Selective Gold and Palladium Adsorption from Standard Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
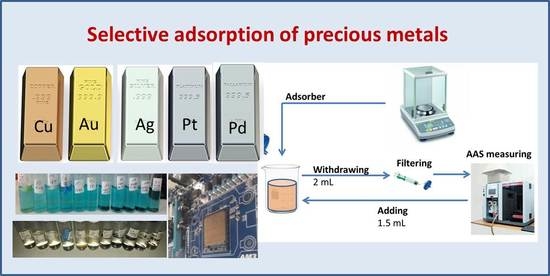
2.2. Adsorption Tests
2.3. Microscopy and Energy Dispersive Spectroscopy Characterization
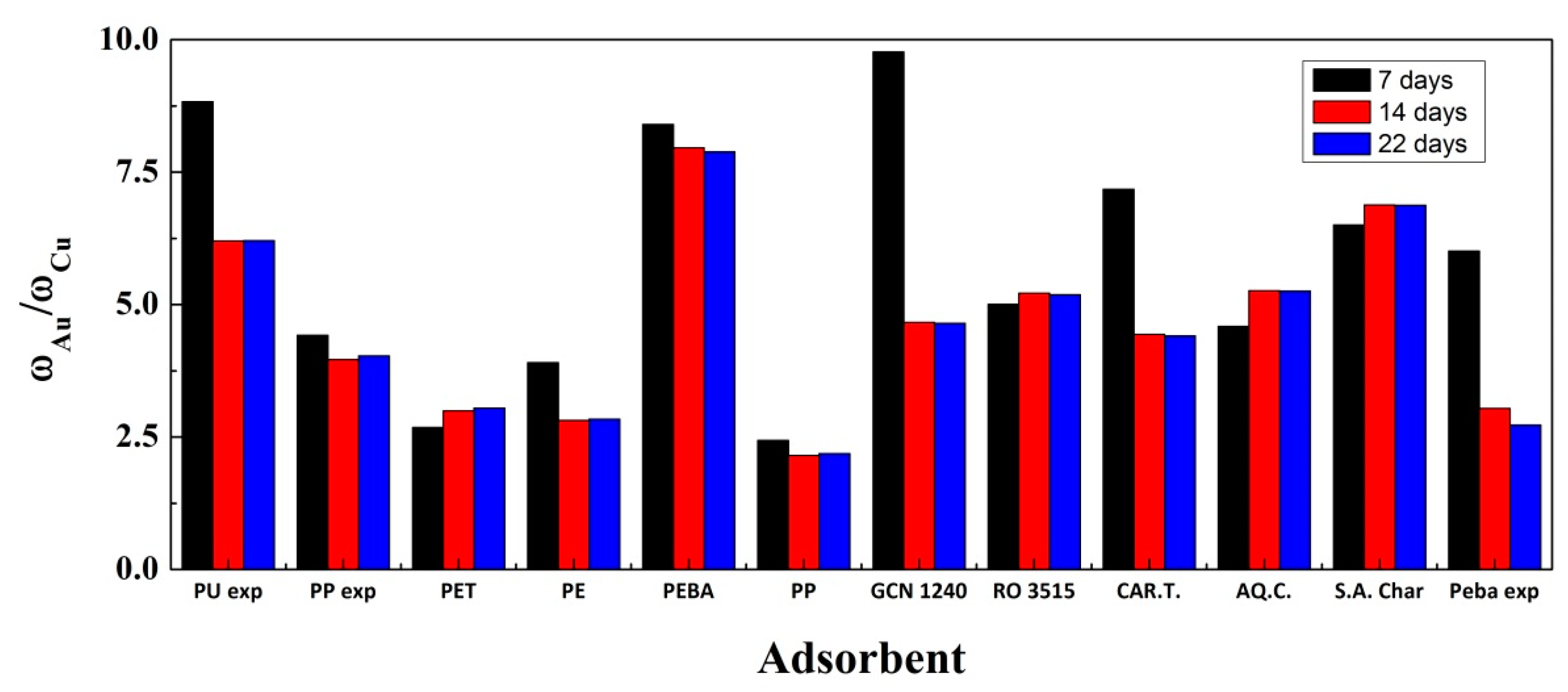
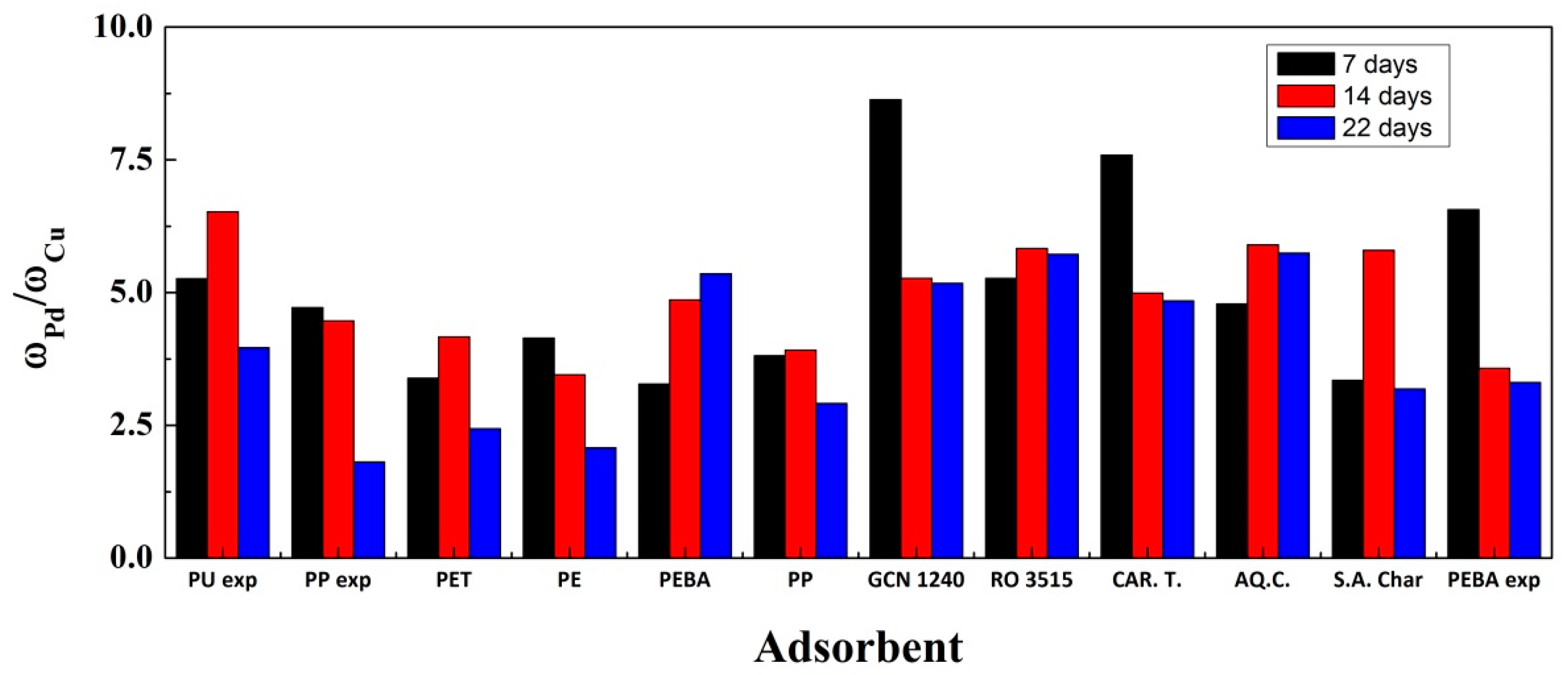
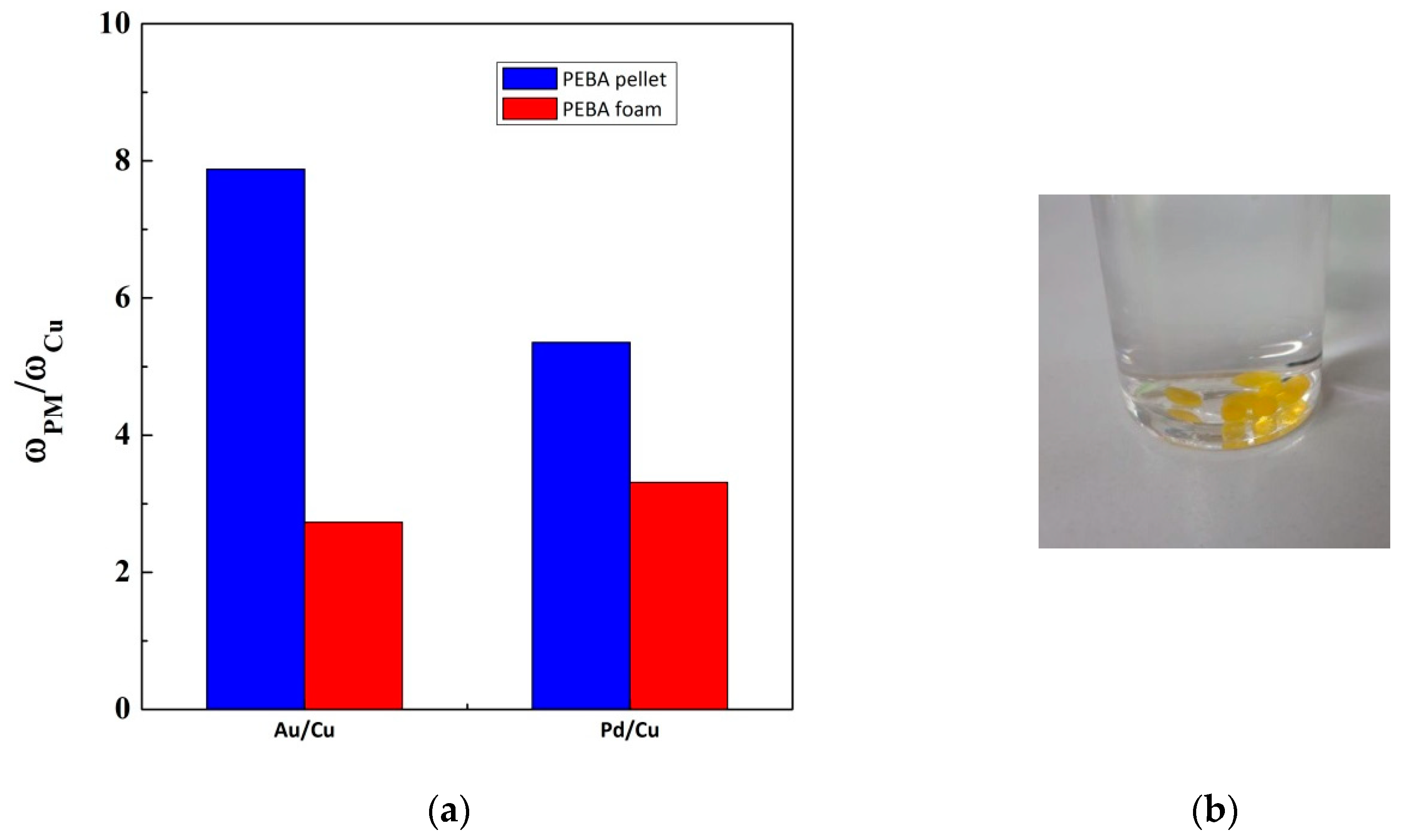
3. Results
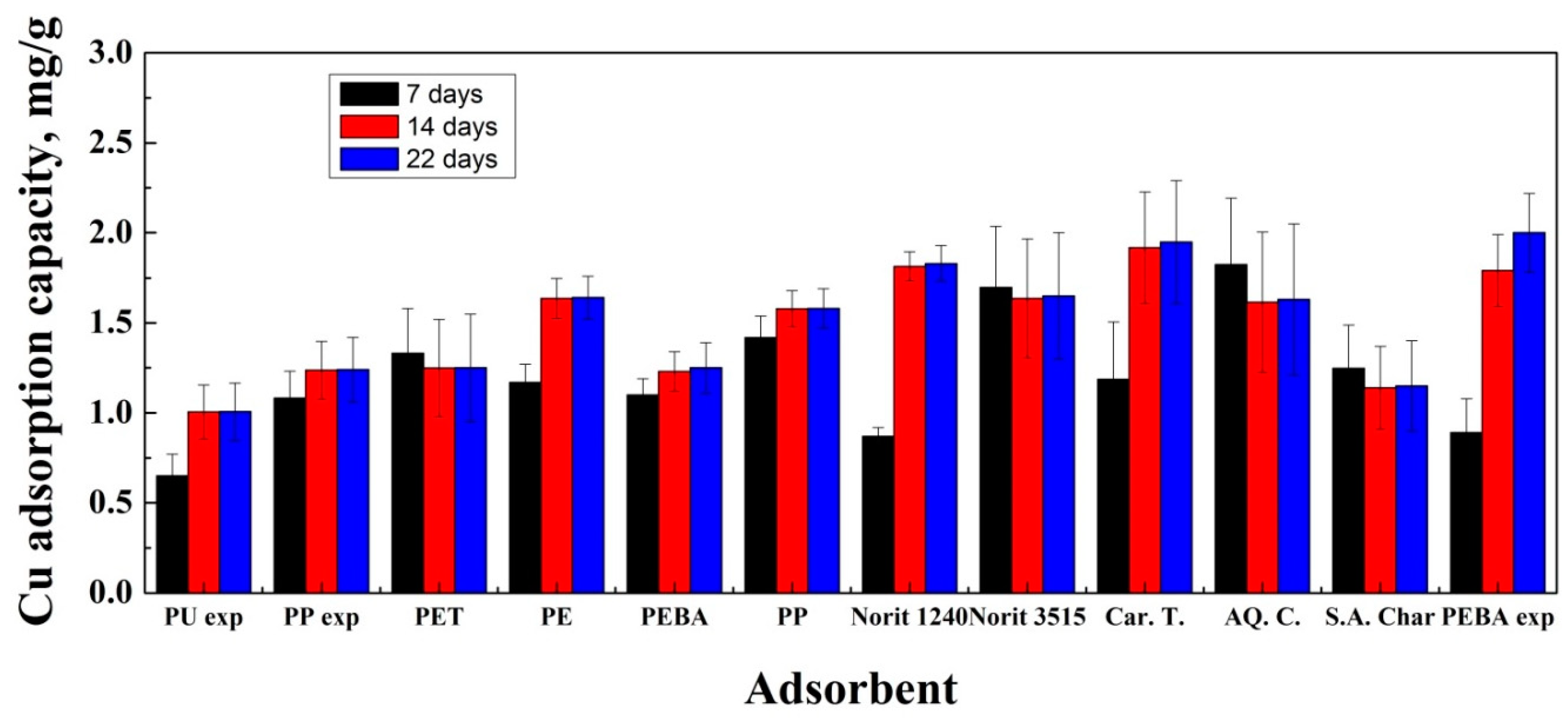
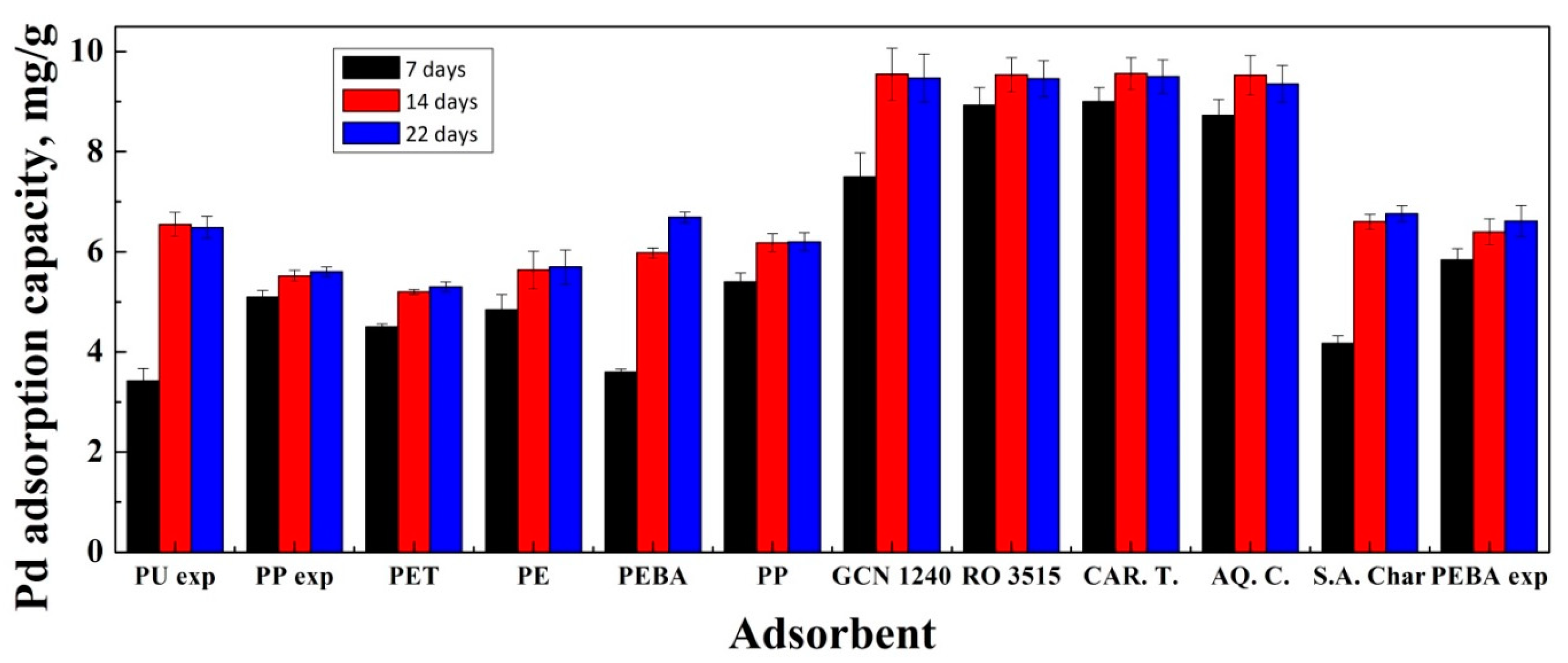
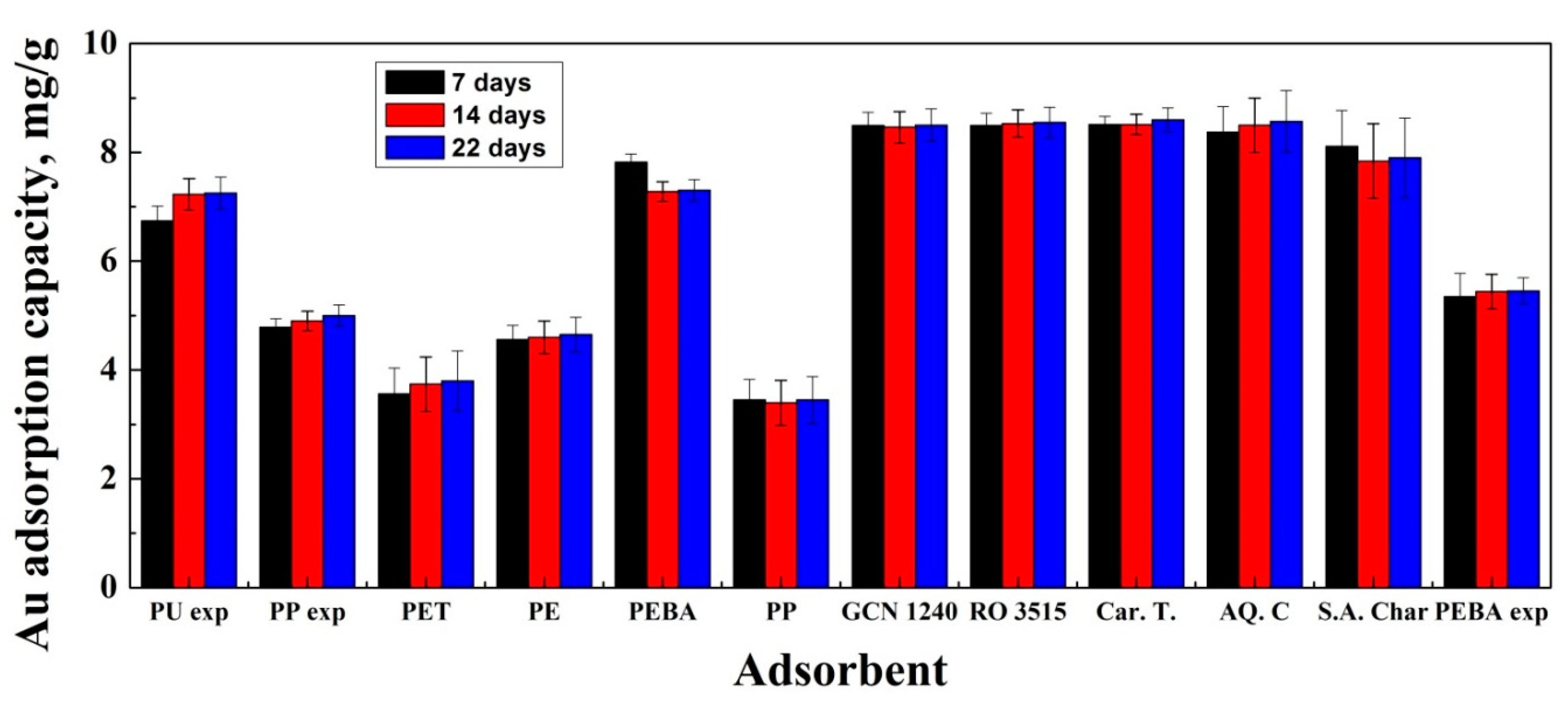
3.1. Adsorption Experiments
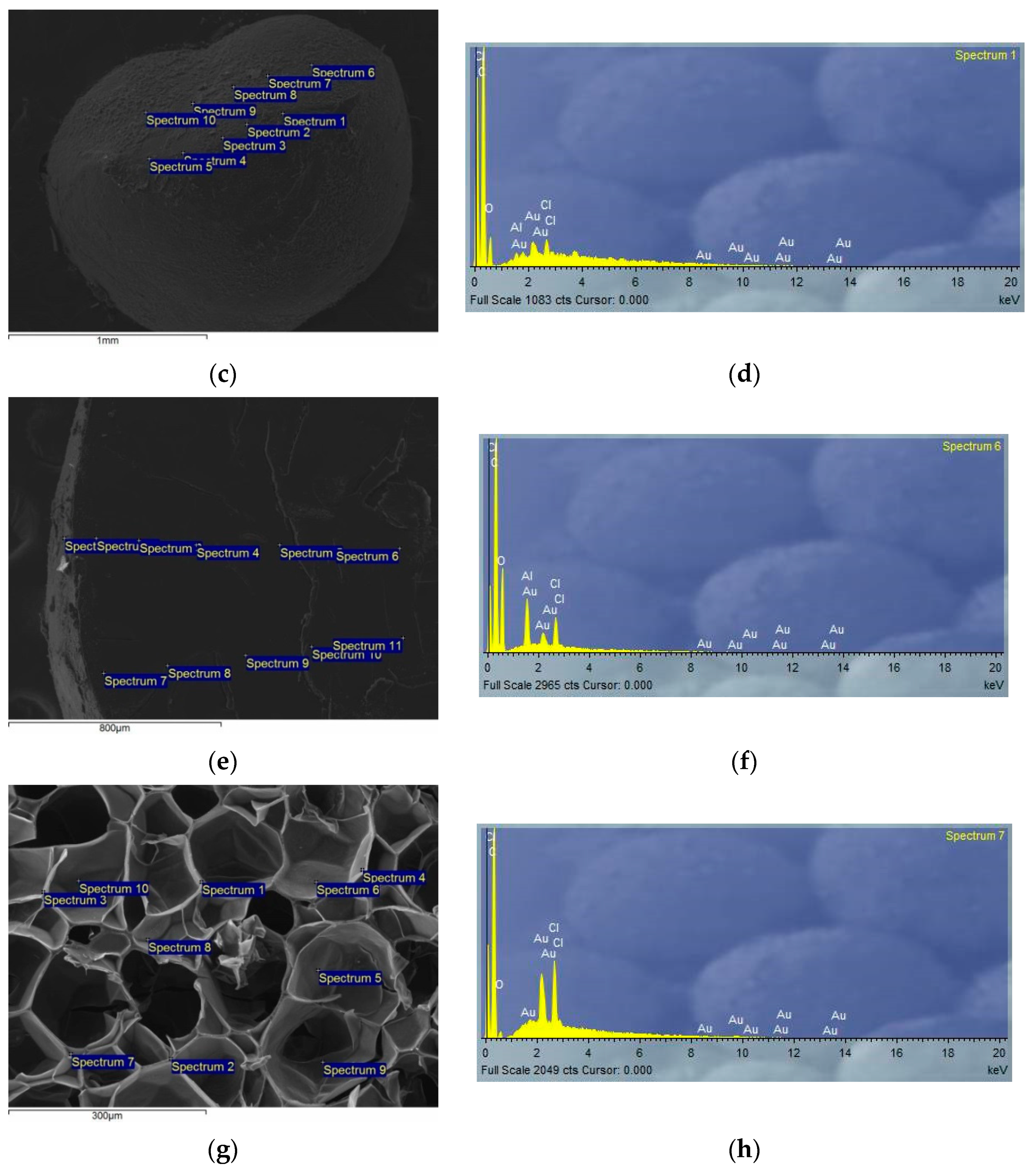
3.2. SEM-EDS Characterizations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Benhabib, J.; Spiegel, M.M. Human capital and technology diffusion. Handb. Econ. Growth 2005, 1, 935–966. [Google Scholar] [CrossRef]
- Köhler, A.; Erdmann, L. Expected environmental impacts of pervasive computing. Hum. Ecol. Risk Assess. 2004, 10, 831–852. [Google Scholar] [CrossRef]
- Ylä-Mella, J.; Poikela, K.; Lehtinen, U.; Keiski, R.L.; Pongrácz, E. Implementation of waste electrical and electronic equipment directive in Finland: Evaluation of the collection network and challenges of the effective WEEE management. Resour. Conserv. Recycl. 2014, 86, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Kilic, H.S.; Cebeci, U.; Ayhan, M.B. Reverse logistics system design for the waste of electrical and electronic equipment (WEEE) in Turkey. Resour. Conserv. Recycl. 2015, 95, 120–132. [Google Scholar] [CrossRef]
- Kaya, M. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manag. 2016, 57, 64–90. [Google Scholar] [CrossRef]
- Ongondo, F.O.; Williams, I.D.; Cherrett, T.J. How are WEEE doing? A global review of the management of electrical and electronic wastes. Waste Manag. 2011, 31, 714–730. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, Y.; Liu, B.; Chang, C.C. Supply and demand of some critical metals and present status of their recycling in WEEE. Waste Manag. 2017, 65, 113–127. [Google Scholar] [CrossRef]
- Chen, D.; Heyer, S.; Ibbotson, S.; Salonitis, K.; Steingrímsson, J.G.; Thiede, S. Direct digital manufacturing: Definition, evolution, and sustainability implications. J. Clean. Prod. 2015, 107, 615–625. [Google Scholar] [CrossRef]
- Alzate, A.; López, M.E.; Serna, C. Recovery of gold from waste electrical and electronic equipment (WEEE) using ammonium persulfate. Waste Manag. 2016, 57, 113–120. [Google Scholar] [CrossRef]
- Ueberschaar, M.; Otto, S.J.; Rotter, V.S. Challenges for critical raw material recovery from WEEE–The case study of gallium. Waste Manag. 2017, 60, 534–545. [Google Scholar] [CrossRef]
- Charles, R.G.; Douglas, P.; Hallin, I.L.; Matthews, I.; Liversage, G. An investigation of trends in precious metal and copper content of RAM modules in WEEE: Implications for long term recycling potential. Waste Manag. 2017, 60, 505–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.P.; Lim, L.L. Recovery of precious metals by an electrochemical deposition method. Chemosphere 2005, 60, 1384–1392. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, Y.J. A novel process of extracting precious metals from waste printed circuit boards: Utilization of gold concentrate as a fluxing material. J. Hazard. Mater. 2019, 365, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Diaz, L.A.; Yang, Z.; Jin, H.; Lister, T.E.; Vahidi, E.; Zhao, F. Comparative life cycle analysis for value recovery of precious metals and rare earth elements from electronic waste. Resour. Conserv. Recycl. 2019, 149, 20–30. [Google Scholar] [CrossRef]
- Ojeda, M.W.; Perino, E.; Ruiz, M.D.C. Gold extraction by chlorination using a pyrometallurgical process. Miner. Eng. 2009, 22, 409–411. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Li, Y.; Sun, X.; Li, J.; Shen, J.; Han, W.L. A novel approach for recovery of metals from waste printed circuit boards and simultaneous removal of iron from steel pickling waste liquor by two-step hydrometallurgical method. Waste Manag. 2018, 71, 411–419. [Google Scholar] [CrossRef]
- Palmieri, R.; Bonifazi, G.; Serranti, S. Recycling-oriented characterization of plastic frames and printed circuit boards from mobile phones by electronic and chemical imaging. Waste Manag. 2014, 34, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Cesaro, A.; Belgiorno, V. Separation efficiency of valuable and critical metals in WEEE mechanical treatments. J. Clean. Prod. 2018, 186, 490–498. [Google Scholar] [CrossRef]
- Lennartsson, A.; Engström, F.; Samuelsson, C.; Björkman, B.; Pettersson, J. Large-Scale WEEE recycling integrated in an ore-based Cu-extraction system. J. Sustain. Metall. 2018, 4, 222–232. [Google Scholar] [CrossRef] [Green Version]
- Muchova, L.; Bakker, E.; Rem, P. Precious metals in municipal solid waste incineration bottom ash. Water Air Soil Pollut. Focus 2009, 9, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Trucillo, P.; Lancia, A.; D’Amore, D.; Brancato, B.; Di Natale, F. Selective Leaching of Precious Metals from Electrical and Electronic Equipment Through Hydrometallurgical Methods. Chem. Eng. Trans. 2021, 86, 1039–1044. [Google Scholar] [CrossRef]
- Saleem, J.; Bazargan, A.; Barford, J.; McKay, G. Oil spill remedy using bi-axially oriented polymer films. WIT Trans. Ecol. Environ. 2014, 182, 145–152. [Google Scholar]
- Worthington, M.J.H.; Shearer, C.J.; Esdaile, L.J.; Campbell, J.A.; Gibson, C.T.; Legg, S.K.; Yin, Y.; Lundquist, N.A.; Gascooke, J.R.; Albuquerque, I.S.; et al. Sustainable polysulfides for oil spill remediation: Repurposing industrial waste for environmental benefit. Adv. Sustain. Syst. 2018, 2, 1800024. [Google Scholar] [CrossRef] [Green Version]
- Hwa Gupta, S.; He, W.-D.; Tai, N.-H. A comparative study on superhydrophobic sponges and their application as fluid channel for continuous separation of oils and organic solvents from water. Compos. Part B Eng. 2016, 101, 99–106. [Google Scholar] [CrossRef]
- Alabi, O.A.; Bakare, A.A.; Xu, X.; Li, B.; Zhang, Y.; Huo, X. Comparative evaluation of environmental contamination and DNA damage induced by electronic-waste in Nigeria and China. Sci. Total Environ. 2012, 423, 62–72. [Google Scholar] [CrossRef]
- Hao, J.; Wang, Y.; Wu, Y.; Guo, F. Metal recovery from waste printed circuit boards: A review for current status and perspectives. Resour. Conserv. Recycl. 2020, 157, 104787. [Google Scholar] [CrossRef]
- Ai, X.; Ju, J. Research on several problems in enrichment and separation of gold with foam plastic. Gold 2008, 29, 46–49. [Google Scholar]
- Xue, D.; Li, T.; Liu, Y.; Yang, Y.; Zhang, Y.; Cui, J.; Guo, D. Selective adsorption and recovery of precious metal ions from water and metallurgical slag by polymer brush graphene–polyurethane composite. React. Funct. Polym. 2019, 136, 138–152. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Saleem, J.; Bazargan, A.; Duarte, J.L.D.S.; McKay, G.; Meili, L. Sorption as a rapidly response for Oil Spill accidents: A material and mechanistic approach. J. Hazard. Mater. 2020, 124842. [Google Scholar] [CrossRef]
- Worthington, M.J.; Kucera, R.L.; Chalker, J.M. Green chemistry and polymers made from sulfur. Green Chem. 2017, 19, 2748–2761. [Google Scholar] [CrossRef] [Green Version]
- Zoeteman, B.C.; Krikke, H.R.; Venselaar, J. Handling WEEE waste flows: On the effectiveness of producer responsibility in a globalizing world. Int. J. Adv. Manuf. Technol. 2010, 47, 415–436. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, G.; Jadhao, P.R.; Pant, K.K.; Nigam, K.D.P. Novel technologies and conventional processes for recovery of metals from waste electrical and electronic equipment: Challenges & opportunities—A review. J. Environ. Chem. Eng. 2018, 6, 1288–1304. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. A review of current progress of recycling technologies for metals from waste electrical and electronic equipment. J. Clean. Prod. 2016, 127, 19–36. [Google Scholar] [CrossRef]
- Di Natale, F.; Lancia, A.; Molino, A.; Musmarra, D. Removal of chromium ions form aqueous solutions by adsorption on activated carbon and char. J. Hazard. Mater. 2007, 145, 381–390. [Google Scholar] [CrossRef]
- Di Natale, F.; Lancia, A.; Molino, A.; Di Natale, M.; Karatza, D.; Musmarra, D. Capture of mercury ions by natural and industrial materials. J. Hazard. Mater. 2006, 132, 220–225. [Google Scholar] [CrossRef]
- Molino, A.; Erto, A.; Di Natale, F.; Donatelli, A.; Iovane, P.; Musmarra, D. Gasification of granulated scrap tires for the production of syngas and a low-cost adsorbent for Cd (II) removal from wastewaters. Ind. Eng. Chem. Res. 2013, 52, 12154–12160. [Google Scholar] [CrossRef]
- Ishikawa, S.I.; Suyama, K.; Arihara, K.; Itoh, M. Uptake and recovery of gold ions from electroplating wastes using eggshell membrane. Bioresour. Technol. 2020, 81, 201–206. [Google Scholar] [CrossRef]
- Gamez, G.; Gardea-Torresdey, J.L.; Tiemann, K.J.; Parsons, J.; Dokken, K.; Yacaman, M.J. Recovery of gold (III) from multi-elemental solutions by alfalfa biomass. Adv. Environ. Res. 2003, 7, 563–571. [Google Scholar] [CrossRef]
- Jansen, M. The chemistry of gold as an anion. Chem. Soc. Rev. 2008, 37, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Baran, T.; Sargin, I.; Kaya, M.; Menteş, A. An environmental catalyst derived from biological waste materials for green synthesis of biaryls via Suzuki coupling reactions. J. Mol. Catal. A Chem. 2016, 420, 216–221. [Google Scholar] [CrossRef]
- Daniel, S.; Rao, P.P.; Rao, T.P. Investigation of different polymerization methods on the analytical performance of palladium (II) ion imprinted polymer materials. Anal. Chim. Acta 2005, 536, 197–206. [Google Scholar] [CrossRef]
- Guibal, E.; Sweeney, N.V.O.; Vincent, T.; Tobin, J.M. Sulfur derivatives of chitosan for palladium sorption. React. Funct. Polym. 2002, 50, 149–163. [Google Scholar] [CrossRef]
- Awual, M.R. A facile composite material for enhanced cadmium (II) ion capturing from wastewater. J. Environ. Chem. Eng. 2019, 7, 103378. [Google Scholar] [CrossRef]
- Okieimen, F.E.; Sogbaike, C.E.; Ebhoaye, J.E. Removal of cadmium and copper ions from aqueous solution with cellulose graft copolymers. Sep. Purif. Technol. 2005, 44, 85–89. [Google Scholar] [CrossRef]
- Awual, M.R. New type mesoporous conjugate material for selective optical copper (II) ions monitoring & removal from polluted waters. Chem. Eng. J. 2017, 307, 85–94. [Google Scholar] [CrossRef]
- Di Natale, F.; Orefice, M.; La Motta, F.; Erto, A.; Lancia, A. Unveiling the potentialities of activated carbon in recovering palladium from model leaching solutions. Sep. Purif. Technol. 2017, 174, 183–193. [Google Scholar] [CrossRef]
- Di Natale, F.; Gargiulo, V.; Alfè, M. Adsorption of heavy metals on silica-supported hydrophilic carbonaceous nanoparticles (SHNPs). J. Hazard. Mater. 2020, 393, 122374. [Google Scholar] [CrossRef]

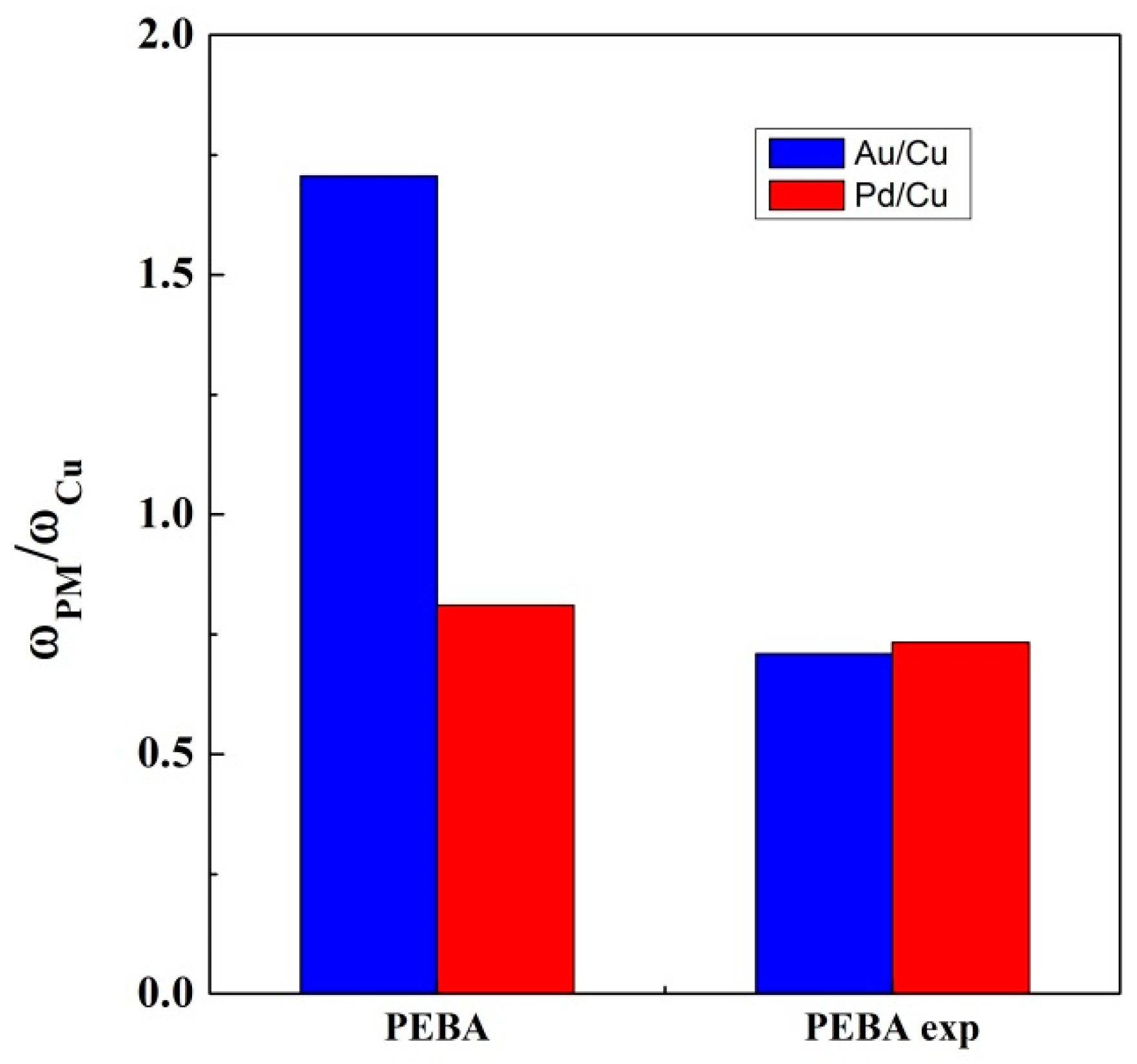
| Ads. Type | Ads. Region | Au, w/w % | Pd, w/w % | Cu, w/w % |
|---|---|---|---|---|
| PEBA | Surface | 0.25–3.79 | 0–0.06 | 0–0.04 |
| Section | 0.26–0.90 | 0–0.12 | 0–0.03 | |
| PEBA foam | Surface | 0.53–7.35 | 0–0.10 | 0–0.16 |
| Section | 0.77–2.76 | 0–0.21 | 0–0.17 |
| Ads. Type | Mixture of Aqueous Solutions | ω (Au) | ω (Pd) | ω (Cu) | ω (Au)/ω (Cu) | ω (Pd)/ω (Cu) |
|---|---|---|---|---|---|---|
| PEBA pellet | Au + Cu | 3.288 | --- | 2.776 | 1.184 | --- |
| Au + Cu + Pd | 2.677 | 1.272 | 1.569 | 1.706 | 0.811 | |
| Pd + Cu | --- | 1.439 | 2.203 | --- | 0.653 | |
| PEBA foam | Pd + Cu | --- | 2.422 | 2.989 | --- | 0.810 |
| Au + Cu | 2.976 | --- | 2.819 | 1.056 | --- | |
| Au + Cu + Pd | 1.013 | 1.047 | 1.428 | 0.709 | 0.733 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trucillo, P.; Di Maio, E.; Lancia, A.; Di Natale, F. Selective Gold and Palladium Adsorption from Standard Aqueous Solutions. Processes 2021, 9, 1282. https://doi.org/10.3390/pr9081282
Trucillo P, Di Maio E, Lancia A, Di Natale F. Selective Gold and Palladium Adsorption from Standard Aqueous Solutions. Processes. 2021; 9(8):1282. https://doi.org/10.3390/pr9081282
Chicago/Turabian StyleTrucillo, Paolo, Ernesto Di Maio, Amedeo Lancia, and Francesco Di Natale. 2021. "Selective Gold and Palladium Adsorption from Standard Aqueous Solutions" Processes 9, no. 8: 1282. https://doi.org/10.3390/pr9081282
APA StyleTrucillo, P., Di Maio, E., Lancia, A., & Di Natale, F. (2021). Selective Gold and Palladium Adsorption from Standard Aqueous Solutions. Processes, 9(8), 1282. https://doi.org/10.3390/pr9081282