Sustainable Syntheses and Sources of Nanomaterials for Microbial Fuel/Electrolysis Cell Applications: An Overview of Recent Progress
Abstract
1. Introduction
2. The Green and Sustainable Approach for Nanoparticles Production
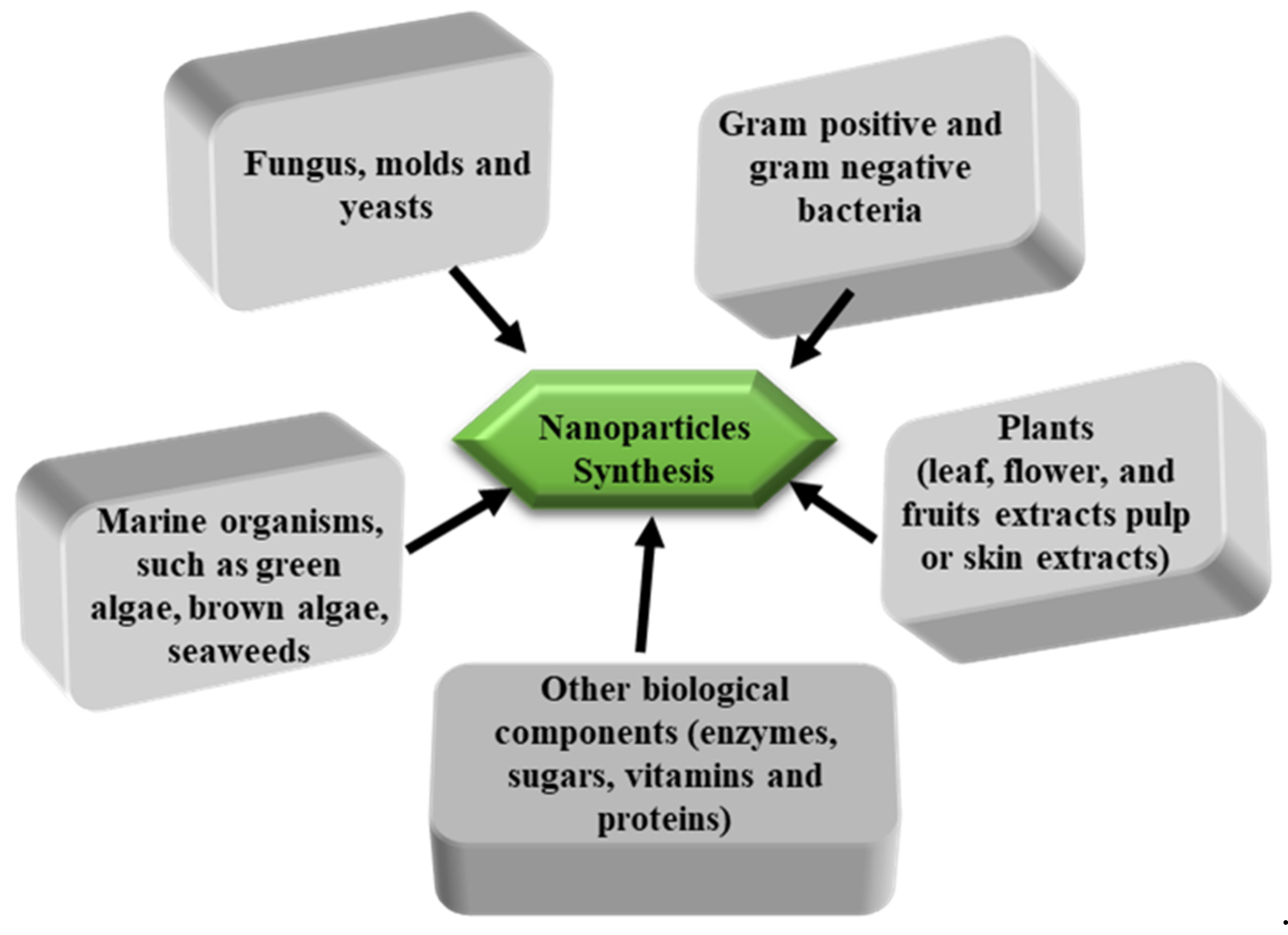
2.1. Potential Renewable and Sustainable Raw Sources for Different Kinds of Nanoparticles
2.2. Green Routes for the Synthesis of Nanoparticles
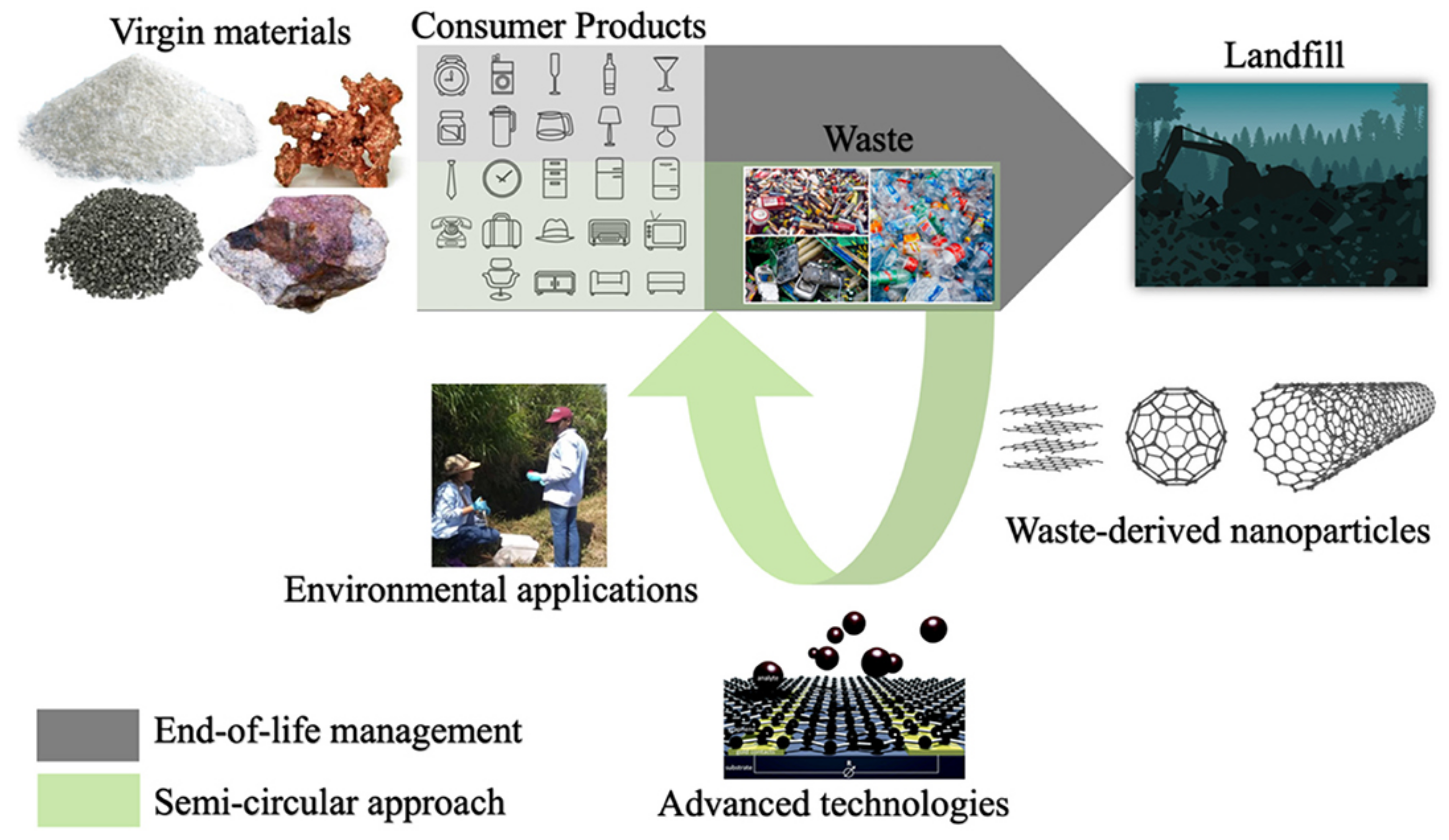
2.3. Other Sustainable Unconventional Sources for Synthesis of Nanoparticles
3. The Use of Green and Sustainable Nanoparticles and Materials in MFCs and MECs
3.1. Metal Oxide and Metal-Based Materials
3.2. Carbon-Sourced Nanomaterials
4. Conclusions and Future Development
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Liu, H.; Hu, H.; Chignell, J.; Fan, Y. Microbial electrolysis: Novel technology for hydrogen production from biomass. Biofuels 2010, 1, 129–142. [Google Scholar] [CrossRef]
- Drendel, G.; Mathews, E.R.; Semenec, L.; Franks, A.E. Microbial Fuel Cells, Related Technologies, and Their Applications. Appl. Sci. 2018, 8, 2384. [Google Scholar] [CrossRef]
- Flimban, S.G.A.; Ismail, I.M.I.; Kim, T.; Oh, S.-E. Overview of Recent Advancements in the Microbial Fuel Cell from Fundamentals to Applications: Design, Major Elements, and Scalability. Energies 2019, 12, 3390. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Kalil, M.S.; Abdeshahian, P.; Hamid, A.A. A review of the substrates used in microbial electrolysis cells (MECs) for producing sustainable and clean hydrogen gas. Renew. Energy 2014, 71, 466–472. [Google Scholar] [CrossRef]
- Gajda, I.; Greenman, J.; Ieropoulos, I.A. Recent advancements in real-world microbial fuel cell applications. Curr. Opin. Electrochem. 2018, 11, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Christwardana, M.; Frattini, D.; Accardo, G.; Yoon, S.P.; Kwon, Y. Early-stage performance evaluation of flowing microbial fuel cells using chemically treated carbon felt and yeast biocatalyst. Appl. Energy 2018, 222, 369–382. [Google Scholar] [CrossRef]
- Jannelli, N.; Nastro, R.A.; Cigolotti, V.; Minutillo, M.; Falcucci, G. Low pH, high salinity: Too much for microbial fuel cells? Appl. Energy 2017, 192, 543–550. [Google Scholar] [CrossRef]
- Iannaci, A.; Myles, A.; Flinois, T.; Behan, J.A.; Barrière, F.; Scanlan, E.M.; Colavita, P.E. Tailored glycosylated anode surfaces: Addressing the exoelectrogen bacterial community via functional layers for microbial fuel cell applications. Bioelectrochemistry 2020, 136, 107621. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Mecheri, B.; Garavaglia, V.; Licoccia, S.; Di Nardo, P.; Traversa, E. Engineering materials and biology to boost performance of microbial fuel cells: A critical review. Energy Environ. Sci. 2008, 1, 417. [Google Scholar] [CrossRef]
- Bajracharya, S.; Srikanth, S.; Mohanakrishna, G.; Zacharia, R.; Strik, D.P.; Pant, D. Biotransformation of carbon dioxide in bioelectrochemical systems: State of the art and future prospects. J. Power Sources 2017, 356, 256–273. [Google Scholar] [CrossRef]
- Pandey, P.; Shinde, V.N.; Deopurkar, R.L.; Kale, S.P.; Patil, S.A.; Pant, D. Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl. Energy 2016, 168, 706–723. [Google Scholar] [CrossRef]
- Christwardana, M.; Frattini, D.; Accardo, G.; Yoon, S.P.; Kwon, Y. Optimization of glucose concentration and glucose/yeast ratio in yeast microbial fuel cell using response surface methodology approach. J. Power Sources 2018, 402, 402–412. [Google Scholar] [CrossRef]
- Andriukonis, E.; Celiesiute-Germaniene, R.; Ramanavicius, S.; Viter, R.; Ramanavicius, A. From Microorganism-Based Amperometric Biosensors towards Microbial Fuel Cells. Sensors 2021, 21, 2442. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2020, 13, 49. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Charge Transfer and Biocompatibility Aspects in Conducting Polymer-Based Enzymatic Biosensors and Biofuel Cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, P.; Frattini, D.; Tagliente, M.; Selleri, F. Electrochemical Deposition of Polypyrrole Nanostructures for Energy Applications: A Review. Curr. Nanosci. 2020, 16, 462–477. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Progress and Insights in the Application of MXenes as New 2D Nano-Materials Suitable for Biosensors and Biofuel Cell Design. Int. J. Mol. Sci. 2020, 21, 9224. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, P.; Frattini, D.; Ghosh, S.; Mohan, A.M.V.; Kumar, Y.; Kwon, Y. Soft Materials for Wearable/Flexible Electrochemical Energy Conversion, Storage, and Biosensor Devices. Materials 2020, 13, 2733. [Google Scholar] [CrossRef] [PubMed]
- Gude, V.G. Wastewater treatment in microbial fuel cells—An overview. J. Clean. Prod. 2016, 122, 287–307. [Google Scholar] [CrossRef]
- Aiken, D.C.; Curtis, T.P.; Heidrich, E.S. Avenues to the financial viability of microbial electrolysis cells [MEC] for domestic wastewater treatment and hydrogen production. Int. J. Hydrogen Energy 2019, 44, 2426–2434. [Google Scholar] [CrossRef]
- Gajda, I.; Stinchcombe, A.; Merino-Jimenez, I.; Pasternak, G.; Sanchez-Herranz, D.; Greenman, J.; Ieropoulos, I.A. Miniaturized ceramic-based microbial fuel cell for efficient power generation from urine and stack development. Front. Energy Res. 2018, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Ghadge, A.N.; Ghangrekar, M.M. Development of low cost ceramic separator using mineral cation exchanger to enhance performance of microbial fuel cells. Electrochim. Acta 2015, 166, 320–328. [Google Scholar] [CrossRef]
- Khalili, H.B.; Mohebbi-Kalhori, D.; Afarani, M.S. Microbial fuel cell (MFC) using commercially available unglazed ceramic wares: Low-cost ceramic separators suitable for scale-up. Int. J. Hydrogen Energy 2017, 42, 8233–8241. [Google Scholar] [CrossRef]
- Frattini, D.; Accardo, G.; Kwon, Y. Perovskite ceramic membrane separator with improved biofouling resistance for yeast-based microbial fuel cells. J. Memb. Sci. 2020, 599, 117843. [Google Scholar] [CrossRef]
- Zhao, C.; Gai, P.; Song, R.; Chen, Y.; Zhang, J.; Zhu, J.-J. Nanostructured material-based biofuel cells: Recent advances and future prospects. Chem. Soc. Rev. 2017, 46, 1545–1564. [Google Scholar] [CrossRef] [PubMed]
- ElMekawy, A.; Hegab, H.M.; Losic, D.; Saint, C.P.; Pant, D. Applications of graphene in microbial fuel cells: The gap between promise and reality. Renew. Sustain. Energy Rev. 2017, 72, 1389–1403. [Google Scholar] [CrossRef]
- Yang, W.; Kim, K.-Y.; Saikaly, P.E.; Logan, B.E. The impact of new cathode materials relative to baseline performance of microbial fuel cells all with the same architecture and solution chemistry. Energy Environ. Sci. 2017, 10, 1025–1033. [Google Scholar] [CrossRef]
- Frattini, D.; Accardo, G.; Ferone, C.; Cioffi, R. Fabrication and characterization of graphite-cement composites for microbial fuel cells applications. Mater. Res. Bull. 2017, 88, 188–199. [Google Scholar] [CrossRef]
- HaoYu, E.; Cheng, S.; Scott, K.; Logan, B. Microbial fuel cell performance with non-Pt cathode catalysts. J. Power Sources 2007, 171, 275–281. [Google Scholar] [CrossRef]
- Ben Liew, K.; Daud, W.R.W.; Ghasemi, M.; Leong, J.X.; Su Lim, S.; Ismail, M. Non-Pt catalyst as oxygen reduction reaction in microbial fuel cells: A review. Int. J. Hydrogen Energy 2014, 39, 4870–4883. [Google Scholar] [CrossRef]
- Duarte, K.D.Z.; Frattini, D.; Kwon, Y. High performance yeast-based microbial fuel cells by surfactant-mediated gold nanoparticles grown atop a carbon felt anode. Appl. Energy 2019, 256, 113912. [Google Scholar] [CrossRef]
- Alatraktchi, F.A.; Zhang, Y.; Angelidaki, I. Nanomodification of the electrodes in microbial fuel cell: Impact of nanoparticle density on electricity production and microbial community. Appl. Energy 2014, 116, 216–222. [Google Scholar] [CrossRef]
- Trapero, J.R.; Horcajada, L.; Linares, J.J.; Lobato, J. Is microbial fuel cell technology ready? An economic answer towards industrial commercialization. Appl. Energy 2017, 185, 698–707. [Google Scholar] [CrossRef]
- Pepè Sciarria, T.; de Oliveira, M.A.C.; Mecheri, B.; D’Epifanio, A.; Goldfarb, J.L.; Adani, F. Metal-free activated biochar as an oxygen reduction reaction catalyst in single chamber microbial fuel cells. J. Power Sources 2020, 462, 228183. [Google Scholar] [CrossRef]
- Strambeanu, N.; Demetrovici, L.; Dragos, D. Natural Sources of Nanoparticles. In Nanoparticles’ Promises and Risks; Springer International Publishing: Cham, Switzerland, 2015; pp. 9–19. [Google Scholar]
- Guo, H.; Barnard, A.S. Naturally occurring iron oxide nanoparticles: Morphology, surface chemistry and environmental stability. J. Mater. Chem. A 2013, 1, 27–42. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Martel, J.; Wong, T.-Y.; Young, D.; Liu, C.-C.; Lin, C.-W.; Young, J.D. Formation and characteristics of biomimetic mineralo-organic particles in natural surface water. Sci. Rep. 2016, 6, 28817. [Google Scholar] [CrossRef]
- McGillicuddy, E.; Murray, I.; Kavanagh, S.; Morrison, L.; Fogarty, A.; Cormican, M.; Dockery, P.; Prendergast, M.; Rowan, N.; Morris, D. Silver nanoparticles in the environment: Sources, detection and ecotoxicology. Sci. Total Environ. 2017, 575, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Hough, R.M.; Noble, R.R.P.; Reich, M. Natural gold nanoparticles. Ore Geol. Rev. 2011, 42, 55–61. [Google Scholar] [CrossRef]
- Terrones, M. Carbon nanotubes: Synthesis and properties, electronic devices and other emerging applications. Int. Mater. Rev. 2004, 49, 325–377. [Google Scholar] [CrossRef]
- Faulstich, L.; Griffin, S.; Nasim, M.J.; Masood, M.I.; Ali, W.; Alhamound, S.; Omran, Y.; Kim, H.; Kharma, A.; Schäfer, K.-H.; et al. Nature’s Hat-trick: Can we use sulfur springs as ecological source for materials with agricultural and medical applications? Int. Biodeterior. Biodegrad. 2017, 119, 678–686. [Google Scholar] [CrossRef]
- Alkadasi, N.A.N. Synthesis of Fe3O4 nanoparticles from Ironstone from The Republic of Yemen. Orient. J. Chem. 2014, 30, 1173–1178. [Google Scholar] [CrossRef][Green Version]
- Manivasakan, P.; Rajendran, V.; Rauta, P.R.; Sahu, B.B.; Sahu, P.; Panda, B.K.; Valiyaveettill, S.; Jegadesan, S. Effect of TiO2 Nanoparticles on Properties of Silica Refractory. J. Am. Ceram. Soc. 2010, 93, 2236–2243. [Google Scholar] [CrossRef]
- Manivasakan, P.; Rajendran, V.; Rauta, P.R.; Sahu, B.B.; Panda, B.K. Effect of mineral acids on the production of alumina nanopowder from raw bauxite. Powder Technol. 2011, 211, 77–84. [Google Scholar] [CrossRef]
- Raman, D.N.; Rajapandi, V.; Sundaramoorthy, A.; Ravisekaran, S.S.; Annadurai, N. Naturally derived FeTiO3 nanoparticles: Analysis of optical properties. J. Mater. Sci. Mater. Electron. 2020, 31, 16951–16958. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Zhai, L.; Luo, T.; Mei, Z.; Liu, H. Achievements of biochar application for enhanced anaerobic digestion: A review. Bioresour. Technol. 2019, 292, 122058. [Google Scholar] [CrossRef]
- Li, D.; Zhao, R.; Peng, X.; Ma, Z.; Zhao, Y.; Gong, T.; Sun, M.; Jiao, Y.; Yang, T.; Xi, B. Biochar-related studies from 1999 to 2018: A bibliometrics-based review. Environ. Sci. Pollut. Res. 2020, 27, 2898–2908. [Google Scholar] [CrossRef]
- Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2015; ISBN 9781849770552. [Google Scholar]
- Allam, F.; Elnouby, M.; El-Khatib, K.M.; El-Badan, D.E.; Sabry, S.A. Water hyacinth (Eichhornia crassipes) biochar as an alternative cathode electrocatalyst in an air-cathode single chamber microbial fuel cell. Int. J. Hydrogen Energy 2020, 45, 5911–5927. [Google Scholar] [CrossRef]
- Yuan, H.; Deng, L.; Qi, Y.; Kobayashi, N.; Tang, J. Nonactivated and Activated Biochar Derived from Bananas as Alternative Cathode Catalyst in Microbial Fuel Cells. Sci. World J. 2014, 2014, 832850. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Yuan, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Yuan, H.; Chen, Y. Alfalfa Leaf-Derived Porous Heteroatom-Doped Carbon Materials as Efficient Cathodic Catalysts in Microbial Fuel Cells. ACS Sustain. Chem. Eng. 2017, 5, 9766–9773. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; Xiao, T.; Wang, S.; Zhang, B.; Chen, D.; Su, M.; Tang, J. Low-cost biochar derived from corncob as oxygen reduction catalyst in air cathode microbial fuel cells. Electrochim. Acta 2018, 283, 780–788. [Google Scholar] [CrossRef]
- Shi, Y.-T.; Yu, Y.-Y.; Xu, Z.-A.; Lian, J.; Yong, Y.-C. Superior carbon belts from Spirogyra for efficient extracellular electron transfer and sustainable microbial energy harvesting. J. Mater. Chem. A 2019, 7, 6930–6938. [Google Scholar] [CrossRef]
- Huggins, T.M.; Pietron, J.J.; Wang, H.; Ren, Z.J.; Biffinger, J.C. Graphitic biochar as a cathode electrocatalyst support for microbial fuel cells. Bioresour. Technol. 2015, 195, 147–153. [Google Scholar] [CrossRef]
- Ye, W.; Tang, J.; Wang, Y.; Cai, X.; Liu, H.; Lin, J.; Van der Bruggen, B.; Zhou, S. Hierarchically structured carbon materials derived from lotus leaves as efficient electrocatalyst for microbial energy harvesting. Sci. Total Environ. 2019, 666, 865–874. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, T.; Wang, D.; Tang, J.; Zhou, S. Sewage sludge biochar as an efficient catalyst for oxygen reduction reaction in an microbial fuel cell. Bioresour. Technol. 2013, 144, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.; Liu, T.; Chen, H. Renewable Coffee Waste-Derived Porous Carbons as Anode Materials for High-Performance Sustainable Microbial Fuel Cells. ACS Sustain. Chem. Eng. 2019, 7, 16991–16999. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Li, Y.; Bao, W.; Fang, Z.; Preston, C.; Vaaland, O.; Ren, Z.; Hu, L. Lightweight, conductive hollow fibers from nature as sustainable electrode materials for microbial energy harvesting. Nano Energy 2014, 10, 268–276. [Google Scholar] [CrossRef]
- Zha, Z.; Zhang, Z.; Xiang, P.; Zhu, H.; Zhou, B.; Sun, Z.; Zhou, S. One-step preparation of eggplant-derived hierarchical porous graphitic biochar as efficient oxygen reduction catalyst in microbial fuel cells. RSC Adv. 2021, 11, 1077–1085. [Google Scholar] [CrossRef]
- Jiao, Y.; Hu, Y.; Han, L.; Zhou, M. Activated Carbon Derived from Rice Husk as Efficient Oxygen Reduction Catalyst in Microbial Fuel Cell. Electroanalysis 2020, 32, 2969–2975. [Google Scholar] [CrossRef]
- Chakraborty, I.; Bhowmick, G.D.; Ghosh, D.; Dubey, B.K.; Pradhan, D.; Ghangrekar, M.M. Novel low-cost activated algal biochar as a cathode catalyst for improving performance of microbial fuel cell. Sustain. Energy Technol. Assess. 2020, 42, 100808. [Google Scholar] [CrossRef]
- Zhong, K.; Li, M.; Yang, Y.; Zhang, H.; Zhang, B.; Tang, J.; Yan, J.; Su, M.; Yang, Z. Nitrogen-doped biochar derived from watermelon rind as oxygen reduction catalyst in air cathode microbial fuel cells. Appl. Energy 2019, 242, 516–525. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, T.; Fu, P.; Tang, J.; Zhou, S. Conversion of sewage sludge into high-performance bifunctional electrode materials for microbial energy harvesting. J. Mater. Chem. A 2015, 3, 8475–8482. [Google Scholar] [CrossRef]
- Yin, C.; Shen, Y.; Yuan, R.; Zhu, N.; Yuan, H.; Lou, Z. Sludge-based biochar-assisted thermophilic anaerobic digestion of waste-activated sludge in microbial electrolysis cell for methane production. Bioresour. Technol. 2019, 284, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Chaves Fernandes, B.C.; Ferreira Mendes, K.; Dias Júnior, A.F.; da Silva Caldeira, V.P.; da Silva Teófilo, T.M.; Severo Silva, T.; Mendonça, V.; de Freitas Souza, M.; Valadão Silva, D. Impact of Pyrolysis Temperature on the Properties of Eucalyptus Wood-Derived Biochar. Materials 2020, 13, 5841. [Google Scholar] [CrossRef]
- Varma, R.S. Greener approach to nanomaterials and their sustainable applications. Curr. Opin. Chem. Eng. 2012, 1, 123–128. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Al-Rajhi, A.M.H.; Al Abboud, M.A.; Alawlaqi, M.M.; Ganash Magdah, A.; Helmy, E.A.M.; Mabrouk, A.S. Recent Advances in Green Synthesis of Silver Nanoparticles and Their Applications: About Future Directions. A Review. Bionanoscience 2018, 8, 5–16. [Google Scholar] [CrossRef]
- Hebbalalu, D.; Lalley, J.; Nadagouda, M.N.; Varma, R.S. Greener Techniques for the Synthesis of Silver Nanoparticles Using Plant Extracts, Enzymes, Bacteria, Biodegradable Polymers, and Microwaves. ACS Sustain. Chem. Eng. 2013, 1, 703–712. [Google Scholar] [CrossRef]
- Uma Suganya, K.S.; Govindaraju, K.; Ganesh Kumar, V.; Stalin Dhas, T.; Karthick, V.; Singaravelu, G.; Elanchezhiyan, M. Blue green alga mediated synthesis of gold nanoparticles and its antibacterial efficacy against Gram positive organisms. Mater. Sci. Eng. C 2015, 47, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Varma, R.S. Beet juice utilization: Expeditious green synthesis of noble metal nanoparticles (Ag, Au, Pt, and Pd) using microwaves. RSC Adv. 2012, 2, 10283. [Google Scholar] [CrossRef]
- Gholami-Shabani, M.; Shams-Ghahfarokhi, M.; Gholami-Shabani, Z.; Akbarzadeh, A.; Riazi, G.; Ajdari, S.; Amani, A.; Razzaghi-Abyaneh, M. Enzymatic synthesis of gold nanoparticles using sulfite reductase purified from Escherichia coli: A green eco-friendly approach. Process Biochem. 2015, 50, 1076–1085. [Google Scholar] [CrossRef]
- Adelere, I.A.; Lateef, A. A novel approach to the green synthesis of metallic nanoparticles: The use of agro-wastes, enzymes, and pigments. Nanotechnol. Rev. 2016, 5. [Google Scholar] [CrossRef]
- Sunkar, S.; Nachiyar, C.V. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac. J. Trop. Biomed. 2012, 2, 953–959. [Google Scholar] [CrossRef]
- Deplanche, K.; Macaskie, L.E. Biorecovery of gold by Escherichia coli and Desulfovibrio desulfuricans. Biotechnol. Bioeng. 2008, 99, 1055–1064. [Google Scholar] [CrossRef]
- Mann, S.; Frankel, R.B.; Blakemore, R.P. Structure, morphology and crystal growth of bacterial magnetite. Nature 1984, 310, 405–407. [Google Scholar] [CrossRef]
- Marshall, M.J.; Beliaev, A.S.; Dohnalkova, A.C.; Kennedy, D.W.; Shi, L.; Wang, Z.; Boyanov, M.I.; Lai, B.; Kemner, K.M.; McLean, J.S.; et al. c-Type Cytochrome-Dependent Formation of U(IV) Nanoparticles by Shewanella oneidensis. PLoS Biol. 2006, 4, e268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Tuan, H.-Y.; Tien, C.-W.; Lo, W.-H.; Liang, H.-C.; Hu, Y.-C. Augmented biosynthesis of cadmium sulfide nanoparticles by genetically engineered Escherichia coli. Biotechnol. Progr. 2009, 25, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, B.K.; Rajasab, A.H. A comparative study on biosynthesis of silver nanoparticles using four different fungal species. Int. J. Pharm. Pharm. Sci. 2014, 6, 372–376. [Google Scholar]
- Gericke, M.; Pinches, A. Microbial production of gold nanoparticles. Gold Bull. 2006, 39, 22–28. [Google Scholar] [CrossRef]
- Senapati, S.; Ahmad, A.; Khan, M.I.; Sastry, M.; Kumar, R. Extracellular Biosynthesis of Bimetallic Au-Ag Alloy Nanoparticles. Small 2005, 1, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Tarafdar, J.C. Biosynthesis and characterization of zinc, magnesium and titanium nanoparticles: An eco-friendly approach. Int. Nano Lett. 2014, 4, 93. [Google Scholar] [CrossRef]
- Raliya, R.; Biswas, P.; Tarafdar, J.C. TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.). Biotechnol. Rep. 2015, 5, 22–26. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Coll. Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef]
- Armendariz, V.; Herrera, I.; Peralta-videa, J.R.; Jose-yacaman, M.; Troiani, H.; Santiago, P.; Gardea-Torresdey, J.L. Size controlled gold nanoparticle formation by Avena sativa biomass: Use of plants in nanobiotechnology. J. Nanoparticle Res. 2004, 6, 377–382. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, Q.; Li, Q.; Song, H. The biosynthesis of palladium nanoparticles by antioxidants in Gardenia jasminoides Ellis: Long lifetime nanocatalysts for p-nitrotoluene hydrogenation. Nanotechnology 2009, 20, 385601. [Google Scholar] [CrossRef]
- Herrera-Becerra, R.; Zorrilla, C.; Rius, J.L.; Ascencio, J.A. Electron microscopy characterization of biosynthesized iron oxide nanoparticles. Appl. Phys. A 2008, 91, 241–246. [Google Scholar] [CrossRef]
- Qu, J.; Luo, C.; Hou, J. Synthesis of ZnO nanoparticles from Zn-hyperaccumulator (Sedum alfredii Hance) plants. Micro Nano Lett. 2011, 6, 174. [Google Scholar] [CrossRef]
- Maensiri, S.; Laokul, P.; Klinkaewnarong, J.; Phokha, S.; Promarak, V.; Seraphin, S. Indium oxide (In2O3) nanoparticles using Aloe vera plant extract: Synthesis and optical properties. J. Optoelectron. Adv. Mater. 2008, 10, 161–165. [Google Scholar]
- Kisieliute, A.; Popov, A.; Apetrei, R.-M.; Cârâc, G.; Morkvenaite-Vilkonciene, I.; Ramanaviciene, A.; Ramanavicius, A. Towards microbial biofuel cells: Improvement of charge transfer by self-modification of microoganisms with conducting polymer—Polypyrrole. Chem. Eng. J. 2019, 356, 1014–1021. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Ramanaviciene, A. Hemoproteins in Design of Biofuel Cells. Fuel Cells 2009, 9, 25–36. [Google Scholar] [CrossRef]
- Andriukonis, E.; Stirke, A.; Garbaras, A.; Mikoliunaite, L.; Ramanaviciene, A.; Remeikis, V.; Thornton, B.; Ramanavicius, A. Yeast-assisted synthesis of polypyrrole: Quantification and influence on the mechanical properties of the cell wall. Coll. Surf. B Biointerfaces 2018, 164, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, A.; Andriukonis, E.; Stirke, A.; Mikoliunaite, L.; Balevicius, Z.; Ramanaviciene, A. Synthesis of polypyrrole within the cell wall of yeast by redox-cycling of [Fe(CN)6]3−/[Fe(CN)6]4−. Enzyme Microb. Technol. 2016, 83, 40–47. [Google Scholar] [CrossRef]
- Stirke, A.; Apetrei, R.-M.; Kirsnyte, M.; Dedelaite, L.; Bondarenka, V.; Jasulaitiene, V.; Pucetaite, M.; Selskis, A.; Carac, G.; Bahrim, G.; et al. Synthesis of polypyrrole microspheres by Streptomyces spp. Polymer 2016, 84, 99–106. [Google Scholar] [CrossRef]
- Apetrei, R.-M.; Carac, G.; Bahrim, G.; Ramanaviciene, A.; Ramanavicius, A. Modification of Aspergillus niger by conducting polymer, Polypyrrole, and the evaluation of electrochemical properties of modified cells. Bioelectrochemistry 2018, 121, 46–55. [Google Scholar] [CrossRef]
- Apetrei, R.-M.; Carac, G.; Ramanaviciene, A.; Bahrim, G.; Tanase, C.; Ramanavicius, A. Cell-assisted synthesis of conducting polymer—Polypyrrole—For the improvement of electric charge transfer through fungal cell wall. Coll. Surf. B Biointerfaces 2019, 175, 671–679. [Google Scholar] [CrossRef]
- United Nations. Solid Waste Management. Available online: https://www.unenvironment.org/explore-topics/resource-efficiency/what-we-do/cities/solid-waste-management (accessed on 30 November 2020).
- Abdelbasir, S.M.; McCourt, K.M.; Lee, C.M.; Vanegas, D.C. Waste-Derived Nanoparticles: Synthesis Approaches, Environmental Applications, and Sustainability Considerations. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Xiu, F.-R.; Zhang, F.-S. Size-controlled preparation of Cu2O nanoparticles from waste printed circuit boards by supercritical water combined with electrokinetic process. J. Hazard. Mater. 2012, 233–234, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Lee, B.-K. Recovery of precious metals from low-grade automobile shredder residue: A novel approach for the recovery of nanozero-valent copper particles. Waste Manag. 2016, 48, 353–365. [Google Scholar] [CrossRef]
- Mdlovu, N.V.; Chiang, C.-L.; Lin, K.-S.; Jeng, R.-C. Recycling copper nanoparticles from printed circuit board waste etchants via a microemulsion process. J. Clean. Prod. 2018, 185, 781–796. [Google Scholar] [CrossRef]
- Shokri, A.; Pahlevani, F.; Levick, K.; Cole, I.; Sahajwalla, V. Synthesis of copper-tin nanoparticles from old computer printed circuit boards. J. Clean. Prod. 2017, 142, 2586–2592. [Google Scholar] [CrossRef]
- Elsayed, D.M.; Abdelbasir, S.M.; Abdel-Ghafar, H.M.; Salah, B.A.; Sayed, S.A. Silver and copper nanostructured particles recovered from metalized plastic waste for antibacterial applications. J. Environ. Chem. Eng. 2020, 8, 103826. [Google Scholar] [CrossRef]
- El-Amir, A.A.M.; Ewais, E.M.M.; Abdel-Aziem, A.R.; Ahmed, A.; El-Anadouli, B.E.H. Nano-alumina powders/ceramics derived from aluminum foil waste at low temperature for various industrial applications. J. Environ. Manag. 2016, 183, 121–125. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zeng, Y.; Li, P.; Xie, T.; Zhang, Y. Bacteria-assisted preparation of nano α-Fe2O3 red pigment powders from waste ferrous sulfate. J. Hazard. Mater. 2016, 317, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Su, M.; Lee, P.-H.; Shih, K. Synthesis of submicron lead oxide particles from the simulated spent lead paste for battery anodes. J. Alloys Compd. 2017, 690, 101–107. [Google Scholar] [CrossRef]
- Xiang, X.; Xia, F.; Zhan, L.; Xie, B. Preparation of zinc nano structured particles from spent zinc manganese batteries by vacuum separation and inert gas condensation. Sep. Purif. Technol. 2015, 142, 227–233. [Google Scholar] [CrossRef]
- Duarte, K.D.Z.; Kwon, Y. In situ carbon felt anode modification via codeveloping Saccharomyces cerevisiae living-template titanium dioxide nanoclusters in a yeast-based microbial fuel cell. J. Power Sources 2020, 474, 228651. [Google Scholar] [CrossRef]
- Duarte, K.D.Z.; Kwon, Y. Enhanced extracellular electron transfer of yeast-based microbial fuel cells via one pot substrate-bound growth iron-manganese oxide nanoflowers. J. Power Sources 2020, 474, 228496. [Google Scholar] [CrossRef]
- Yang, S.; Chung, Y.; Lee, K.-S.; Kwon, Y. Enhancements in catalytic activity and duration of PdFe bimetallic catalysts and their use in direct formic acid fuel cells. J. Ind. Eng. Chem. 2020, 90, 351–357. [Google Scholar] [CrossRef]
- Costa de Oliveira, M.A.; D’Epifanio, A.; Ohnuki, H.; Mecheri, B. Platinum Group Metal-Free Catalysts for Oxygen Reduction Reaction: Applications in Microbial Fuel Cells. Catalysts 2020, 10, 475. [Google Scholar] [CrossRef]
- Zhao, N.; Liang, D.; Meng, S.; Li, X. Bibliometric and content analysis on emerging technologies of hydrogen production using microbial electrolysis cells. Int. J. Hydrogen Energy 2020, 45, 33310–33324. [Google Scholar] [CrossRef]
- Kim, C.; Kim, J.R.; Heo, J. Enhancement of bioelectricity generation by a microbial fuel cell using Ti nanoparticle-modified carbon electrode. J. Chem. Technol. Biotechnol. 2019, 94, 1622–1627. [Google Scholar] [CrossRef]
- Wu, X.; Xiong, X.; Owens, G.; Brunetti, G.; Zhou, J.; Yong, X.; Xie, X.; Zhang, L.; Wei, P.; Jia, H. Anode modification by biogenic gold nanoparticles for the improved performance of microbial fuel cells and microbial community shift. Bioresour. Technol. 2018, 270, 11–19. [Google Scholar] [CrossRef]
- Stephen, A.J.; Rees, N.V.; Mikheenko, I.; Macaskie, L.E. Platinum and Palladium Bio-Synthesized Nanoparticles as Sustainable Fuel Cell Catalysts. Front. Energy Res. 2019, 7, 66. [Google Scholar] [CrossRef]
- Saravanakumar, K.; MubarakAli, D.; Kathiresan, K.; Thajuddin, N.; Alharbi, N.S.; Chen, J. Biogenic metallic nanoparticles as catalyst for bioelectricity production: A novel approach in microbial fuel cells. Mater. Sci. Eng. B 2016, 203, 27–34. [Google Scholar] [CrossRef]
- Harshiny, M.; Samsudeen, N.; Kameswara, R.J.; Matheswaran, M. Biosynthesized FeO nanoparticles coated carbon anode for improving the performance of microbial fuel cell. Int. J. Hydrogen Energy 2017, 42, 26488–26495. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, Q.; Jin, X.; Chen, Z. The Effects of Nanoscale Zerovalent Iron on Microbial Fuel Cells in the Start-up Process. Adv. Sustain. Syst. 2018, 2, 1700181. [Google Scholar] [CrossRef]
- Matsena, M.T.; Tichapondwa, S.M.; Chirwa, E.M.N. Synthesis of Biogenic Palladium Nanoparticles Using Citrobacter sp. for Application as Anode Electrocatalyst in a Microbial Fuel Cell. Catalysts 2020, 10, 838. [Google Scholar] [CrossRef]
- Tahernia, M.; Mohammadifar, M.; Feng, S.; Choi, S. Biogenic Palladium Nanoparticles for Improving Bioelectricity Generation in Microbial Fuel Cells. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; pp. 425–428. [Google Scholar]
- Karim, M.R.; Han, T.H.; Sawant, S.Y.; Shim, J.; Lee, M.Y.; Kim, W.K.; Kim, J.S.; Cho, M.H. Microbial fuel cell-assisted biogenic synthesis of gold nanoparticles and its application to energy production and hydrogen peroxide detection. Korean J. Chem. Eng. 2020, 37, 1241–1250. [Google Scholar] [CrossRef]
- Mateo, S.; Cañizares, P.; Rodrigo, M.A.; Fernandez-Morales, F.J. Driving force of the better performance of metal-doped carbonaceous anodes in microbial fuel cells. Appl. Energy 2018, 225, 52–59. [Google Scholar] [CrossRef]
- Quan, X.; Sun, B.; Xu, H. Anode decoration with biogenic Pd nanoparticles improved power generation in microbial fuel cells. Electrochim. Acta 2015, 182, 815–820. [Google Scholar] [CrossRef]
- Lefebvre, O.; Tan, Z.; Shen, Y.; Ng, H.Y. Optimization of a microbial fuel cell for wastewater treatment using recycled scrap metals as a cost-effective cathode material. Bioresour. Technol. 2013, 127, 158–164. [Google Scholar] [CrossRef]
- Xu, H.; Quan, X.; Xiao, Z.; Chen, L. Effect of anodes decoration with metal and metal oxides nanoparticles on pharmaceutically active compounds removal and power generation in microbial fuel cells. Chem. Eng. J. 2018, 335, 539–547. [Google Scholar] [CrossRef]
- Liu, J.; Vipulanandan, C. Effects of Fe, Ni, and Fe/Ni metallic nanoparticles on power production and biosurfactant production from used vegetable oil in the anode chamber of a microbial fuel cell. Waste Manag. 2017, 66, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Ayyaru, S.; Mahalingam, S.; Ahn, Y.H. A non-noble V2O5 nanorods as an alternative cathode catalyst for microbial fuel cell applications. Int. J. Hydrogen Energy 2019, 44, 4974–4984. [Google Scholar] [CrossRef]
- Guo, W.; Pi, Y.; Song, H.; Tang, W.; Sun, J. Layer-by-layer assembled gold nanoparticles modified anode and its application in microbial fuel cells. Coll. Surf. A Physicochem. Eng. Asp. 2012, 415, 105–111. [Google Scholar] [CrossRef]
- Liu, X.W.; Sun, X.F.; Huang, Y.X.; Sheng, G.P.; Zhou, K.; Zeng, R.J.; Dong, F.; Wang, S.G.; Xu, A.W.; Tong, Z.H.; et al. Nano-structured manganese oxide as a cathodic catalyst for enhanced oxygen reduction in a microbial fuel cell fed with a synthetic wastewater. Water Res. 2010, 44, 5298–5305. [Google Scholar] [CrossRef]
- Varanasi, J.L.; Nayak, A.K.; Sohn, Y.; Pradhan, D.; Das, D. Improvement of power generation of microbial fuel cell by integrating tungsten oxide electrocatalyst with pure or mixed culture biocatalysts. Electrochim. Acta 2016, 199, 154–163. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, S.; Schaller, R.; Jiao, J.; Chaplen, F.; Liu, H. Nanoparticle decorated anodes for enhanced current generation in microbial electrochemical cells. Biosens. Bioelectron. 2011, 26, 1908–1912. [Google Scholar] [CrossRef]
- Heydorn, R.L.; Engel, C.; Krull, R.; Dohnt, K. Strategies for the Targeted Improvement of Anodic Electron Transfer in Microbial Fuel Cells. ChemBioEng Rev. 2020, 7, 4–17. [Google Scholar] [CrossRef]
- Heydorn, R.L.; Engel, C.; Krull, R.; Dohnt, K. Strategien zur gezielten Verbesserung des anodenseitigen Elektronentransfers in mikrobiellen Brennstoffzellen. Chem. Ing. Tech. 2019, 91, 744–757. [Google Scholar] [CrossRef]
- Qiao, Y.; Bao, S.-J.; Li, C.M.; Cui, X.-Q.; Lu, Z.-S.; Guo, J. Nanostructured Polyaniline/Titanium Dioxide Composite Anode for Microbial Fuel Cells. ACS Nano 2008, 2, 113–119. [Google Scholar] [CrossRef]
- Ma, M.; Dai, Y.; Zou, J.; Wang, L.; Pan, K.; Fu, H. Synthesis of Iron Oxide/Partly Graphitized Carbon Composites as a High-Efficiency and Low-Cost Cathode Catalyst for Microbial Fuel Cells. ACS Appl. Mater. Interfaces 2014, 6, 13438–13447. [Google Scholar] [CrossRef]
- Di Palma, L.; Bavasso, I.; Sarasini, F.; Tirillò, J.; Puglia, D.; Dominici, F.; Torre, L. Synthesis, characterization and performance evaluation of Fe3O4/PES nano composite membranes for microbial fuel cell. Eur. Polym. J. 2018, 99, 222–229. [Google Scholar] [CrossRef]
- Huang, Y.X.; Liu, X.W.; Sun, X.F.; Sheng, G.P.; Zhang, Y.Y.; Yan, G.M.; Wang, S.G.; Xu, A.W.; Yu, H.Q. A new cathodic electrode deposit with palladium nanoparticles for cost-effective hydrogen production in a microbial electrolysis cell. Int. J. Hydrogen Energy 2011, 36, 2773–2776. [Google Scholar] [CrossRef]
- Hennebel, T.; Benner, J.; Clauwaert, P.; Vanhaecke, L.; Aelterman, P.; Callebaut, R.; Boon, N.; Verstraete, W. Dehalogenation of environmental pollutants in microbial electrolysis cells with biogenic palladium nanoparticles. Biotechnol. Lett. 2011, 33, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wen, Z.; Ci, S.; Chen, J.; He, Z. Carbon/iron-based nanorod catalysts for hydrogen production in microbial electrolysis cells. Nano Energy 2012, 1, 751–756. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, C.; Jeon, Y.; Kim, S. Hydrogen Production from Makgeolli Wastewater Using a Single-Chamber Microbial Electrolysis Cell. Bull. Korean Chem. Soc. 2019, 12–14. [Google Scholar] [CrossRef]
- Choi, M.J.; Yang, E.; Yu, H.W.; Kim, I.S.; Oh, S.E.; Chae, K.J. Transition metal/carbon nanoparticle composite catalysts as platinum substitutes for bioelectrochemical hydrogen production using microbial electrolysis cells. Int. J. Hydrogen Energy 2019, 44, 2258–2265. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; He, Z. Bioelectrochemical deposition of palladium nanoparticles as catalysts by Shewanella oneidensis MR-1 towards enhanced hydrogen production in microbial electrolysis cells. Electrochim. Acta 2019, 318, 794–800. [Google Scholar] [CrossRef]
- Zakaria, B.S.; Barua, S.; Sharaf, A.; Liu, Y.; Dhar, B.R. Impact of antimicrobial silver nanoparticles on anode respiring bacteria in a microbial electrolysis cell. Chemosphere 2018, 213, 259–267. [Google Scholar] [CrossRef]
- Hu, J.; Zeng, C.; Liu, G.; Luo, H.; Qu, L.; Zhang, R. Magnetite nanoparticles accelerate the autotrophic sulfate reduction in biocathode microbial electrolysis cells. Biochem. Eng. J. 2018, 133, 96–105. [Google Scholar] [CrossRef]
- Yuan, H.; Deng, L.; Cai, X.; Zheng, T.; Zhou, S.; Chen, Y.; Yuan, Y. Recycling electroplating sludge to produce sustainable electrocatalysts for the efficient conversion of carbon dioxide in a microbial electrolysis cell. Electrochim. Acta 2016, 222, 177–184. [Google Scholar] [CrossRef]
- Jayabalan, T.; Matheswaran, M.; Radhakrishnan, T.K.; Naina Mohamed, S. Influence of Nickel molybdate nanocatalyst for enhancing biohydrogen production in microbial electrolysis cell utilizing sugar industrial effluent. Bioresour. Technol. 2021, 320, 124284. [Google Scholar] [CrossRef]
- Ngaw, C.K.; Zhao, C.; Wang, V.B.; Kjelleberg, S.; Yang Tan, T.T.; Zhang, Q.; Joachim Loo, S.C. A graphene/carbon nanotube biofilm based solar-microbial fuel device for enhanced hydrogen generation. Sustain. Energy Fuels 2017, 1, 191–198. [Google Scholar] [CrossRef]
- Sharma, T.; Mohanareddy, A.; Chandra, T.; Ramaprabhu, S. Development of carbon nanotubes and nanofluids based microbial fuel cell. Int. J. Hydrogen Energy 2008, 33, 6749–6754. [Google Scholar] [CrossRef]
- Vishwanathan, A.S.; Aiyer, K.S.; Chunduri, L.A.A.; Venkataramaniah, K.; Siva Sankara Sai, S.; Rao, G. Carbon quantum dots shuttle electrons to the anode of a microbial fuel cell. 3 Biotech 2016, 6, 228. [Google Scholar] [CrossRef]
- Christwardana, M.; Kwon, Y. Yeast and carbon nanotube based biocatalyst developed by synergetic effects of covalent bonding and hydrophobic interaction for performance enhancement of membraneless microbial fuel cell. Bioresour. Technol. 2017, 225, 175–182. [Google Scholar] [CrossRef]
- Chakraborty, I.; Sathe, S.M.; Dubey, B.K.; Ghangrekar, M.M. Waste-derived biochar: Applications and future perspective in microbial fuel cells. Bioresour. Technol. 2020, 312, 123587. [Google Scholar] [CrossRef]
- Shen, R.; Zhao, L.; Yao, Z.; Feng, J.; Jing, Y.; Watson, J. Efficient Treatment of Wood Vinegar via Microbial Electrolysis Cell with the Anode of Different Pyrolysis Biochars. Front. Energy Res. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Keruthiga, K.; Mohamed, S.N.; Gandhi, N.N.; Muthukumar, K. Sugar industry waste-derived anode for enhanced biohydrogen production from rice mill wastewater using artificial photo-assisted microbial electrolysis cell. Int. J. Hydrogen Energy 2021. [Google Scholar] [CrossRef]
- Jiang, B.; Zeng, Q.; Liu, J.; Hou, Y.; Xu, J.; Li, H.; Shi, S.; Ma, F. Enhanced treatment performance of phenol wastewater and membrane antifouling by biochar-assisted EMBR. Bioresour. Technol. 2020, 306, 123147. [Google Scholar] [CrossRef]
- Chen, S.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Liu, F.; Fan, W.; Nevin, K.P.; Lovley, D.R. Promoting Interspecies Electron Transfer with Biochar. Sci. Rep. 2015, 4, 5019. [Google Scholar] [CrossRef] [PubMed]
- Christwardana, M.; Frattini, D.; Duarte, K.D.Z.; Accardo, G.; Kwon, Y. Carbon felt molecular modification and biofilm augmentation via quorum sensing approach in yeast-based microbial fuel cells. Appl. Energy 2019, 238, 239–248. [Google Scholar] [CrossRef]
- Christwardana, M.; Frattini, D.; Accardo, G.; Yoon, S.P.; Kwon, Y. Effects of methylene blue and methyl red mediators on performance of yeast based microbial fuel cells adopting polyethylenimine coated carbon felt as anode. J. Power Sources 2018, 396, 1–11. [Google Scholar] [CrossRef]
- Esmaeili, C.; Ghasemi, M.; Heng, L.Y.; Hassan, S.H.A.; Abdi, M.M.; Daud, W.R.W.; Ilbeygi, H.; Ismail, A.F. Synthesis and application of polypyrrole/carrageenan nano-bio composite as a cathode catalyst in microbial fuel cells. Carbohydr. Polym. 2014, 114, 253–259. [Google Scholar] [CrossRef]
- Hernández, L.A.; Riveros, G.; González, D.M.; Gacitua, M.; del Valle, M.A. PEDOT/graphene/nickel-nanoparticles composites as electrodes for microbial fuel cells. J. Mater. Sci. Mater. Electron. 2019, 30, 12001–12011. [Google Scholar] [CrossRef]
- Yang, W.; Lu, J.E.; Zhang, Y.; Peng, Y.; Mercado, R.; Li, J.; Zhu, X.; Chen, S. Cobalt oxides nanoparticles supported on nitrogen-doped carbon nanotubes as high-efficiency cathode catalysts for microbial fuel cells. Inorg. Chem. Commun. 2019, 105, 69–75. [Google Scholar] [CrossRef]


| N. | Source Material | Surface Area (m2 g−1) | Avg. Pore Size (nm) | Process Conditions | Trace Elements | Ref. |
|---|---|---|---|---|---|---|
| 1 | Water hyacinth (Eichhornia crassipes) | 25.9 (BET) * | 5.6 (BET) | Washing, sunlight drying, drying at 80 °C for 1 h, pyrolysis at 900 °C for 2 h at a rate of 25 °C min−1 | Al, Mg, Si, P, S, O, K, Ca, Fe, Cu, Zn (EDS) * | [50] |
| 2 | Bananas | 105.1–172.3 (BET) | - | Hydrothermal treatment at 180 °C for 12 h, filtration, washing, drying at 60 °C, pyrolysis at 900 °C for 2 h in Ar, washing, drying at 100 °C for 12 h, activation with KOH | O, N (EDS) | [51] |
| 3 | Alfalfa leaves | 148–883 (BET) | - | Pyrolysis at 250 °C for 2 h in N2, FeCl3/KOH/ZnCl2 activation, pyrolysis at 900 °C for 2 h in N2 | O, N, P, S, Fe (EDS) | [52] |
| 4 | Corncob | 68.5–655.9 (CV) * | - | Sunlight drying for 5 days and pyrolysis at 750 °C for 2 h in N2 | O, K, N, P (EDS) | [53] |
| 5 | Green algae (Spirogyra) | 258 (BET) | 4 (BET) 50–200 μm (SEM) | Washing, shaping, vacuum filtration, freeze-drying, and pyrolysis at 1100 °C for 2 h in N2 | - | [54] |
| 6 | Pinewood lumber | 42.4 (BET) | - | Gasification at 1000 °C at a rate of 17 °C min−1 in air, sonication 30 min, stirring at 80 °C for 2 h in 3 M KOH, filtering, washing, vacuum drying at 50 °C for 3 h, functionalization with Mn | Al, Ca, K, Mg, Mn, P, Si, Ti (ICP) * | [55] |
| 7 | Lotus leaves | 611–909 (BET) | 0.49–1.23 (BET) | Washing, ultrasonication for 2 h, drying at 40 °C for 12 h, hydrothermal treatment with ZnCl2/(NH4)2SO4 at 160 °C for 2 h, drying 60 °C for 24 h, pyrolysis at 1000 °C for 2 h in N2, washing, drying at 60 °C for 24 h | Ca, N, O, S (XPS) * | [56] |
| 8 | Sewage Sludge | 44 (BET) | 0.8–8.6 (BET) | Drying, pyrolysis at 900 °C for 2 h at a rate of 5 °C min−1 in N2 | N, O, Fe (XPS) | [57] |
| 9 | Coffee waste | 3.5–428 (BET) | 7.9–23.2 (BET) | Washing, filtering, drying, activation in KOH for 24 h, pyrolysis at 900 °C for 1 h in N2, washing, drying for 24 h | - | [58] |
| 10 | Kapok tree fibres | - | 10–20 μm (SEM) * | Washing, shaping, pyrolysis at 1100 °C for 2 h at a rate of 30/200 °C h−1 in 95% Ar and 5% H2, washing, filtering | - | [59] |
| 11 | Eggplant | 637–1181 (BET) | - | Peeling, shredding, washing, drying at 100 °C for 8 h, activation in K3[Fe(C2O4)3], drying, pyrolysis at 800 °C for 1 h at a rate of 5 °C min−1 N2, washing in 2 M of HCl, washing, drying at 70 °C for 8 h | N, O (EDS) | [60] |
| 12 | Rice husk | 1164–1809 (BET) | 12.4–23.1 (BET) | Grinding, washing, drying at 105 °C for 12 h, washing in 98–54% wt H2SO4 at 55 °C, filtration, washing, drying at 60 °C for 2 h, drying at 95 °C for 6 h, washing, drying at 120 °C for 12 h, pyrolysis at 500 °C for 0.5 h in N2, activation with NaOH/KOH, pyrolysis at 400 °C for 0.5 h and then 800 °C for 1 h | - | [61] |
| 13 | Mixed microalgae | 21 (BET) | - | Filtering, washing with HCl at 1 mM, centrifugation, drying at 60 °C, pyrolysis at 400 °C for 2 h at a rate of 10 °C min−1, washing, drying at 60 °C, pyrolysis at 900 °C for 1 h at a rate of 5 °C min−1 in Ar, washing, drying at 60 °C overnight | N, O, P (XPS) | [62] |
| 14 | Watermelon rind | 78–659 (CV) | - | Washing, drying at 40 °C for 72 h, sonication in 1 M of HCl for 5 h, filtering, washing, drying at 85 °C for 12 h, pyrolysis at 700 °C for 2 h at a rate of 5 °C min−1 in N2 | N, O (XPS, EDS) | [63] |
| 15 | Sewage sludge/coconut shell | 16–54 (BET) | - | Grinding, shaping, drying at 50 °C, pyrolysis at 900 °C for 2 h at a rate of 5 °C min−1 in N2 | O, N, Fe, P (XPS, EDS) | [64] |
| 16 | Sludge from wastewater treatment | 41.8 (BET) | - | Drying at 105 °C, pyrolysis at 500 °C for 1.5 h at a rate of 10 °C min−1 in N2, HCl activation, drying at 105 °C | N, S, Si, Fe, Al, Ca, Mg, K, Na (elemental analysis) | [65] |
| N. | Source Material | Nanoparticle | Size (nm) | Shape | Purity (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Bacterium (Bacillus cereus) | Ag | 20–40 | Spherical | 80 | [74] |
| 2 | Bacterium (Desulfovvibrio desulfuricans) | Au | 5–50 | Nanorods or nanoprisms | 80 | [75] |
| 3 | Bacterium (Aquaspirillum magnetotacticum) | Fe3O4 | 10 | Octahedral prism | 70 | [76] |
| 4 | Bacterium (Shewanella oneidensis) | UO2 | - | - | - | [77] |
| 5 | Bacterium (E. coli) | CdS | 2.9 | Spherical | 100 | [78] |
| 6 | Fungus (Rhizopus nigricans) | Ag | 13–74 | Spherical | 100 | [79] |
| 7 | Fungus (Verticillium luteoalbum) | Au | ~100 | Spherical, triangular, hexagonal | - | [80] |
| 8 | Fungus (Fusarium oxysporum) | Au-Ag alloy | 8–141 | Spherical | 98 | [81] |
| 9 | Fungus (Aspergillus terreus) | ZnO | 8.2 | Spherical | 100 | [82] |
| 10 | Fungus (Aspergillus flavus TFR7) | TiO2 | 12–15 | Spherical | 80 | [83] |
| 11 | Plant (Acalypha indica) | Ag | 20–30 | Spherical | 80 | [84] |
| 12 | Plant (Avena sativa) | Au | 25–85 | Spherical, triangular, hexagonal | 78 | [85] |
| 13 | Plant (Gardenia jasminoides Ellis) | Pd | 3–5 | Spherical, rod and three-dimensional polyhedral | 80 | [86] |
| 14 | Plant (Medicago sativa) | FeO | 1–10 | Spherical | - | [87] |
| 15 | Plant (Sedum alfredii Hance) | ZnO | 53.7 | Spherical | - | [88] |
| 16 | Plant (Aloe barbadensis Miller) | In2O3 | 5–50 | Spherical | - | [89] |
| N. | Nanomaterial | Green/Sustainable (Y/N) | MFC Type | Component | Best Power Density (mW m−2) | Control Power Density (mW m−2) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Pd NPs on carbon cloth | Y | Dual chamber | Anode | 605 | 534 | [123] |
| 2 | W/Co scraps | Y | Dual chamber | Cathode catalyst | 36 W m−3 | 3 W m−3 | [124] |
| 3 | Pd NPs on carbon cloth | Y | Dual chamber | Anode | 824 ± 36 | 680 ± 28 | [125] |
| 4 | MnO2 NPs on carbon cloth | N | Dual chamber | Anode | 782 ± 37 | ||
| 5 | Fe3O4 NPs on carbon cloth | N | Dual chamber | Anode | 728 ± 33 | ||
| 6 | Fe nanorods | N | Dual chamber | Cathode catalyst | 66.4 mW m−3 | 10.6 mW m−3 | [126] |
| 7 | V2O5 nanorods | N | Single chamber | Cathode catalyst | 1073 ± 18 | 2067 ± 25 | [127] |
| 8 | Au NPs on carbon paper | N | Dual chamber | Anode | 346 | 174 | [128] |
| 9 | MnOx nanorods | N | Single chamber | Cathode catalyst | 772.8 mW m−3 | 236.7 mW m−3 | [129] |
| 10 | WO3 NPs on carbon felt | N | Single chamber | Anode | 1280 | 490 | [130] |
| N. | Source Material | MFC/MEC Type | Component | Best Power Density (mW m−2) | Control Power Density (mW m−2) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Water hyacinth (Eichhornia crassipes) | Single chamber | Cathode catalyst | 12.3 | 24.7 | [50] |
| 2 | Bananas | Single chamber | Cathode catalyst | 528.2 | 695 | [51] |
| 3 | Alfalfa leaves | Single chamber | Cathode catalyst | 1328.9 | 1337.7 | [52] |
| 4 | Corncob | Single chamber | Cathode catalyst | 458.8 mW m−3 | - | [53] |
| 5 | Green algae (Spirogyra) | Dual chamber | Anode electrode | 408 ± 12 | 62 | [54] |
| 6 | Pinewood lumber | Single chamber | Cathode catalyst | 146.7 | 156.8 | [55] |
| 7 | Lotus leaves | Single chamber | Cathode catalyst | 511.5 ± 25.6 | 486.7 ± 23.3 | [56] |
| 8 | Sewage Sludge | Single chamber | Cathode catalyst | 500 ± 17 | 625 ± 17 | [57] |
| 9 | Coffee waste | Single chamber | Anode electrode | 3927 | 975 | [58] |
| 10 | Kapok tree fibres | Single chamber | Anode | 1738.1 | 1689.8 | [59] |
| 11 | Eggplant | Single chamber | Cathode catalyst | 667 | 621 | [60] |
| 12 | Rice husk | Single chamber | Cathode catalyst | 317.7 ± 0.4 | 367.8 ± 7.8 | [61] |
| 13 | Mixed microalgae | Dual chamber | Cathode catalyst | 12.86 ± 0.35 W m−3 | 13.52 ± 0.05 W m−3 | [62] |
| 14 | Watermelon rind | Single chamber | Cathode catalyst | 262 mW m−3 | - | [63] |
| 15 | Sewage sludge/coconut shell | Single chamber | Anode and cathode catalysts | 969 ± 28 | 1069 ± 15 | [64] |
| N. | Source Material | MEC type | Component | Best performance | Control Performance | |
| 16 | Sludge from wastewater treatment | Single chamber | Anode catalyst | (CH4 prod.) 110 mLCH4 g−1VSadded | (CH4 prod.) 80 mLCH4 g−1VSadded | [65] |
| 17 | Coconut shell | Dual chamber | Anode catalyst | (COD rem.) 71.4% | (COD rem.) 59.6% | [152] |
| 18 | Sugar industry filter cake | Dual chamber | Anode catalyst | (H2 prod.) 3.6 ± 0.4 mLH2 L−1h−1 | - | [153] |
| 19 | Straw | Dual chamber | Anode catalyst | (COD rem.) 87.94% | (COD rem.) 57.58% | [154] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frattini, D.; Karunakaran, G.; Cho, E.-B.; Kwon, Y. Sustainable Syntheses and Sources of Nanomaterials for Microbial Fuel/Electrolysis Cell Applications: An Overview of Recent Progress. Processes 2021, 9, 1221. https://doi.org/10.3390/pr9071221
Frattini D, Karunakaran G, Cho E-B, Kwon Y. Sustainable Syntheses and Sources of Nanomaterials for Microbial Fuel/Electrolysis Cell Applications: An Overview of Recent Progress. Processes. 2021; 9(7):1221. https://doi.org/10.3390/pr9071221
Chicago/Turabian StyleFrattini, Domenico, Gopalu Karunakaran, Eun-Bum Cho, and Yongchai Kwon. 2021. "Sustainable Syntheses and Sources of Nanomaterials for Microbial Fuel/Electrolysis Cell Applications: An Overview of Recent Progress" Processes 9, no. 7: 1221. https://doi.org/10.3390/pr9071221
APA StyleFrattini, D., Karunakaran, G., Cho, E.-B., & Kwon, Y. (2021). Sustainable Syntheses and Sources of Nanomaterials for Microbial Fuel/Electrolysis Cell Applications: An Overview of Recent Progress. Processes, 9(7), 1221. https://doi.org/10.3390/pr9071221









