Study of the Microstructural and First Hydrogenation Properties of TiFe Alloy with Zr, Mn and V as Additives
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Morphology
3.2. Crystal Structure
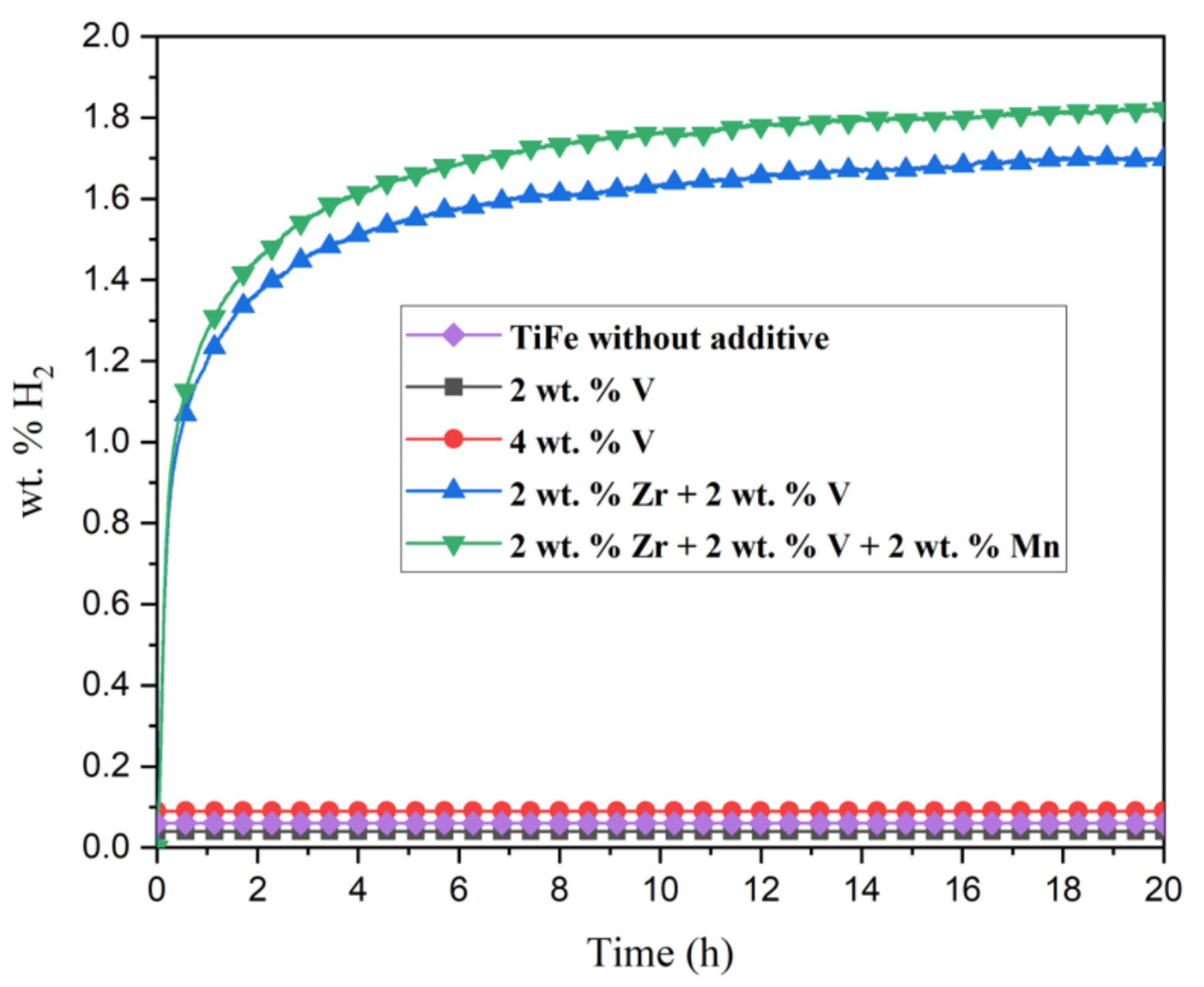
3.3. Activation Kinetics
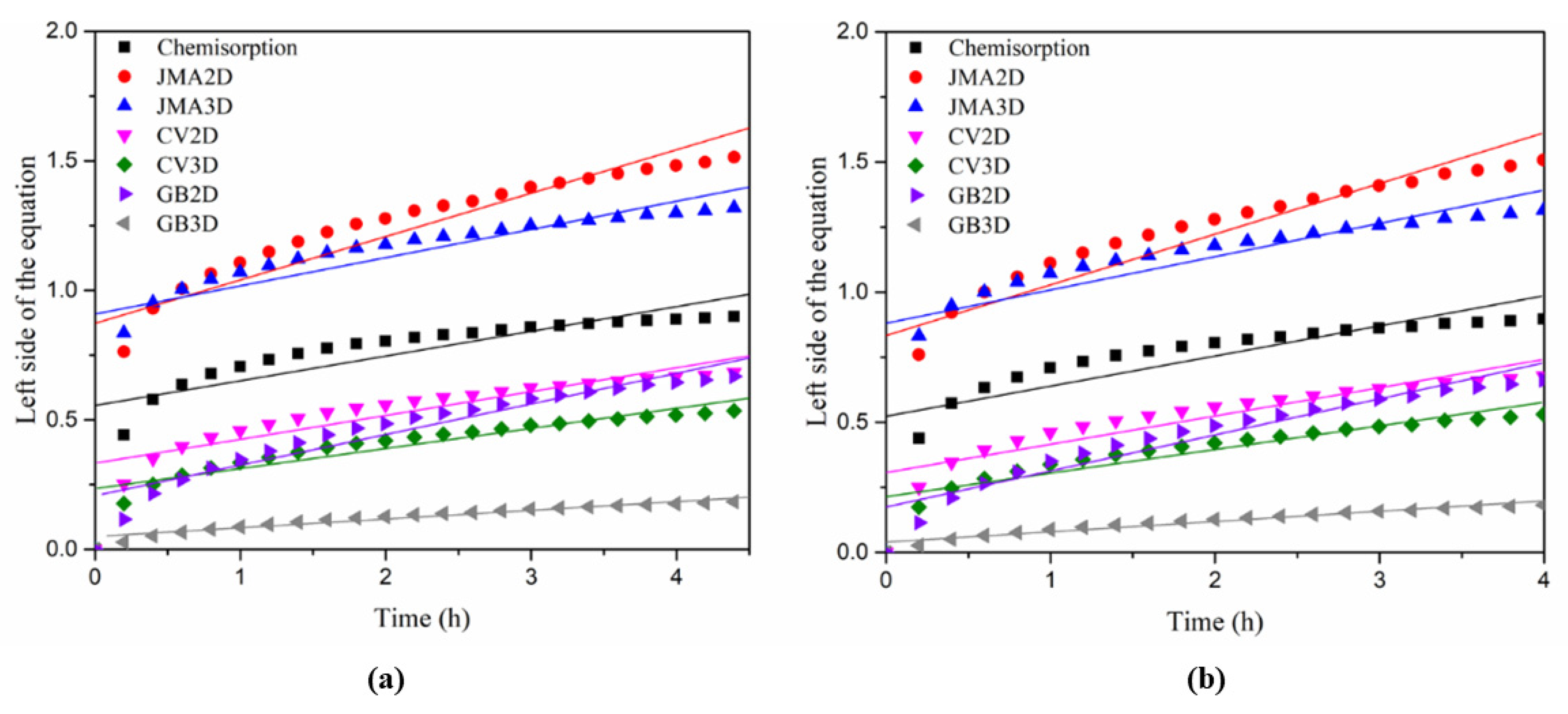
3.4. Rate-Limiting Step of First Hydrogenation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Colbe, J.B.; Ares, J.-R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Endo, N.; Suzuki, S.; Goshome, K.; Maeda, T. Operation of a bench-scale TiFe-based alloy tank under mild conditions for low-cost stationary hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 5246–5251. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Formation and properties of iron titanium hydride. Inorg. Chem. 1974, 13, 218–222. [Google Scholar] [CrossRef]
- Sandrock, G.D.; Goodell, P.D. Surface poisoning of LaNi5, FeTi and (Fe,Mn)Ti by O2, Co and H2O. J. Less Common Met. 1980, 73, 161–168. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuo, M.; Emami, H.; Itano, S.; Alhamidi, A.; Staykov, A.; Smith, D.J.; Orimo, S.-I.; Akiba, E.; Horita, Z. Impact of severe plastic deformation on microstructure and hydrogen storage of titanium-iron-manganese intermetallics. Scr. Mater. 2016, 124, 108–111. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuda, J.; Yanagida, A.; Akiba, E.; Horita, Z. Activation of TiFe for hydrogen storage by plastic deformation using groove rolling and high-pressure torsion: Similarities and differences. Int. J. Hydrogen Energy 2014, 39, 15589–15594. [Google Scholar] [CrossRef]
- Falcão, R.B.; Dammann, E.D.C.C.; Rocha, C.J.; Durazzo, M.; Ichikawa, R.U.; Martinez, L.G.; Botta, W.J.; Leal Neto, R.M. An alternative route to produce easily activated nanocrystalline TiFe powder. Int. J. Hydrogen Energy 2018, 43, 16107–16116. [Google Scholar] [CrossRef]
- Hosni, B.; Fenineche, N.; ElKedim, O.; Khaldi, C.; Lamloumi, J. Structural and electrochemical properties of TiFe alloys synthesized by ball milling for hydrogen storage. J. Solid State Electrochem. 2018, 22, 17–29. [Google Scholar] [CrossRef]
- Aoyagi, H.; Aoki, K.; Masumoto, T. Effect of ball milling on hydrogen absorption properties of FeTi, Mg2Ni and LaNi5. J. Alloys Compd. 1995, 231, 804–809. [Google Scholar] [CrossRef]
- Emami, H.; Edalati, K.; Matsuda, J.; Akiba, E.; Horita, Z. Hydrogen storage performance of TiFe after processing by ball milling. Acta Mater. 2015, 88, 190–195. [Google Scholar] [CrossRef]
- Haraki, T.; Oishi, K.; Uchida, H.; Miyamoto, Y.; Abe, M.; Kokaji, T.; Uchida, S. Properties of hydrogen absorption by nano-structured FeTi alloys. Int. J. Mater. Res. 2008, 99, 507–512. [Google Scholar] [CrossRef]
- Bellosta von Colbe, J.M.; Puszkiel, J.; Capurso, G.; Franz, A.; Benz, H.U.; Zoz, H.; Klassen, T.; Dornheim, M. Scale-up of milling in a 100 L device for processing of TiFeMn alloy for hydrogen storage applications: Procedure and characterization. Int. J. Hydrogen Energy 2019, 44, 29282–29290. [Google Scholar] [CrossRef]
- Jain, P.; Gosselin, C.; Huot, J. Effect of Zr, Ni and Zr7Ni10 alloy on hydrogen storage characteristics of TiFe alloy. Int. J. Hydrogen Energy 2015, 40, 16921–16927. [Google Scholar] [CrossRef]
- Lv, P.; Huot, J. Hydrogen storage properties of Ti0.95FeZr0.05, TiFe0.95Zr0.05 and TiFeZr0.05 alloys. Int. J. Hydrogen Energy 2016, 41, 22128–22133. [Google Scholar] [CrossRef]
- Patel, A.K.; Sharma, P.; Huot, J. Effect of annealing on microstructure and hydrogenation properties of TiFe + X wt% Zr (X = 4, 8). Int. J. Hydrogen Energy 2018, 43, 6238–6243. [Google Scholar] [CrossRef]
- Guéguen, A.; Latroche, M. Influence of the addition of vanadium on the hydrogenation properties of the compounds TiFe0.9Vx and TiFe0.8Mn0.1Vx (x = 0, 0.05 and 0.1). J. Alloys Compd. 2011, 509, 5562–5566. [Google Scholar] [CrossRef]
- Chung, H.S.; Lee, J.Y. Effect of partial substitution of Mn and Ni for Fe in FeTi on hydriding kinetics. Int. J. Hydrogen Energy 1986, 11, 335–339. [Google Scholar] [CrossRef]
- Lee, S.M.; Perng, T.P. Effect of the second phase on the initiation of hydrogenation of TiFe1−xMx (M = Cr,Mn) alloys. Int. J. Hydrogen Energy 1994, 19, 259–263. [Google Scholar] [CrossRef]
- Ali, W.; Hao, Z.; Li, Z.; Chen, G.; Wu, Z.; Lu, X.; Li, C. Effects of Cu and Y substitution on hydrogen storage performance of TiFe0.86Mn0.1Y0.1−xCux. Int. J. Hydrogen Energy 2017, 42, 16620–16631. [Google Scholar] [CrossRef]
- Lee, S.M.; Perng, T.P. Correlation of substitutional solid solution with hydrogenation properties of TiFe1-xMx (M = Ni, Co, Al) alloys. J. Alloys Compd. 1999, 291, 254–261. [Google Scholar] [CrossRef]
- Patel, A.K.; Duguay, A.; Tougas, B.; Schade, C.; Sharma, P.; Huot, J. Microstructure and first hydrogenation properties of TiFe alloy with Zr and Mn as additives. Int. J. Hydrogen Energy 2020, 45, 787–797. [Google Scholar] [CrossRef]
- Patel, A.K.; Tougas, B.; Sharma, P.; Huot, J. Effect of cooling rate on the microstructure and hydrogen storage properties of TiFe with 4 wt% Zr as an additive. J. Mater. Res. Technol. 2019, 8, 5623–5630. [Google Scholar] [CrossRef]
- Contieri, R.J.; Lopes, E.S.N.; Taquire de La Cruz, M.; Costa, A.M.; Afonso, C.R.M.; Caram, R. Microstructure of directionally solidified Ti–Fe eutectic alloy with low interstitial and high mechanical strength. J. Cryst. Growth 2011, 333, 40–47. [Google Scholar] [CrossRef]
- Dong, C.; Hei, Z.K.; Wang, L.B.; Song, Q.H.; Wu, Y.K.; Kuo, K.H. A new icosahedral quasicrystal in rapidly solidified FeTi2. Scr. Metall. Mater. 1986, 20, 1155–1158. [Google Scholar] [CrossRef]
- Fedorov, T.F.; Kuzma, Y.B. The Titanium-Iron-Boron System. Inorg. Mater. 1967, 8, 1307–1308. [Google Scholar] [CrossRef]
- Faisal, M.; Suh, J.-Y.; Lee, Y.-S. Understanding first cycle hydrogenation properties of Ti–Fe–Zr ternary alloys. Int. J. Hydrogen Energy 2021, 46, 4241–4251. [Google Scholar] [CrossRef]
- Cheary, R.W.; Coelho, A.A.; Cline, J.P. Fundamental Parameters Line Profile Fitting in Laboratory Diffractometers. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 1–25. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Mintz, M.H.; Zeiri, Y. Hydriding kinetics of powders. J. Alloys Compd. 1995, 216, 159–175. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. I: General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Pang, Y.; Li, Q. A review on kinetic models and corresponding analysis methods for hydrogen storage materials. Int. J. Hydrogen Energy 2016, 41, 18072–18087. [Google Scholar] [CrossRef]
- Lv, P.; Guzik, M.N.; Sartori, S.; Huot, J. Effect of ball milling and cryomilling on the microstructure and first hydrogenation properties of TiFe+4wt.% Zr alloy. J. Mater. Res. Technol. 2019, 8, 1828–1834. [Google Scholar] [CrossRef]
- Ulate-Kolitsky, E.; Tougas, B.; Neumann, B.; Schade, C.; Huot, J. First hydrogenation of mechanically processed TiFe-based alloy synthesized by gas atomization. Int. J. Hydrogen Energy 2021, 46, 7381–7389. [Google Scholar] [CrossRef]


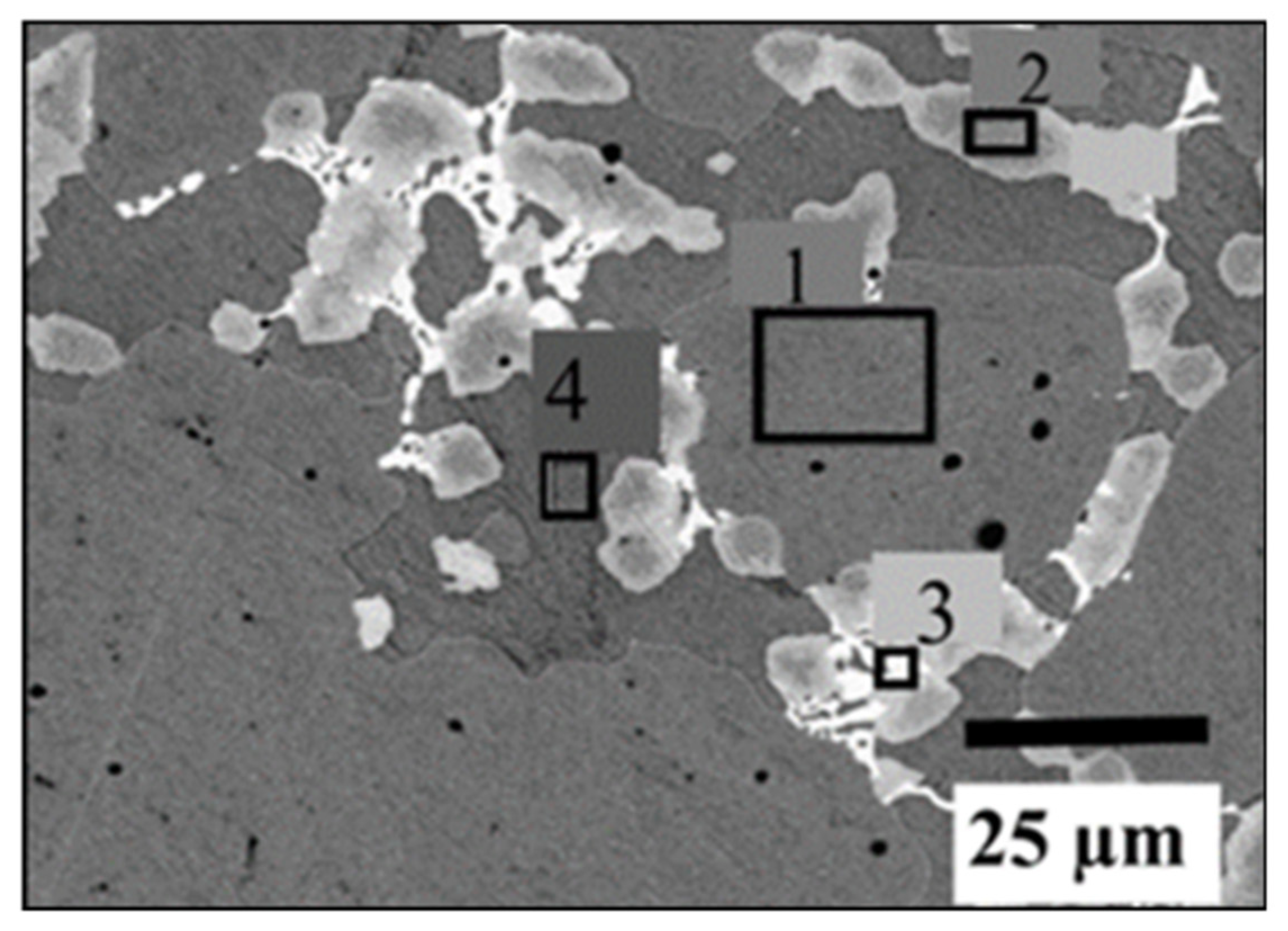


| Alloy | Ti (at.%) | Fe (at.%) | Zr (at.%) | V (at.%) | Mn (at.%) | |
|---|---|---|---|---|---|---|
| TiFe+ 2 wt.% V | Nominal composition Measurement | 49 49 | 49 48 | 2 2 | ||
| TiFe+ 4 wt.% V | Nominal composition Measurement | 48 49 | 48 47 | 4 4 | ||
| TiFe+ 2 wt.% Zr + 2 wt.% V | Nominal composition Measurement | 48 54 | 48 43 | 1 1 | 2 2 | |
| TiFe+ 2 wt.% Zr + 2 wt.% Mn + 2 wt.% V | Nominal composition Measurement | 48 53 | 47 41 | 1 1 | 2 2 | 2 2 |
| Alloys | Ti (at.%) | Fe (at.%) | V (at.%) |
|---|---|---|---|
| TiFe + 2 wt.% V | |||
| Area 1 (grey phase) | 49 | 48 | 3 |
| Area 2 (light grey phase) | 61 | 34 | 6 |
| Area 3 (dark grey phase) | 72 | 21 | 7 |
| TiFe + 4 wt.% V | |||
| Area 1 (grey phase) | 48 | 49 | 4 |
| Area 2 (light grey phase) | 58 | 34 | 8 |
| Area 3 (dark grey phase) | 67 | 22 | 11 |
| Area 4 (bright phase, TiFe2) | 38 | 56 | 6 |
| Alloy | Ti (at.%) | Fe (at.%) | V (at.%) | Zr (at.%) |
|---|---|---|---|---|
| TiFe + 2 wt.% Zr + 2 wt.% V | ||||
| Area 1(grey phase) | 50 | 48 | 2 | |
| Area 2 (light grey phase) | 61 | 33 | 4 | 3 |
| Area 3 (bright phase) | 54 | 29 | 4 | 14 |
| Area 4 (dark grey phase) | 72 | 22 | 5 | 2 |
| Alloy | Ti (at.%) | Fe (at.%) | V (at.%) | Zr (at.%) | Mn (at.%) |
|---|---|---|---|---|---|
| TiFe + 2 wt.% Zr + 2 wt.% V + 2 wt.% Mn | |||||
| Area 1 (grey phase) | 50 | 47 | 2 | <1 | 2 |
| Area 2 (light grey phase) | 57 | 32 | 3 | 7 | 2 |
| Area 3 (bright phase) | 45 | 33 | 5 | 14 | 3 |
| Area 4 (dark grey phase) | 78 | 17 | 5 | 3 | 2 |
| Phase Name | Phase Abundance (%) | Lattice Parameter (Å) | Cell Volume (Å3) | Crystallite Size (nm) | Microstrain (%) |
|---|---|---|---|---|---|
| TiFe without additive [21] | |||||
| TiFe | 93(1) | a = 2.9773(2) | 26.39(6) | 31(1) | 0.08(4) |
| TiFe2 | 7(1) | a = 4.709(2) c = 7.968(1) | 153.0(2) | 36(2) | --- |
| 2 wt.% V | |||||
| TiFe | 90(2) | a = 2.9810(4) | 26.49(1) | 35(2) | 0.145(3) |
| (Ti,V)2Fe | 10(2) | a = 10.533(3) | 1168(1) | 18(3) | --- |
| 4 wt.% V | |||||
| TiFe | 91(2) | a = 2.9806(4) | 26.48(1) | 29(1) | 0.161(4) |
| (Ti,V)2Fe | 9(1) | a = 10.532(3) | 1168(1) | 18(3) | --- |
| 2 wt.% Zr + 2 wt.% V | |||||
| TiFe | 68(2) | a = 2.9856(3) | 26.613(7) | 32(1) | 0.068(4) |
| Ti | 9(1) | a = 2.991(2) c = 4.678(5) | 36.25(6) | 18(3) | --- |
| (Ti,V,Zr)2Fe | 9(1) | a = 10.522(3) | 1167.0(9) | 22(4) | --- |
| (Ti,V,Zr)2Fe-Zr-rich | 14(1) | a = 11.411(3) | 1486(1) | 17(2) | --- |
| 2 wt.% Zr + 2 wt.% V+ 2 wt.% Mn | |||||
| TiFe | 69(2) | a = 2.9878(3) | 26.671(7) | 24.8(9) | 0.02(1) |
| Ti | 6(1) | a = 2.991(2) c = 4.685(5) | 36.29(6) | 32(12) | --- |
| (Ti,V,Zr,Mn)2Fe | 8(1) | a = 10.543(3) | 1172(1) | 29(7) | --- |
| (Ti,V,Zr,Mn)2Fe-Zr-rich | 18(1) | a = 11.438(2) | 1496.4(8) | 24(3) | --- |
| Rate Limiting Step Model Name | Model Equation | Description |
|---|---|---|
| Chemisorption | α = kt | Surface controlled [29] |
| Nucleation-growth-impingement model (JMA2D) | [−ln(1 − α)]1/2 = kt | 2D growth of existing nuclei with constant velocity [29,30] |
| Nucleation-growth-impingement model (JMA3D) | [−ln(1 − α)]1/3 = kt | 3D growth of existing nuclei with constant velocity [29,30] |
| Contracting volume (CV2D) | 1 − (1 − α)1/2 = kt | 2D growth with constant interface velocity [29] |
| Contracting volume (CV3D) | 1 − (1 − α)1/3 = kt | 3D growth with constant interface velocity [29] |
| Ginstling–Brounstein model (GB2D) | (1 − α) ln(1 − α) + α = kt | 2D growth, diffusion controlled with decreasing interface velocity [31] |
| Ginstling–Brounstein model (GB3D) | 1 − (2α/3) − (1 − α)2/3 = kt | 3D growth, diffusion controlled with decreasing interface velocity [29] |
| Adjusted R2 | Chemisorption | JMA2D | JMA3D | CV2D | CV3D | GB2D | GB3D |
|---|---|---|---|---|---|---|---|
| 2 wt.% Zr + 2 wt.% V | 0.661 | 0.791 | 0.713 | 0.818 | 0.861 | 0.912 | 0.942 |
| 2 wt.% Zr + 2 wt.% V + 2 wt.% Mn | 0.670 | 0.794 | 0.717 | 0.818 | 0.859 | 0.917 | 0.942 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, A.K.; Duguay, A.; Tougas, B.; Neumann, B.; Schade, C.; Sharma, P.; Huot, J. Study of the Microstructural and First Hydrogenation Properties of TiFe Alloy with Zr, Mn and V as Additives. Processes 2021, 9, 1217. https://doi.org/10.3390/pr9071217
Patel AK, Duguay A, Tougas B, Neumann B, Schade C, Sharma P, Huot J. Study of the Microstructural and First Hydrogenation Properties of TiFe Alloy with Zr, Mn and V as Additives. Processes. 2021; 9(7):1217. https://doi.org/10.3390/pr9071217
Chicago/Turabian StylePatel, Abhishek Kumar, Alexandre Duguay, Bernard Tougas, Bettina Neumann, Chris Schade, Pratibha Sharma, and Jacques Huot. 2021. "Study of the Microstructural and First Hydrogenation Properties of TiFe Alloy with Zr, Mn and V as Additives" Processes 9, no. 7: 1217. https://doi.org/10.3390/pr9071217






