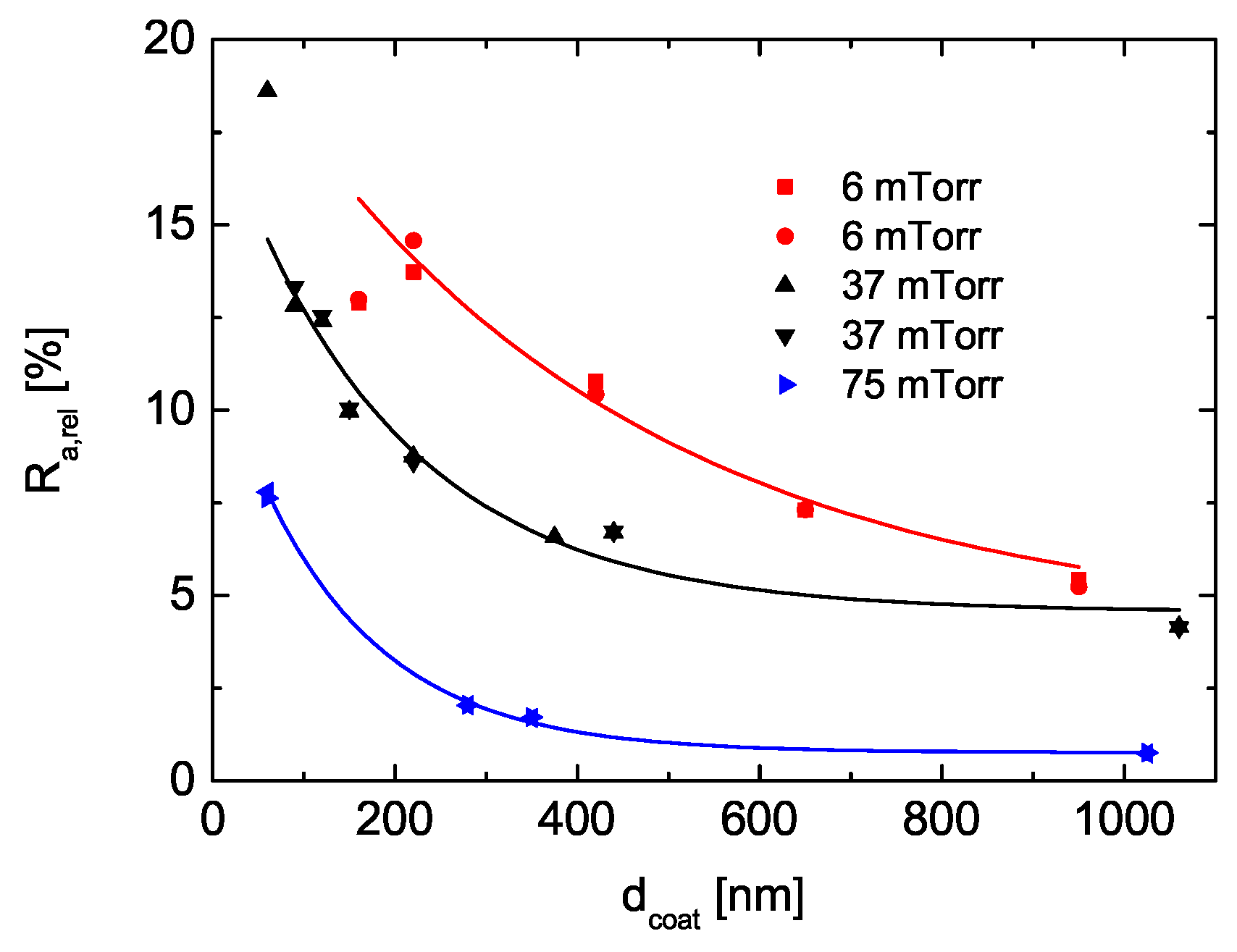
3.5.2. Surface Roughness
- polar groups which terminate the chains at the surface, and
- the smoother surfaces of PECVD layers,
3.6. Functionalization of Surfaces
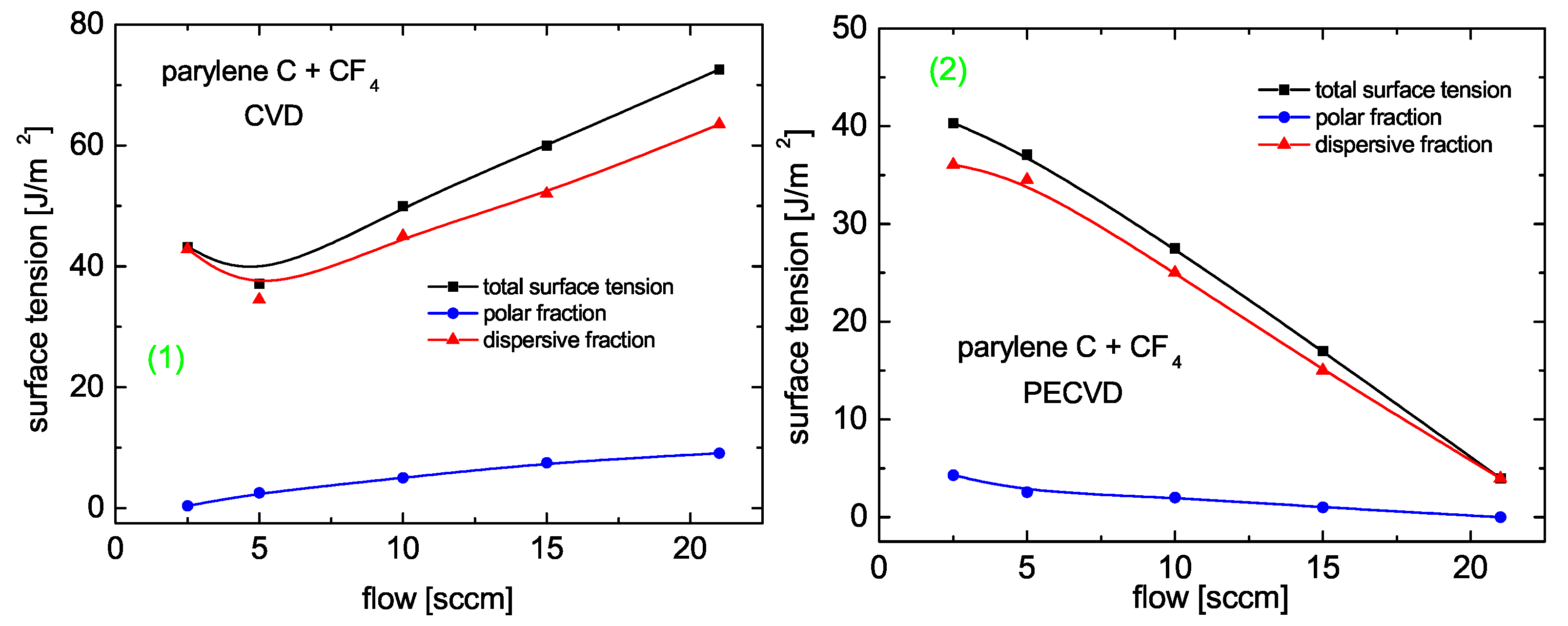
3.6.1. Parylene, Copolymerized with Cf
Contact Angle and Surface Energy
SEM
4. Conclusions
5. Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Davey, J.E.; Pankey, T. Epitaxial GaAs Films Deposited by Vacuum Evaporation. J. Appl. Phys. 1968, 39, 1941–1948. [Google Scholar] [CrossRef]
- Cho, A.Y.; Arthur, J.R., Jr. Molecular Beam Epitaxy. Prog. Solid State Chem. 1975, 10, 157–192. [Google Scholar] [CrossRef]
- Gorham, W.F. Para-Xylylene Polymers. U.S. Patent 3,342,754, 19 September 1967. [Google Scholar]
- Franz, G.; Rauter, F.; Dribinskiy, S.F. Characterization of microwave plasmas for deposition of polyparylene. J. Vac. Sci. Technol. 2009, A27, 1035. [Google Scholar] [CrossRef]
- Schäfer, H. Chemical Transport Reactions; Academic Press: New York, NY, USA; London, UK, 1964. [Google Scholar]
- Ganguli, S.; Agrawal, H.; Wang, B.; McDonald, J.F.; Lu, T.-M.; Yang, G.-R.; Gill, W.N. Improved growth and thermal stability of parylene films. J. Vac. Sci. Technol. 1997, A15, 3138–3142. [Google Scholar] [CrossRef]
- Errede, L.A.; Hoyt, J.M. The Chemistry of Xylylenes. III. Some Reactions of p-Xylylene that Occur by Free Radical Intermediates. J. Am. Chem. Soc. 1960, 82, 436–439. [Google Scholar] [CrossRef]
- Errede, L.A.; Gregorian, R.S.; Hoyt, J.M. The Chemistry of Xylylenes. VI. The Polymerization of p-Xylylene. J. Amer. Chem. Soc. 1960, 82, 5218–5223. [Google Scholar] [CrossRef]
- Kramer, P.; Sharma, A.K.; Hennecke, E.E.; Yasuda, H. Polymerization of Para-Xylylene Derivatives Parylene Polymerization. I. Deposition Kinetics for Parylene N and Parylene C. J. Polym. Sci. 1984, 22, 475–491. [Google Scholar] [CrossRef]
- Yasuda, H.K.; Yeh, Y.S.; Fusselman, S. A growth mechanism for the vacuum deposition of polymeric materials. Pure Appl. Chem. 1990, 62, 1689–1698. [Google Scholar] [CrossRef]
- Boehm, G.; Katz, G.S.; Meyer, R.; Amann, M.-C. AlInAs-GaInAs strain-compensated active regions for injectorless quantum cascade lasers. J. Cryst. Growth 2008, 311, 1932–1934. [Google Scholar] [CrossRef]
- Schamberger, F.; Ziegler, A.; Franz, G. Influence of film thickness and deposition rate on surface quality of polyparylene coatings. J. Vac. Sci. Technol. 2012, B30, 051801. [Google Scholar] [CrossRef]
- Franz, G. Low Pressure Plasmas and Microstructuring Technology; Springer: Berlin/Heidelberg, Germany, 2009; Chapter 8. [Google Scholar]
- Franz, G. Low Pressure Plasmas and Microstructuring Technology; Springer: Berlin/Heidelberg, Germany, 2009; Chapters 5 and 6. [Google Scholar]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing, 1st ed.; Wiley: New York, NY, USA, 1994; pp. 301–326. [Google Scholar]
- Bogart, K.H.A.; Dalleska, N.F.; Bogart, G.R.; Fisher, E.R. Plasma enhanced chemical vapor deposition of SiO2 using novel alkoxysilane precursors. J. Vac. Sci. Technol. 1995, A13, 476–480. [Google Scholar] [CrossRef]
- Coburn, J.W.; Kay, E. Some Chemical Aspects of the Fluorocarbon Plasma Etching of Silicon and Its Compounds. IBM J. Res. Develop. 1979, 23, 33–41. [Google Scholar] [CrossRef]
- Coburn, J.W.; Winters, H.F. Plasma Etching—A Discussion of Mechanisms. J. Vac. Sci. Technol. 1979, 16, 391–403. [Google Scholar] [CrossRef]
- Franz, G. Low Pressure Plasmas and Microstructuring Technology; Springer: Berlin/Heidelberg, Germany, 2009; Chapter 7. [Google Scholar]
- Franz, G. Low Pressure Plasmas and Microstructuring Technology; Springer: Berlin/Heidelberg, Germany, 2009; Chapters 3 and 9. [Google Scholar]
- Dorai, R.; Kushner, M.J. A model for plasma modification of polypropylene using atmospheric pressure discharges. J. Phys. D Appl. Phys. 2003, 36, 666–685. [Google Scholar] [CrossRef]
- Yasuda, H.; Hirotsu, T. Critical Evaluation of Conditions of Plasma Polymerization. J. Polym. Sci. 1978, 16, 743–759. [Google Scholar] [CrossRef]
- Yasuda, H. Plasma Polymerization; Academic Press: Orlando, FL, USA, 1985. [Google Scholar]
- Koch, R. The intrinsic stress of poylcrysatlline and epitaxial thin metal films. J. Phys. Condens. Matter 1994, 6, 9519–9550. [Google Scholar] [CrossRef]
- Koch, R. Stress in Evaporated and Sputtered Thin Films—A Comparison. Surf. Coat. Technol. 2010, 204, 1973–1982. [Google Scholar] [CrossRef]
- Klemberg-Sapieha, J.E.; Martinu, L.; Wertheimer, M.R.; Günther, P.; Schellin, R.; Thielemann, C.; Sessler, G.M. Plasma deposition of low-stress electret films for electroacoustic and solar cell application. J. Vac. Sci. Technol. 1996, A14, 2775–2779. [Google Scholar] [CrossRef]
- Whitfield, R.; Parkatzidis, K.; Truong, N.P.; Junkers, T.; Anastasaki, A. Tailoring Polymer Dispersity by RAFT Polymerization: A Versatile Approach. Chem 2020, 6, 1340–1352. [Google Scholar] [CrossRef]
- Jensen, R.J.; Bell, A.T.; Soong, D.S. Plasma Polymerization of Ethane. I. Experimental Studies of Effluent Gas Composition and Polymer Deposition Rates. Plasma Chem. Plasma Process. 1983, 3, 139–161. [Google Scholar] [CrossRef]
- Jensen, R.J.; Bell, A.T.; Soong, D.S. Plasma Polymerization of Ethane. II. Theoretical Analysis of Effluent Gas Composition and Polymer Deposition Rates. Plasma Chem. Plasma Process. 1983, 3, 163–192. [Google Scholar] [CrossRef]
- Aisenberg, S.; Chabot, R.W. Physics of Ion Plating and Ion Beam Deposition. J. Vac. Sci. Technol. 1973, 10, 104–107. [Google Scholar] [CrossRef]
- Spitsyn, B.V.; Buyilov, L.L.; Derjaguin, B.V. Vapor Growth of Diamond on Diamond and other Surfaces. J. Cryst. Growth 1981, 52, 219–226. [Google Scholar] [CrossRef]
- Bachmann, P.K. Diamond thin film technology I. Diamond deposition. Adv. Mater. 1990, 2, 195–199. [Google Scholar] [CrossRef]
- Awakowicz, P. Niederdruckplasmen: Modelle, Diagnostikmethoden und Anwendungen. Habilitation Thesis, Technische Universität München, Bavaria, Germany, 1998. [Google Scholar]
- Krätschmer, W.; Wagner, B. Molekül-Linien in den Spektren von im Labor produzierten Kohlenstoff-Staubteilchen. In Jahresbericht des Max-Planck-Instituts für Kernphysik Heidelberg 1988; Klapdor, H.V., Jessberger, E.K., Eds.; MPI: Heidelberg, Germany, 1989. [Google Scholar]
- Iijima, S. Helical microtubes of graphitic carbon. Nature 1991, 354, 115–122. [Google Scholar] [CrossRef]
- Seidel, R.; Duesberg, G.S.; Unger, E.; Graham, A.P.; Liebau, M.; Kreupl, F. Chemical Vapor Deposition Growth of Single-Walled Carbon Nanotubes at 600 °C and a Simple Growth Model. J. Phys. Chem. B 2004, 108, 1888–1893. [Google Scholar] [CrossRef]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Ijima, S.I. Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef]
- Stehl, C.; Fischer, M.; Gsell, S.; Berdermann, E.; Rahman, M.S.; Traeger, M.; Klein, O.; Schreck, M. Efficiency of dislocation density reduction during heteroepitaxial growth of diamond for detector applications. Appl. Phys. Lett. 2013, 103, 151905. [Google Scholar] [CrossRef]
- Melville, A.; Mairoser, T.; Schmehl, A.; Fischer, M.; Gsell, S.; Schreck, M.; Awschalom, D.D.; Heeg, T.; Holländer, B.; Schubert, J.; et al. Epitaxial growth of europium monoxide on diamond. Appl. Phys. Lett. 2013, 103, 222402. [Google Scholar] [CrossRef]
- Thomsen, C.; Reich, S. Raman Scattering in Carbon Nanotubes. In Light Scattering in Solid IX; Cardona, M., Merlin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 108, pp. 115–232. [Google Scholar]
- Ge, C.; Li, Y.; Yin, J.-J.; Liu, Y.; Wang, L.; Zhao, Y.; Chen, C. The contributions of metal impurities and tube structure to the toxicity of carbon nanotube materials. NPG Asia Mater. 2012, 4, e32. [Google Scholar] [CrossRef]
- Bachmann, P.K.; Leers, D.; Wiechert, D.U. Diamond Thin Films: Preparation, Characterization and Selected Applications—Progress Report. Ber. Bunsenges. Phys. Chem. 1991, 95, 1390–1400. [Google Scholar] [CrossRef]
- Spear, K.E. Diamond—Ceramic Coating of the Future. J. Am. Ceram. Soc. 1989, 72, 171–191. [Google Scholar] [CrossRef]
- Reich, S.; Thomsen, C.; Maultzsch, J. Carbon Nanotubes: Basic Concepts and Physical Properties; Wiley-VCH: Berlin, Germany, 2004. [Google Scholar]
- Caccamo, M.T.; Mavilia, G.; Magazù, S. Thermal Investigations on Carbon Nanotubes by Spectroscopic Techniques. Appl. Sci. 2020, 10, 8159. [Google Scholar] [CrossRef]
- Rao, A.M.; Richter, E.; Bandow, S.; Chase, B.; Eklund, P.C.; Williams, K.A.; Fang, S.; Subbaswamy, K.R.; Menon, M.; Thess, A.; et al. Diameter-selective Raman scattering from vibrational modes in carbon nanotubes. Science 1997, 275, 187. [Google Scholar] [CrossRef]
- Maultzsch, J.; Reich, S.; Thomsen, C. Raman scattering in carbon nanotubes revisited. Phys. Rev. 2002, B65, 233402. [Google Scholar] [CrossRef]
- d’Agostino, R. Plasma Deposition, Treatment, and Etching of Polymers; d’Agostino, R., Flamm, D., Auciello, O., Eds.; Academic Press, Inc.: New York, NY, USA, 1990. [Google Scholar]
- Möller, W. Plasma and Surface Modeling of the Deposition of Hydrogenated Carbon Films from Low-Pressure Methane Plasmas. Appl. Phys. 1993, A56, 527–546. [Google Scholar] [CrossRef]
- Fortin, J.B.; Lu, T.-M. The Growth and Properties of Parylene Thin Films; Springer Science: New York, NY, USA, 2004. [Google Scholar]
- Franz, G. Low Pressure Plasmas and Microstructuring Technology; Springer: Berlin/Heidelberg, Germany, 2009; p. 409, for Ta/Si sputtering in oxygen-doped Ar atmospheres. [Google Scholar]
- Bayer, C. Aufbringung von Siliziumoxidschichten durch Plasma-CVD und Anwendung auf Beschichtungen in einer Plasma-Wirbelschicht. Ph.D. Thesis, ETH, Zurich, Switzerland, 1998. [Google Scholar]
- Soll, C. Plasmapolymerisation von Hexamethyldisolxan zur Abscheidung von Quarzähnlichen Schichten bei Gepulster Leistungszufuhr. Ph.D. Thesis, Bergische Universität, Wuppertal, Germany, 2000. [Google Scholar]
- Rauf, S.; Kushner, M.J. Controller design issues in the feedback control of radio frequency plasma processing reactors. J. Vac. Sci. Technol. 1999, A17, 704–712. [Google Scholar] [CrossRef]
- Kinder, R.L.; Kushner, M.J. Wave propagation and power deposition in magnetically enhanced inductively coupled and helicon plasma sources. J. Vac. Sci. Technol. 2001, A19, 76–86. [Google Scholar] [CrossRef]
- Franz, G.; Schamberger, F. Evaporation and thermal cracking of dimeric parylenes. J. Vac. Sci. Technol. 2013, A31, 061602. [Google Scholar] [CrossRef]
- Cariou, F.E.; Valley, D.J.; Loeb, W.E. Poly-para-xylylene in thin film application. IEEE Trans. Parts 1965, 1, 54–62. [Google Scholar] [CrossRef]
- Beach, W.F. A Model for the Vapor Deposition Polymerization of p-Xylylene. Macromolecules 1978, 11, 72–76. [Google Scholar] [CrossRef]
- Fortin, J.B.; Lu, T.-M. A Model for the Chemical Vapor Deposition of Poly para-xylylene Parylene Thin Films. Chem. Mater. 2002, 14, 1945–1949. [Google Scholar] [CrossRef]
- Semlyen, J.A. Ring-chain equilibria and their conformations of polymer chains. Adv. Polym. Sci. 1976, 22, 41–75. [Google Scholar]
- Reichel, A.; Franz, G.; Amann, M.-C. Correlation of Growth and Surface Properties of Polyp-xylylenes to Reaction Conditions. Coatings 2015, 5, 142–171. [Google Scholar] [CrossRef]
- Fortin, J.B.; Lu, T.-M. The Growth and Properties of Parylene Thin Films; Springer Science: New York, NY, USA, 2004; Chapter 5. [Google Scholar]
- Rogojevic, S.; Moore, J.A.; Gill, W.N. Modeling vapor deposition of low-K polymers: Parylene and polynaphtalene. J. Vac. Sci. Technol. 1999, A17, 266–274. [Google Scholar] [CrossRef]
- Yasuda, H.; Chun, B.H.; Cho, D.L.; Lin, T.J.; Yang, D.J.; Antonelli, J.A. Interface-Engineered Parylene C Coating for Corrosion Protection of Cold-Rolled Steel. Corrosion 1996, 52, 169. [Google Scholar] [CrossRef]
- Blackburn, E.V.; Timmons, C.J. The photocyclisation of stilbene analogues. Quart. Rev. 1969, 23, 482. [Google Scholar] [CrossRef]
- Olson, R. Xylylene Polymers. In Encyclopedia of Polymer Science and Engineering, 2nd ed.; Wiley: Hoboken, NJ, USA, 1989; Volume 17, pp. 990–1024. [Google Scholar]
- Streitwieser, A., Jr.; Ward, H.R. Organic Compounds in Microwave Discharges. II. Initial Sudies with Toluene and Related Hydrocarbons. J. Am. Chem. Soc. 1963, 85, 539–542. [Google Scholar] [CrossRef]
- Stahl, U. Entwicklung eines Verfahrens zur Stabilisierung von Polymerschichten auf OFW-Sensoren für die Analytik von organischen Gasen. Ph.D. Thesis, TU Karlsruhe, Karlsruhe, Germany, 1999. [Google Scholar]
- Wertheimer, M.R.; Moisan, M. Comparison of Microwave and Lower Frequency Plasmas for Thin Film Deposition and Etching. J. Vac. Sci. Technol. 1985, A3, 2643–2649. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, C.; Claude, R.; Ferreira, C.M.; Margot, J.; Paraszczak, J.; Sá, A.B.; Sauvé, G.; Wertheimer, M.R. Radio frequency or microwave plasma reactors? Factors determining the optimum frequency of operation. J. Vac. Sci. Technol. 1991, B9, 8–25. [Google Scholar] [CrossRef]
- Kaelble, D.H. Physical Chemistry of Adhesion; John Wiley: New York, NY, USA, 1971. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–550. [Google Scholar] [CrossRef]
- Hwang, K.S.; Park, J.H.; Lee, J.H.; Yoon, D.S.; Kim, T.S.; Han, I.; Noh, J.H. Effect of Atmospheric-Plasma Treatment for Enhancing Adhesion of Au on Parylene-C-Coated Protein Chips. J. Korean Phys. Soc. 2004, 44, 1168–1172. [Google Scholar]
- Senkevich, J.J.; Mitchell, C.J.; Vijayaraghavan, A.; Barnat, E.V.; McDonald, J.F. Unique structure/properties of chemical vapor depositied parylene E. J. Vac. Sci. Technol. 2002, A20, 1445. [Google Scholar] [CrossRef]
- Shin, Y.S.; Cho, K.; Lim, S.H.; Chung, S.; Park, S.-J.; Chung, C.; Han, D.-C.; Chang, J.K. PDMS-based micro PRC chip with Parylene coating. J. Micromech. Microeng. 2003, 13, 768–774. [Google Scholar] [CrossRef]
- Pruden, K.G.; Sinclair, K.; Beaudoin, S. Characterization of Parylene-N and Parylene-C Photooxidation. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1486–1496. [Google Scholar] [CrossRef]
- Zhuang, Y.X.; Menon, A. Wettability and thermal stability of fluorocarbon films deposited by deep reactive ion etching. J. Vac. Sci. Technol. 2005, A23, 434–439. [Google Scholar] [CrossRef]
- Liston, E.M.; Martinu, L.; Wertheimer, M.R. Plasma surface modification of polymers for improved adhesion: A critical review. J. Adhes. Sci. Technol. 1993, 7, 1091–1127. [Google Scholar] [CrossRef]
- Liston, E.M. Plasma treatment for Improved Bonding: A Review. J. Adhes. 1989, 30, 199–218. [Google Scholar] [CrossRef]
- Bi, X.; Crum, B.P.; Li, W. Super Hydrophobic Parylene-C Produced by Consecutive O2 and SF6 Plasma Treatment. J. Microelectromech. Syst. 2014, 23, 628–635. [Google Scholar] [CrossRef]
- Tsougeni, K.; Petrou, P.S.; Tserepi, A.; Kakabakos, S.E.; Gogolides, E. Nano-texturing of polymethyl metacrylate polymer using plasma processes and applications in wetting control and protein absorption. Microelectron. Eng. 2009, 86, 1424–1427. [Google Scholar] [CrossRef]
- Zare, H.H.; Düttmann, O.; Vass, A.; Franz, G.; Jocham, D. Silver ions eluted from partially protected silver nanoparticles. Biointerphases 2016, 11, 031002. [Google Scholar] [CrossRef]
- Zare, H.H.; Juhart, V.; Vass, A.; Franz, G.; Jocham, D. Efficacy of silver/hydrophilic polyp-xylylene on preventing bacterial growth and biofilm formation in urinary catheters. Biointerphases 2017, 12, 011001. [Google Scholar] [CrossRef] [PubMed]
- Franz, G.; Schamberger, F.; Zare, H.H.; Bröskamp, S.F.; Jocham, D. Bi-layer sandwich film for antibacterial catheters. Beilstein J. Nanotechnol. 2017, 8, 1982. [Google Scholar] [CrossRef] [PubMed]
- Tserepi, A.D.; Vlachopoulou, M.-E.; Gogolides, E. Nanotexturing of polydimethylsiloxane in plasmas for creating robust super-hydrophobic surfaces. Nanotechnology 2006, 17, 3977–3983. [Google Scholar] [CrossRef]
- Kokkoris, G.; Constantoudis, V.; Angelikopoulos, P.; Boulousis, G.; Gogolides, E. Dual nanoscale roughness on plasma-etched Si-surfaces: Role of etch inhibitors. Phys. Rev. 2007, B76, 193405–193408. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.-O. Superhydrophobicity. In Waterproof and Water Repellent Textiles and Clothing; The Textile Institute Book Series; Elsevier: Amsterdam, The Netherlands, 2018; pp. 267–297. [Google Scholar]
- Rioboo, R.; Demnati, I.; Ali, M.A.; Sevkan, R.; de Coninck, J. Superhydrophobicity of composite surfaces created from polymer blends. J. Coll. Interf. Sci. 2020, 560, 596–605. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franz, G. Plasma Enhanced Chemical Vapor Deposition of Organic Polymers. Processes 2021, 9, 980. https://doi.org/10.3390/pr9060980
Franz G. Plasma Enhanced Chemical Vapor Deposition of Organic Polymers. Processes. 2021; 9(6):980. https://doi.org/10.3390/pr9060980
Chicago/Turabian StyleFranz, Gerhard. 2021. "Plasma Enhanced Chemical Vapor Deposition of Organic Polymers" Processes 9, no. 6: 980. https://doi.org/10.3390/pr9060980
APA StyleFranz, G. (2021). Plasma Enhanced Chemical Vapor Deposition of Organic Polymers. Processes, 9(6), 980. https://doi.org/10.3390/pr9060980




