The Osteogenic Function of Danggui Buxue Tang, a Herbal Decoction Containing Astragali Radix and Angelicae Sinensis Radix, Is Optimized by Boiling the Two Herbs Together: Signaling Analyses Revealed by Systems Biology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Quality Control of Herbal Medicines
2.2. Rat Osteoblast and Micromass Culture
2.3. Alkaline Phosphatase (ALP) and Collagen Quantification
2.4. Adhesion Assay
2.5. Shotgun Proteomics
2.6. Protein Identification and Quantification
2.7. Lipidomics Analysis
2.8. Data Extraction and Processing
2.9. Pathway and Statistical Analysis
3. Results
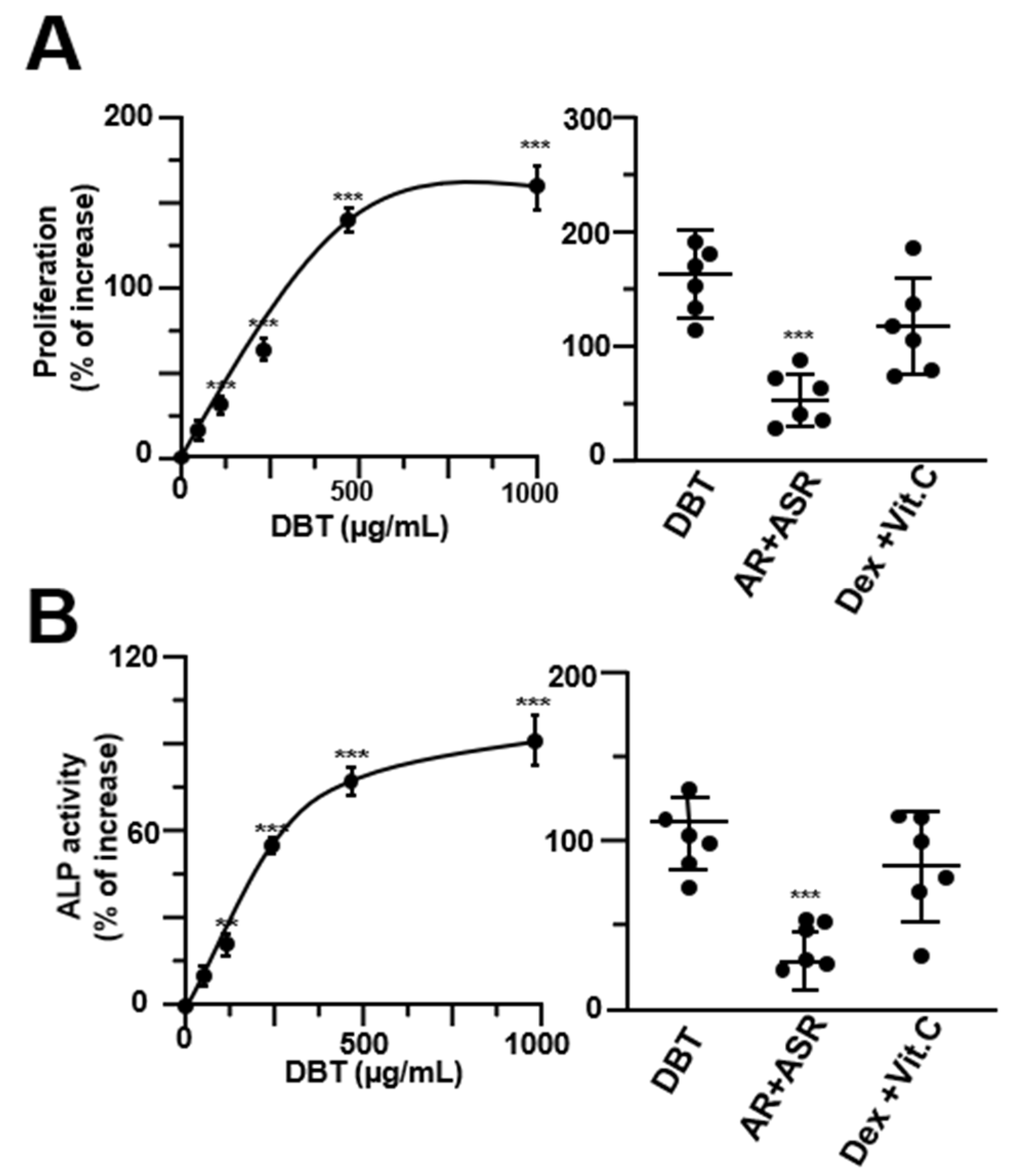
3.1. The Osteoblastic Function of DBT Requires Boiling Herbs Together
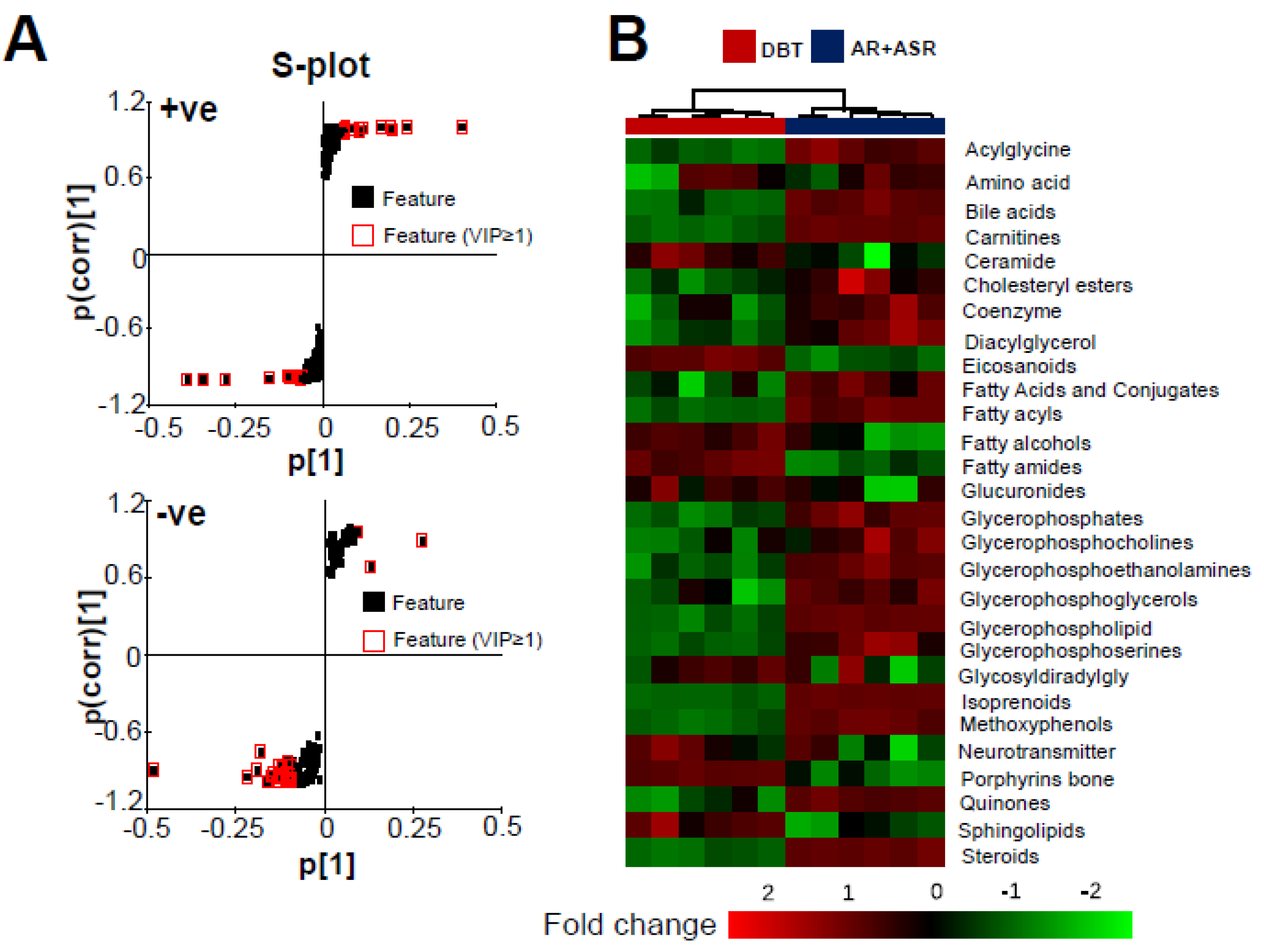
3.2. Proteomics Analysis of Herbal-Extract-Treated Osteoblasts
3.3. Lipidomics Analysis of Herbal-Extract-Treated Osteoblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Statements
References
- Wu, X.; Wang, S.; Lu, J.; Jing, Y.; Li, M.; Cao, J.; Bian, B.; Hu, C. Seeing the unseen of Chinese herbal medicine processing (Paozhi): Advances in new perspectives. Chin. Med. 2018, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.T.; Zhao, K.J.; Gao, Q.T.; Ji, Z.N.; Zhu, T.T.; Li, J.; Duan, R.; Cheung, A.W.; Tsim, K.W. Chemical and biological assessment of a Chinese herbal decoction containing Radix Astragali and Radix Angelicae Sinensis: Determination of drug ratio in having optimized properties. J. Agric. Food Chem. 2006, 5, 2767–2774. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.T.; Cheung, J.K.; Choi, R.C.; Cheung, A.W.; Li, J.; Jiang, Z.Y.; Duan, R.; Zhao, K.J.; Ding, A.W.; Dong, T.T.; et al. A Chinese herbal decoction prepared from Radix Astragali and Radix Angelicae Sinensis induces the expression of erythropoietin in cultured Hep3B cells. Planta Med. 2008, 74, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.Y.; Choi, R.C.; Xie, H.Q.; Cheung, A.W.; Guo, A.J.; Leung, K.W.; Chen, V.P.; Bi, C.W.; Zhu, K.Y.; Chan, G.K.; et al. The expression of erythropoietin triggered by Danggui Buxue Tang, a Chinese herbal decoction prepared from Radix Astragali and Radix Angelicae Sinensis, is mediated by the hypoxia-inducible factor in cultured HEK293T cells. J. Ethnopharmacol. 2010, 28, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.G.; Lau, K.M.; Xu, M.L.; Lin, H.Q.; Dong, T.T.; Zheng, K.Y.; Zhao, K.J.; Tsim, K.W. The estrogenic properties of Danggui Buxue Tang, a Chinese herbal decoction, are triggered predominantly by calycosin in MCF-7 cells. J. Ethnopharmacol. 2016, 2, 81–89. [Google Scholar] [CrossRef]
- Lin, H.Q.; Gong, A.G.; Wang, H.Y.; Duan, R.; Dong, T.T.; Zhao, K.J.; Tsim, K.W. Danggui Buxue Tang (Astragali Radix and Angelicae Sinensis Radix) for menopausal symptoms: A review. J. Ethnopharmacol. 2017, 6, 205–210. [Google Scholar] [CrossRef]
- Song, Z.H.; Ji, Z.N.; Lo, C.K.; Dong, T.T.; Zhao, K.J.; Li, O.T.; Haines, C.J.; Kung, S.D.; Tsim, K.W. Chemical and biological assessment of a traditional Chinese herbal decoction prepared from Radix Astragali and Radix Angelicae Sinensis: Orthogonal array design to optimize the extraction of chemical constituents. Planta Med. 2014, 70, 1222–1225. [Google Scholar] [CrossRef]
- Paananen, J.; Fortino, V. An omics perspective on drug target discovery platforms. Brief. Bioinform. 2020, 1, 1937–1953. [Google Scholar] [CrossRef] [Green Version]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]
- Gong, A.G.; Lau, K.M.; Zhang, L.M.; Lin, H.Q.; Dong, T.T.; Tsim, K.W. Danggui Buxue Tang, Chinese herbal decoction containing Astragali Radix and Angelicae Sinensis Radix, induces production of nitric oxide in endothelial cells: Signaling mediated by phosphorylation of endothelial nitric oxide synthase. Planta Med. 2016, 82, 418–423. [Google Scholar] [CrossRef]
- Yu, A.X.; Xu, M.L.; Yao, P.; Kwan, K.K.; Liu, Y.X.; Duan, R.; Dong, T.T.; Ko, R.K.; Tsim, K.W. Corylin, a flavonoid derived from Psoralea Fructus, induces osteoblastic differentiation via estrogen and Wnt/β-catenin signaling pathways. FASEB J. 2020, 34, 4311–4328. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef] [Green Version]
- Choi, R.C.; Gao, Q.T.; Cheung, A.W.; Zhu, J.T.; Lau, F.T.; Li, J.; Li, W.Z.; Chu, G.K.; Duan, R.; Cheung, J.K.; et al. A Chinese herbal decoction, Danggui Buxue Tang, stimulates proliferation, differentiation and gene expression of cultured osteosarcoma cells: Genomic approach to reveal specific gene activation. Evid. Based Complement. Alternat. Med. 2011, 307548. [Google Scholar] [CrossRef]
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef]
- Jun, D.W.; Tak, W.Y.; Bae, S.H.; Lee, Y.J. Recent trends in the treatment of chronic hepatitis C. Korean J. Hepatol. 2012, 18, 22–28. [Google Scholar] [CrossRef]
- Huck, K.; Sens, C.; Wuerfel, C.; Zoeller, C.; Nakchbandi, I.A. The Rho GTPase RAC1 in osteoblasts controls their function. Int. J. Mol. Sci. 2020, 21, 385. [Google Scholar] [CrossRef] [Green Version]
- Dallas, S.L.; Bonewald, L.F. Dynamics of the transition from osteoblast to osteocyte. Ann. N. Y. Acad. Sci. 2020, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Langeland, N. In vitro studies on collagen metabolism in metaphyseal rat bone. A. The effect of pre-treatment with oestradiol-17 beta. Acta Endocrinol. 1975, 80, 775–783. [Google Scholar] [CrossRef]
- Suzuki, A.; Minamide, M.; Iwaya, C.; Ogata, K.; Iwata, J. Role of metabolism in bone development and homeostasis. Int. J. Mol. Sci. 2020, 21, 8992. [Google Scholar] [CrossRef]
- Esen, E.; Lee, S.Y.; Wice, B.M.; Long, F. PTH promotes bone anabolism by stimulating aerobic glycolysis via IGF signaling. J. Bone Miner. Res. 2015, 30, 2137. [Google Scholar] [CrossRef]
- Chang, W.C.; Yen, C.C.; Cheng, C.P.; Wu, Y.T.; Hsu, M.C. 2020, Chinese herbal decoction (Danggui Buxue Tang) supplementation augments physical performance and facilitates physiological adaptations in swimming rats. Pharm. Biol. 2020, 58, 545–552. [Google Scholar] [CrossRef]
- Kwan, K.K.L.; Huang, Y.; Leung, K.W.; Dong, T.T.X.; Tsim, K.W.K. Danggui Buxue Tang, a Chinese herbal decoction containing Astragali Radix and Angelicae Sinensis Radix, modulates mitochondrial bioenergetics in cultured cardiomyoblasts. Front Pharmacol. 2019, 10, 614. [Google Scholar] [CrossRef]
- Meier, U.; Gressner, A.M. Endocrine regulation of energy metabolism: Review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin. Chem. 2004, 50, 1511–1525. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.H.; Zhang, W.L.; Cheung, C.Y.; Tsim, K.W.; Lam, H. Quality control of Danggui Buxue Tang, a traditional Chinese medicine decoction, by 1H-NMR metabolic profiling. Evid. Based Complement. Alternat. Med. 2014, 2014, 567893. [Google Scholar] [CrossRef]
- Duarte, T.M.; Carinhas, N.; Barreiro, L.C.; Carrondo, M.J.; Alves, P.M.; Teixeira, A.P. Metabolic responses of CHO cells to limitation of key amino acids. Biotechnol. Bioeng. 2014, 111, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 2016, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Elliott, J.T.; Tona, A.; Woodward, J.T.; Meuse, C.W.; Elgendy, H.M.; Plant, A.L. Osteoblast cell membrane hybrid bilayers for studying cell-cell interactions. IEE Proc. Nanobiotechnol. 2004, 151, 75–81. [Google Scholar] [CrossRef]
- Bieberich, E. Sphingolipids and lipid rafts: Novel concepts and methods of analysis. Chem. Phys. Lipids 2018, 114–131. [Google Scholar] [CrossRef]
- Wang, H.; Ye, J. Regulation of energy balance by inflammation: Common theme in physiology and pathology. Rev. Endocr. Metab. Disord. 2015, 16, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Frey, J.L.; Kim, S.P.; Li, Z.; Wolfgang, M.J.; Riddle, R.C. β-Catenin directs long-chain fatty acid catabolism in the osteoblasts of male mice. Endocrinology 2018, 159, 272–284. [Google Scholar] [CrossRef] [Green Version]
- Rong, P.; Zhang, X.; Liu, Q.; Ma, R.; Li, L.; Yan, H.; Xin, D.; Xue, Z.; Ren, X.; Wu, Z.; et al. Clinical practices of traditional Chinese medicine for acute upper respiratory tract infection in children. J. Tradit. Chin. Med. 2018, 38, 504–510. [Google Scholar]
- Zhu, K.Y.; Xu, S.L.; Choi, R.C.; Yan, A.L.; Dong, T.T.; Tsim, K.W. Kai-Xin-San, a Chinese herbal decoction containing Ginseng Radix et Rhizoma, Polygalae Radix, Acori Tatarinowii Rhizoma, and Poria, stimulates the expression and secretion of neurotrophic factors in cultured astrocytes. Evid. Based Complement. Alternat. Med. 2013, 731385. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.Y.; Choi, R.C.; Guo, A.J.; Bi, C.W.; Zhu, K.Y.; Du, C.Y.; Zhang, Z.X.; Lau, D.T.; Dong, T.T.; Tsim, K.W. The membrane permeability of Astragali Radix-derived formononetin and calycosin is increased by Angelicae Sinensis Radix in Caco-2 cells: A synergistic action of an ancient herbal decoction Danggui Buxue Tang. J. Pharm. Biomed. Anal. 2012, 2012, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xue, Z.; Zhao, L.; Yang, X.; Yang, Y.; Zhou, X.; Feng, S.; Chen, K. Calycosin stimulates the osteogenic differentiation of rat calvarial osteoblasts by activating the IGF1R/PI3K/Akt signaling pathway. Cell Biol. Int. 2019, 43, 323–332. [Google Scholar] [CrossRef]
- Gautam, A.K.; Bhargavan, B.; Tyagi, A.M.; Srivastava, K.; Yadav, D.K.; Kumar, M.; Singh, A.; Mishra, J.S.; Singh, A.B.; Sanyal, S.; et al. Differential effects of formononetin and cladrin on osteoblast function, peak bone mass achievement and bioavailability in rats. J. Nutr. Biochem. 2011, 22, 318–327. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwan, K.K.L.; Wong, T.Y.; Yu, A.X.D.; Dong, T.T.X.; Lam, H.H.L.; Tsim, K.W.K. The Osteogenic Function of Danggui Buxue Tang, a Herbal Decoction Containing Astragali Radix and Angelicae Sinensis Radix, Is Optimized by Boiling the Two Herbs Together: Signaling Analyses Revealed by Systems Biology. Processes 2021, 9, 1119. https://doi.org/10.3390/pr9071119
Kwan KKL, Wong TY, Yu AXD, Dong TTX, Lam HHL, Tsim KWK. The Osteogenic Function of Danggui Buxue Tang, a Herbal Decoction Containing Astragali Radix and Angelicae Sinensis Radix, Is Optimized by Boiling the Two Herbs Together: Signaling Analyses Revealed by Systems Biology. Processes. 2021; 9(7):1119. https://doi.org/10.3390/pr9071119
Chicago/Turabian StyleKwan, Kenneth K L, Tin Yan Wong, Anna X. D. Yu, Tina T. X. Dong, Henry H. L. Lam, and Karl W. K. Tsim. 2021. "The Osteogenic Function of Danggui Buxue Tang, a Herbal Decoction Containing Astragali Radix and Angelicae Sinensis Radix, Is Optimized by Boiling the Two Herbs Together: Signaling Analyses Revealed by Systems Biology" Processes 9, no. 7: 1119. https://doi.org/10.3390/pr9071119




