Effect of Coated Cow Dung on Fluidization Reduction of Fine Iron Ore particles
Abstract
:1. Introduction
2. Experiment
2.1. Experimental Materials
2.2. Experimental Equipment
2.3. Experimental Scheme and Steps
3. Experimental Results and Discussion
3.1. Optimum Operating Parameters
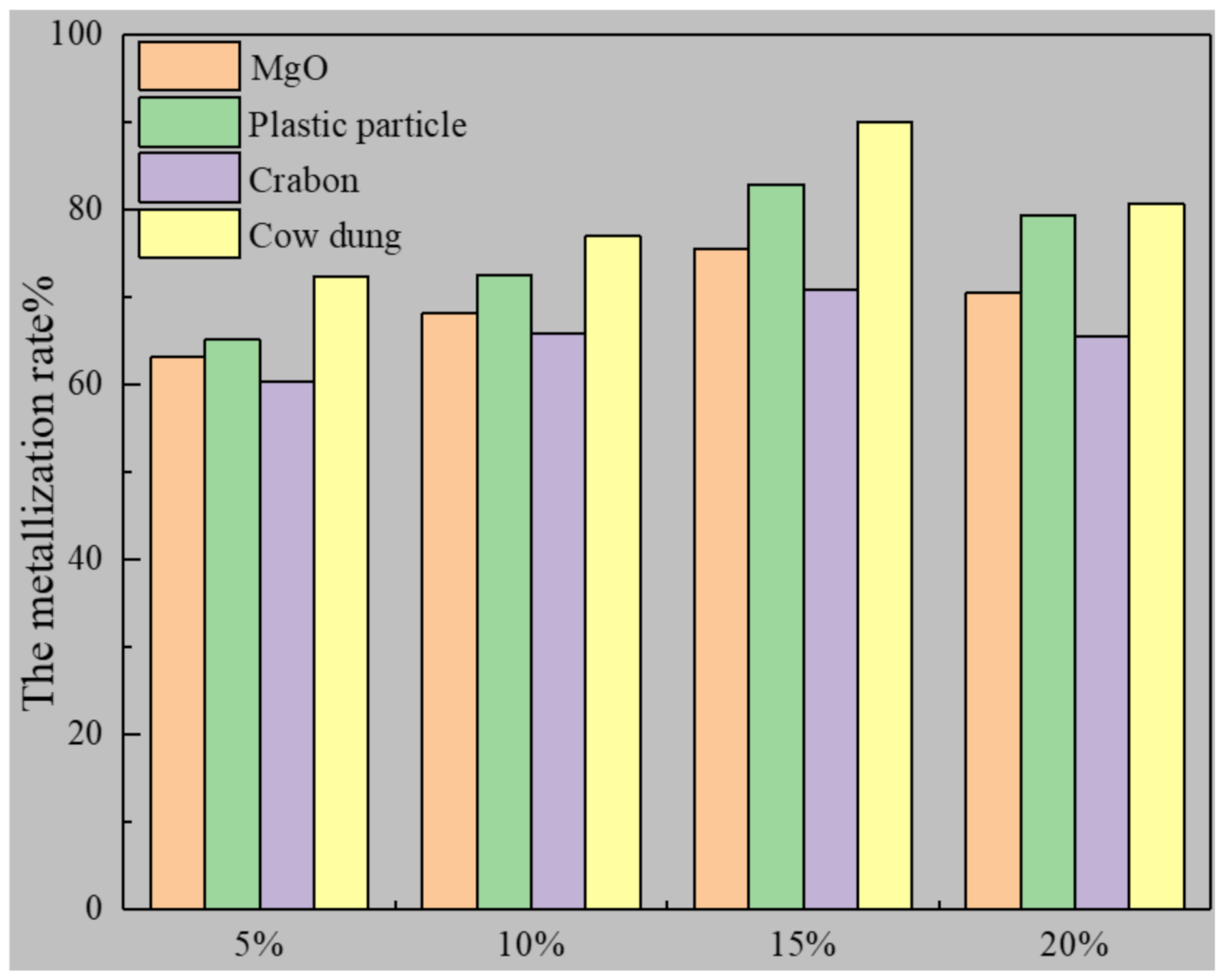
3.2. Study of Inhibition of Bond Loss by Cow Dung
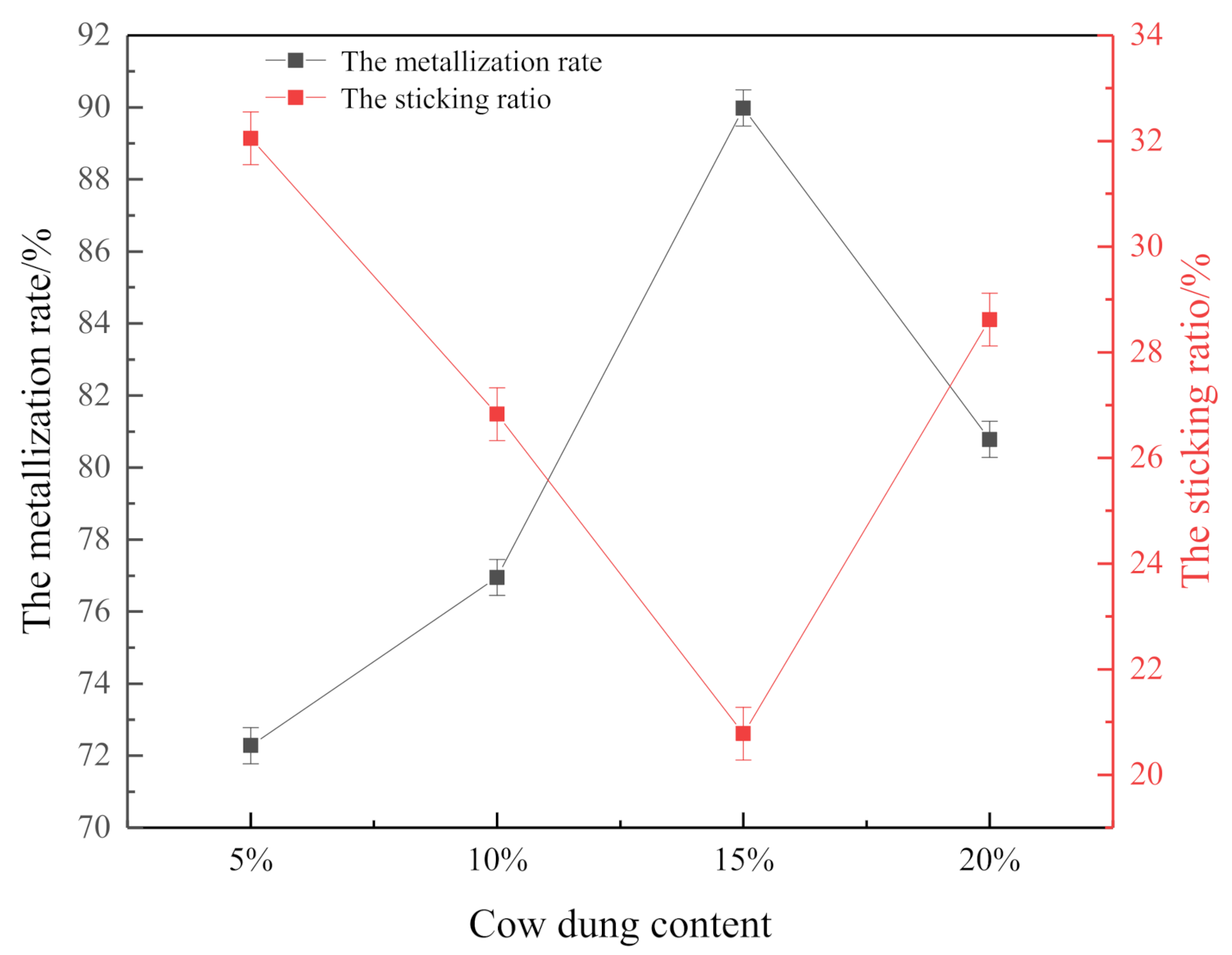
3.2.1. Effect of Coated Cow Dung on the Metallization Rate and Sticking Ratio
3.2.2. Effect of Coated Cow Dung on Phase Structure
4. Analysis of the Mechanism of Fine Iron Ore Particles Bond Loss
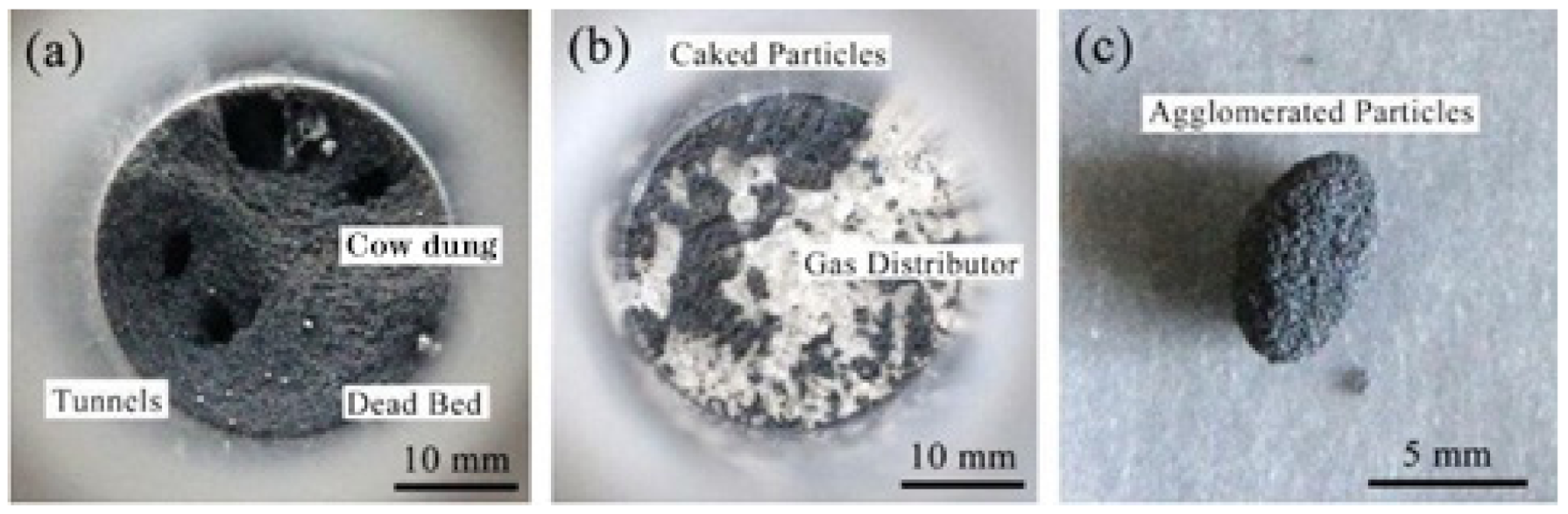
4.1. Characteristics of Fe2O3 Particles Bond Loss Behavior in Fluidization Reduction
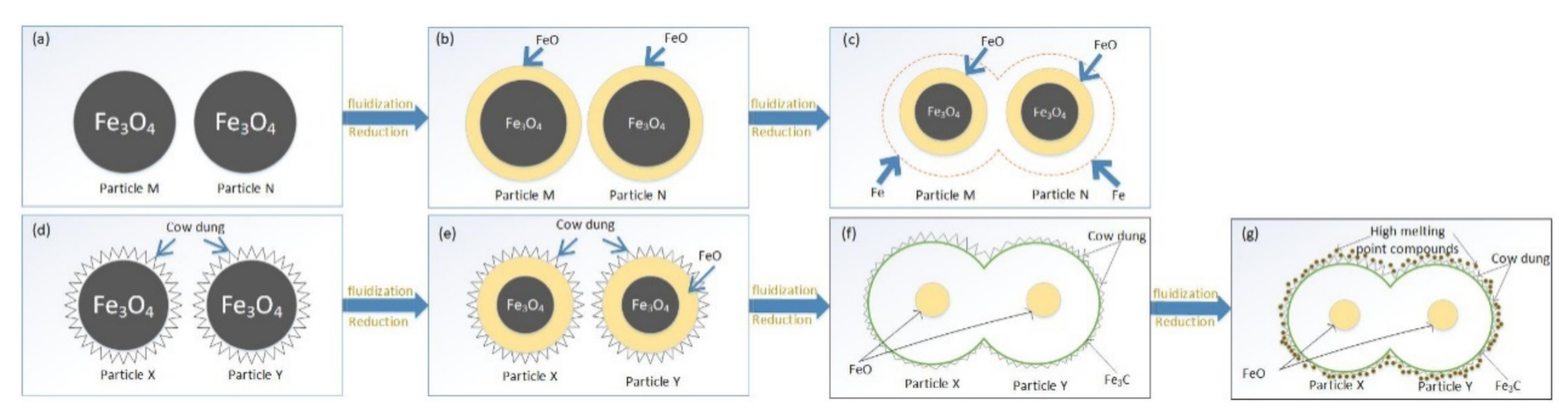
4.2. Mechanism of the Inhibition of the Loss of Fine Iron Ore by Cow Dung Coating
4.2.1. Effects of Cow Dung on Thermodynamics and Kinetics
4.2.2. Model of Inhibition of Fine Iron Ore Particles Bond Loss by Cow Dung
5. Conclusions
- The addition of a coating component increases the metallization rate, reduces the sticking ratio, and improves the fluidization state.
- The optimal operating parameters for the addition of cow dung are as follows: reduction temperature of 923–1023 K, reducing gas velocity of 0.6 m/s, reduction time of 40–60 min, reduction pressure of 0.2 MPa, H2 as the reducing gas, and cow dung content of 16%.
- At high temperature, cow dung decomposes a large amount of hydrogen that reacts with iron oxide, absorbs a high amount of energy and inhibits the growth of iron whiskers, thus inhibiting the loss of adhesion and improving the fluidization quality.
- At high temperature, cow dung reacts with the oxide in fine iron ore particles to form a high melting point substance, thus forming a certain isolation layer that reduces the likelihood of direct contact of the reduced iron between the ore powder particles and effectively inhibits the bond loss.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pang, P.M.; Guo, Z.P. Reductionof1—3 mm iron ore by CO on fluidized bed. J. Iron Steel Res. Int. 2011, 18, 1–5. [Google Scholar] [CrossRef]
- Zheng, Z.; Lin, L.C.; Chen, Z.W.; Lim, J.; Grace, J. A dual-scale lattice gas automata model for gas–solid two-phase flow in bubbling fluidized beds. Comput. Math. Appl. 2011, 61, 3593–3605. [Google Scholar] [CrossRef] [Green Version]
- Dang, J.; Zhang, G.H.; Chou, K.C. Kinetics and mechanism of hydrogen reduction of ilmenite powders. J. Alloys Compd. 2015, 619, 443–451. [Google Scholar] [CrossRef]
- Bonalde, A.; Henriquez, A.; Manrique, M. Kinetic analysis of the iron oxide reduction using hydrogen-carbon monoxide mixtures as reducing agent. ISIJ Int. 2005, 45, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Hou, B.L.; Zhang, H.Y.; Li, H.Z.; Zhu, Q.S. Study on Kinetics of Iron Oxide Reduction by Hydrogen. Chin. J. Chem. Eng. 2012, 20, 10–17. [Google Scholar] [CrossRef]
- Liang, Z.J.; Yusheng, Z.Z.Z.; Yu, A. Preliminary calculation of reduction kinetics of iron concentrate powder in fluidized bed. J. Process Eng. 2008, 8, 119–122. [Google Scholar]
- Bohn, C.D.; Cleeton, J.P.; Ller, C.R.M.; Davidson, J.F.; Hayhurst, A.N.; Dennis, S.A.S.S. The Kinetics of the reduction of iron oxide by carbon monoxide mixed with carbon dioxide. AICHE J. 2010, 56, 1016–1029. [Google Scholar] [CrossRef]
- Chironea, R.; Massimilla, L.; Russoa, S. Bubble-free fluidization of a cohesive powder in an acoustic field. Chem. Eng. Sci. 1993, 48, 41–52. [Google Scholar] [CrossRef]
- Zhou, L.; Diao, R.; Zhou, T.; Wang, H.; Kage, H.; Mawatari, Y. Behavior of magnetic Fe3O4 nano-particles in magnetically assisted gas-fluidized beds. Adv. Powder Technol. 2011, 22, 427–432. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, H. Research and application of magnetic field fluidization technology. Chem. Metall. 1995, 16, 271–281. [Google Scholar]
- Xu, Q.; Gu, Z.; Li, Z.; Zhang, Y.; Wang, H. Effects of pressure and plastic addition on sticking of fine iron ore particles in fluidized bed reduction. J. Iron Steel Res. Int. 2020, prepublish. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Z.; Gu, Z.; Faux, D.; Cardea, S. Experimental Investigation of the Fluidization Reduction Characteristics of Iron Particles Coated with Carbon Powder under Pressurized Conditions. Molecules 2020, 25, 1810. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Liu, Z.; Li, Z.; Wang, J.; Zhou, L. The Effect of Carbon Dissection of Waste Plastics on Inhibiting the Adhesion of Fine Iron Ore Particles during Hydrogen Reduction. Metals 2018, 8, 523. [Google Scholar] [CrossRef] [Green Version]
- Geldart, D. Type of fluidization. Powder Technol. 1973, 7, 285–292. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Z.; Guo, Z.; Tang, Q. Defluidization behavior of iron powders at elevated temperature: Influence of fluidizing gas and particle adhesion. Powder Technol. 2012, 230, 225–231. [Google Scholar] [CrossRef]
- Cao, H.; Li, G.; Huang, S.; Li, Y.; Liu, Y.; Xin, Y.; Yuan, Q. Kinetic characteristics of cattle manure pyrolysis based on equal conversion rate method. Acta Sol. Sin. 2015, 36, 1773–1778. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-depth investigation of biomass pyrolysis based on threemajor components:Hemicelluloses, cellulose and lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Li, K.; Zhang, S.; Chen, H. Effectof temperature on product property during biomass ploy-generation based on cotton stalk pyrolysis. Proc. CSEE 2012, 17, 117–124. [Google Scholar]
- Li, Q.; Rudolph, V.; Peukert, W. London-van der Waals adhesiveness of rough particles. Powder Technol. 2006, 161, 248–255. [Google Scholar] [CrossRef]
- John, D.H.S.; Hayes, P.C. Microstructural features produced by the reduction of wustite in H2/H2O gas mixtures. Metall. Trans. B 1982, 13, 117–124. [Google Scholar] [CrossRef]
- Farren, M.; Matthew, S.P.; Hayes, P.C. Reduction of solid wustite in H2/H2O/CO/CO2 gas mixtures. Metall. Trans. B 1990, 21, 135–139. [Google Scholar] [CrossRef]


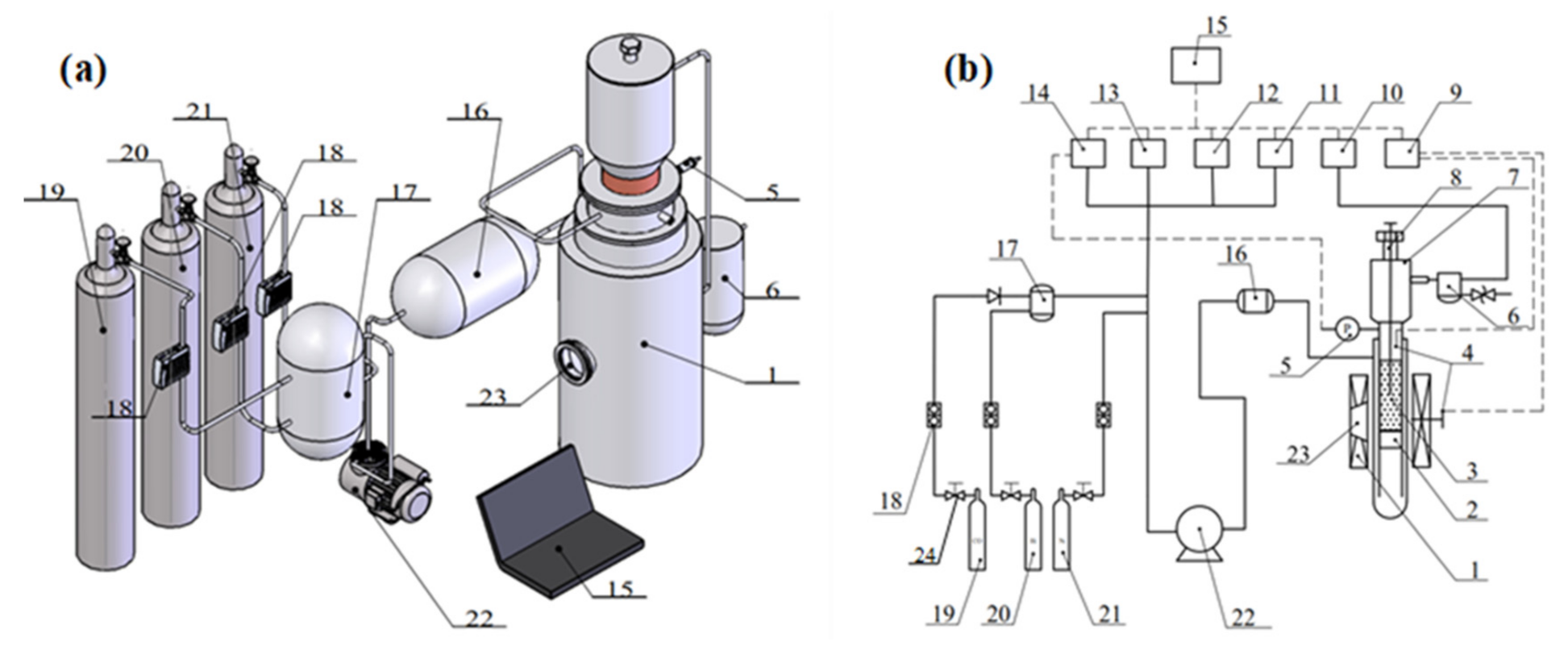





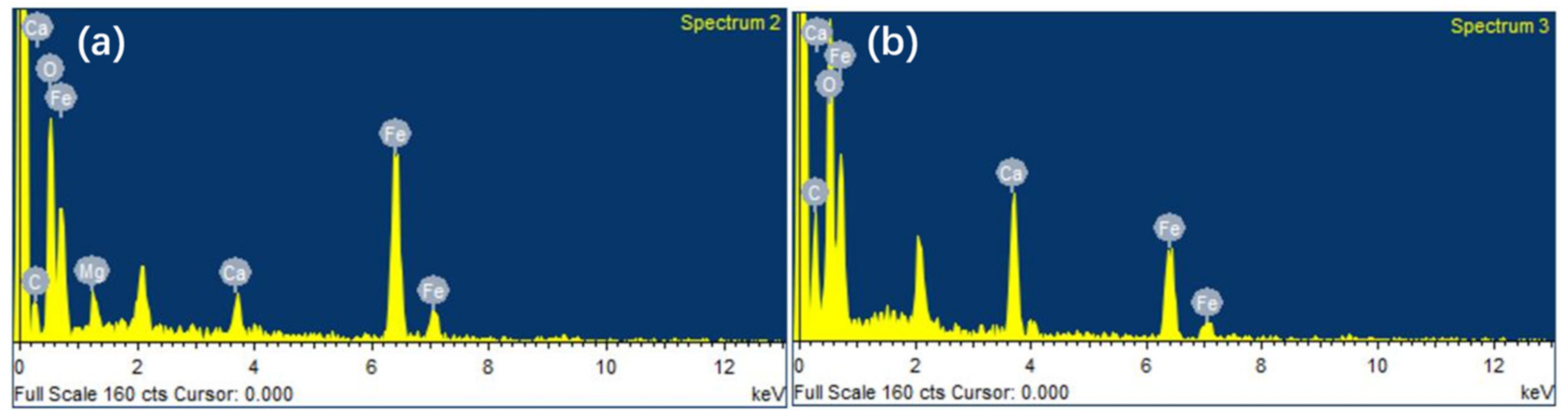

| Composition | TFe | SiO2 | CaO | MgO | Al2O3 | S | P |
| Content | 62.5 | 4.37 | 0.07 | 0.10 | 2.08 | 0.099 | 0.088 |
| Composition | C | N | H | S | O |
| Content | 36.21 | 2.39 | 5.14 | 1.32 | 54.95 |
| A Temperature/K | B Reduction Time/min | C The Reducing Pressure/MPa | D Linear Velocity/m/s | E Type of Reducing Gas | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | B1 | B2 | B3 | C1 | C2 | C3 | D1 | D2 | D3 | E1 | E2 | E3 |
| 923 | 1023 | 1123 | 20 | 40 | 60 | 0.1 | 0.2 | 0.3 | 0.4 | 0.6 | 0.8 | H2 | CO | Mixture |
| Reduction Temperature/K | Reduction Time/min | Reduction Pressure/MPa | Linear Velocity/m/s | Type of Reducing Gas | Metallization Rate/% | Sticking Ratio /% | |
|---|---|---|---|---|---|---|---|
| 1 | 923 | 20 | 0.1 | 0.4 | H2 | 77.67 | 27.98 |
| 2 | 923 | 40 | 0.2 | 0.6 | CO | 51.17 | 20.12 |
| 3 | 923 | 60 | 0.3 | 0.8 | H2 + CO | 73.58 | 29.36 |
| 4 | 1023 | 20 | 0.1 | 0.6 | CO | 56.87 | 26.36 |
| 5 | 1023 | 40 | 0.2 | 0.8 | H2 + CO | 69.04 | 32.47 |
| 6 | 1023 | 60 | 0.3 | 0.4 | H2 | 83.44 | 48.63 |
| 7 | 1123 | 20 | 0.2 | 0.4 | H2 + CO | 65.34 | 33.67 |
| 8 | 1123 | 40 | 0.3 | 0.6 | H2 | 91.1 | 45.73 |
| 9 | 1123 | 60 | 0.1 | 0.8 | CO | 49.2 | 38.92 |
| 10 | 923 | 20 | 0.3 | 0.8 | CO | 40.29 | 17.98 |
| 11 | 923 | 40 | 0.1 | 0.4 | H2 + CO | 63.15 | 25.58 |
| 12 | 923 | 60 | 0.2 | 0.6 | H2 | 89.61 | 35.59 |
| 13 | 1023 | 20 | 0.2 | 0.8 | H2 | 89.7 | 35.15 |
| 14 | 1023 | 40 | 0.3 | 0.4 | CO | 60.46 | 24.97 |
| 15 | 1023 | 60 | 0.1 | 0.6 | H2 + CO | 67.69 | 34.25 |
| 16 | 1123 | 20 | 0.3 | 0.6 | H2 + CO | 67.98 | 34.53 |
| 17 | 1123 | 40 | 0.1 | 0.8 | H2 | 87.45 | 46.64 |
| 18 | 1123 | 60 | 0.2 | 0.4 | CO | 62.87 | 37.16 |
| Factor | Indicators | A | B | C | D | E |
|---|---|---|---|---|---|---|
| Metallization Rate % | K1 | 395.47 | 397.85 | 402.03 | 412.93 | 518.97 |
| K2 | 427.2 | 422.37 | 427.73 | 424.42 | 320.86 | |
| K3 | 423.94 | 426.39 | 416.85 | 409.26 | 406.78 | |
| k1 | 65.91 | 66.31 | 67.01 | 68.82 | 86.50 | |
| k2 | 71.20 | 70.40 | 71.29 | 70.74 | 53.48 | |
| k3 | 70.66 | 71.07 | 69.48 | 68.21 | 67.80 | |
| R | 5.29 | 4.76 | 4.28 | 2.53 | 33.02 | |
| Primary and Secondary Factors EABCD Optimization Scheme E1A2B3C2D2 | ||||||
| Factor | Indicators | A | B | C | D | E |
|---|---|---|---|---|---|---|
| Sticking Ratio % | K1 | 156.61 | 175.67 | 199.73 | 197.99 | 239.72 |
| K2 | 201.83 | 195.51 | 194.16 | 196.58 | 165.51 | |
| K3 | 236.65 | 223.91 | 201.2 | 200.52 | 189.86 | |
| k1 | 26.10 | 29.28 | 33.29 | 33.00 | 39.95 | |
| k2 | 33.64 | 32.59 | 32.36 | 32.76 | 27.59 | |
| k3 | 39.44 | 37.32 | 33.53 | 33.42 | 31.64 | |
| R | 13.34 | 8.04 | 1.17 | 0.66 | 12.37 | |
| Primary and Secondary Factors AEBCD Optimization Scheme A1E2B1C2D2 | ||||||
| Content | 5% | 10% | 15% | 20% | |
|---|---|---|---|---|---|
| Component | |||||
| MgO | 63.11 | 68.22 | 75.53 | 70.58 | |
| Plastic Particles | 65.16 | 72.56 | 82.95 | 79.42 | |
| Carbon | 60.34 | 65.88 | 70.89 | 65.54 | |
| Cow dung | 72.28 | 76.95 | 89.98 | 80.78 | |
| Content | 5% | 10% | 15% | 20% | |
|---|---|---|---|---|---|
| Component | |||||
| MgO | 39.05 | 30.54 | 25.26 | 35.78 | |
| Plastic particles | 35.58 | 28.46 | 23.41 | 32.64 | |
| Carbon | 40.55 | 36.74 | 28.04 | 38.89 | |
| Cow dung | 32.05 | 26.83 | 20.78 | 28.62 | |
| Content | 10% | 12% | 14% | 16% | 18% | 20% | |
|---|---|---|---|---|---|---|---|
| Index | |||||||
| Metallization Rate | 76.95 | 80.84 | 85.78 | 91.35 | 84.12 | 80.78 | |
| Sticking Ratio | 26.83 | 24.32 | 21.63 | 20.02 | 24.68 | 28.62 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Gu, Z.; Wan, Z.; Huangfu, M.; Meng, Q.; Liao, Z.; Wu, B. Effect of Coated Cow Dung on Fluidization Reduction of Fine Iron Ore particles. Processes 2021, 9, 1175. https://doi.org/10.3390/pr9071175
Xu Q, Gu Z, Wan Z, Huangfu M, Meng Q, Liao Z, Wu B. Effect of Coated Cow Dung on Fluidization Reduction of Fine Iron Ore particles. Processes. 2021; 9(7):1175. https://doi.org/10.3390/pr9071175
Chicago/Turabian StyleXu, Qiyan, Zhanghan Gu, Ziwei Wan, Mingzhu Huangfu, Qingmin Meng, Zhiyou Liao, and Baoguo Wu. 2021. "Effect of Coated Cow Dung on Fluidization Reduction of Fine Iron Ore particles" Processes 9, no. 7: 1175. https://doi.org/10.3390/pr9071175
APA StyleXu, Q., Gu, Z., Wan, Z., Huangfu, M., Meng, Q., Liao, Z., & Wu, B. (2021). Effect of Coated Cow Dung on Fluidization Reduction of Fine Iron Ore particles. Processes, 9(7), 1175. https://doi.org/10.3390/pr9071175





