Scaling-Up and Semi-Continuous Cultivation of Locally Isolated Marine Microalgae Tetraselmis striata in the Subtropical Island of Gran Canaria (Canary Islands, Spain)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae and Cultivation Conditions
2.2. Measurements of the Optical Density, Quantum Productivity and Biomass Concentration of the Cultures
2.3. Experimental Setup
2.4. Semi-Continuous Pond Operation, Definitions and Calculations
- is the combustion enthalpy of algal biomass (22.5 kJ/g) [24];
- A is the open pond illuminated surface (m2).
2.5. Determination of FA Profile and Heavy Metal Content in Algal Biomass
2.6. Statistical Treatment of the Data
3. Results and Discussion
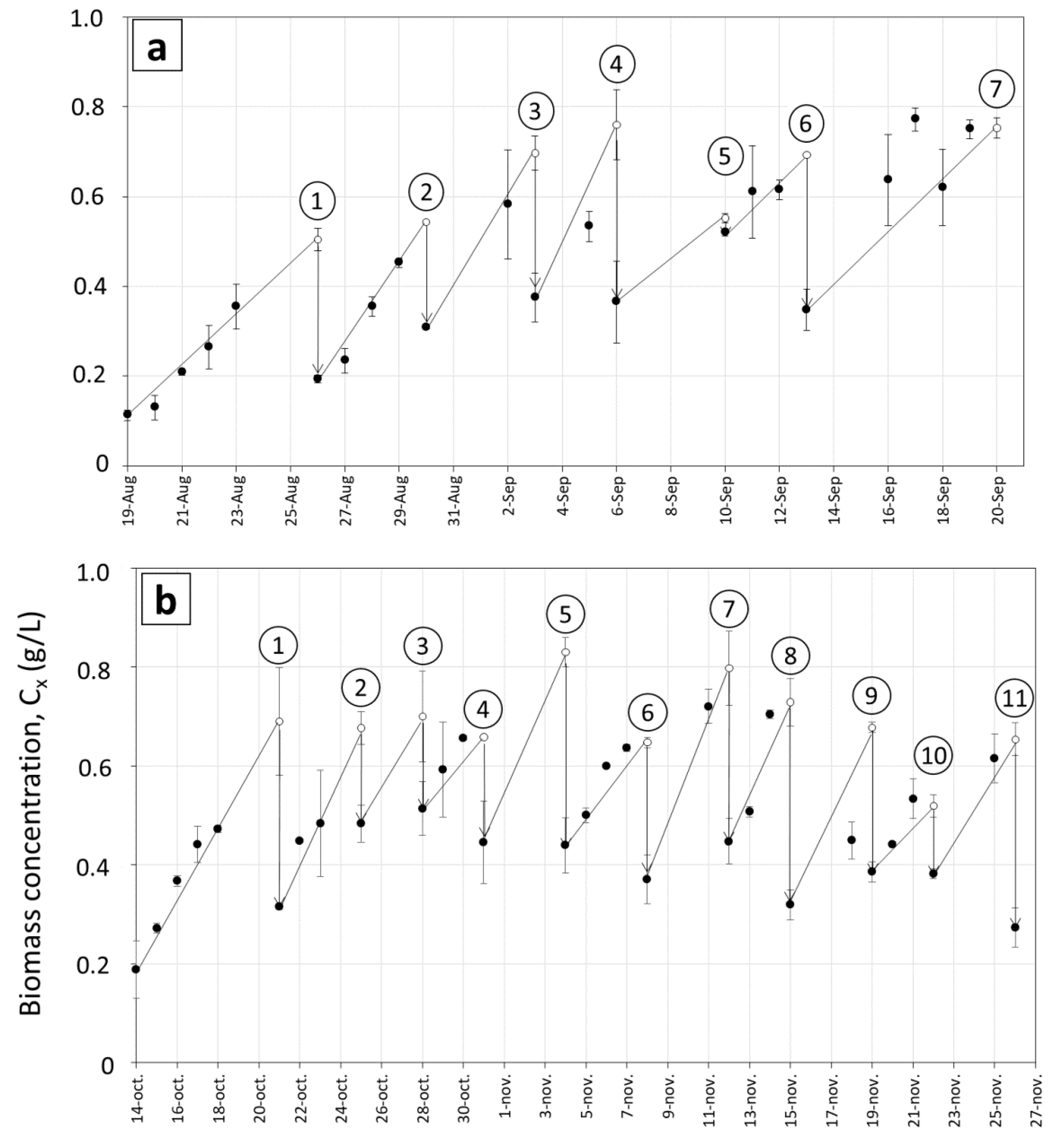
3.1. Biomass Productivity during Long Term Cultivations in Various Ponds
3.2. Growth Rates and Photosynthetic Efficiency of Open Pond Cultures
3.3. Biomass Quality, FA Profile and Heavy Metal Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A



| RW76out (8000 L–76 m2) | RW300out (45,000 L–300 m2) | ||||
|---|---|---|---|---|---|
| Harvest | Paste Humidity (% H2O) | Conc. Factor | Harvest | Paste Humidity (% H2O) | Conc. Factor |
| 21-Oct | 83.5 ± 0.62 | 239.3 ± 0.16 | 28-Nov | 87.5 ± 0.00 | 193.3 ± 0.02 |
| 25-Oct | 87.5 ± 0.14 | 187.4 ± 0.05 | 2-Dec | 87.5 ± 0.01 | 300.6 ± 0.02 |
| 31-Oct | 85.1 ± 0.10 | 226.0 ± 0.00 | 5-Dec | 86.8 ± 0.01 | 328.2 ± 0.01 |
| 4-Nov | 80.0 ± 0.05 | 241.2 ± 0.04 | 9-Dec | 83.3 ± 0.01 | 270.9 ± 0.03 |
| 8-Nov | 87.9 ± 0.05 | 187.5 ± 0.02 | 12-Dec | 86.4 ± 0.18 | 195.5 ± 0.00 |
| 12-Nov | 83.2 ± 0.04 | 210.0 ± 0.09 | 16-Dec | 88.2 ± 0.00 | 183.1 ± 0.02 |
| 15-Nov | 87.8 ± 0.00 | 167.1 ± 0.07 | 18-Dec | 89.6 ± 0.02 | 147.9 ± 0.07 |
| 19-Nov | 88.0 ± 0.04 | 177.5 ± 0.02 | 20-Dec | 84.9 ± 0.01 | 231.0 ± 0.04 |
| 26-Nov | 84.5 ± 0.01 | 237.4 ± 0.05 | - | - | - |
| Average | 85.3 ± 0.65 | 207.9 ± 0.21 | Average | 86.8 ± 0.18 | 231.3 ± 0.09 |

| Element | RW80in | RW76out | RW76out |
|---|---|---|---|
| Units: ng/gDW | 10-Sep | 22-Nov | 26-Nov |
| Co | 0 | 589 ± 26 | 618 ± 26 |
| Cd | 0 | 54 ± 0.5 | 54 ± 0.9 |
| Cr | 165 ± 8 | 3584 ± 324 | 3632 ± 260 |
| Ni | 0 | 4616 ± 409 | 4178 ± 263 |
| Se | 0 | 4627 ± 546 | 4971 ± 227 |
| Al | 0 | 49 ± 1.5 | 49 ± 0.6 |
| Si | 0 | 25 ± 2.1 | 26 ± 4.0 |
| Mo | 402 ± 20 | 0 | 0 |
| As, Hg, Pb | 0 | 0 | 0 |
References
- Boopathy, B.A.; Jayakumar, T.; Chinnasamy, S.; Rajaram, G.M.; Mohan, N.; Nagaraj, S.; Rengasamy, R.; Manubolu, M.; Sheu, J.-R.; Chang, C.-C. Biomass and lipid production potential of an indian marine algal isolate tetraselmis striata BBRR1. Energies 2020, 13, 341. [Google Scholar] [CrossRef] [Green Version]
- Lage, S.; Gojkovic, Z.; Funk, C.; Gentili, G.F. Algal biomass from wastewater and flue gases as a source of bioenergy. Energies 2018, 11, 664. [Google Scholar] [CrossRef] [Green Version]
- Pereira, H.; Páramo, J.; Silva, J.; Marques, A.; Barros, A.; Maurício, D.; Santos, T.; Schulze, P.; Barros, R.; Gouveia, L.; et al. Scale-up and large-scale production of tetraselmis sp. CTP4 (Chlorophyta) for CO2 mitigation: From an agar plate to 100-M3 industrial photobioreactors. Sci. Rep. 2018, 8, 5112. [Google Scholar] [CrossRef] [PubMed]
- Razzak, S.A.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; de Lasa, H. Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renew. Sustain. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Arora, M.; Anil, A.C.; Leliaert, F.; Delany, J.; Mesbahi, E. Tetraselmis Indica (chlorodendrophyceae, chlorophyta), a new species isolated from salt pans in Goa, India. Eur. J. Phycol. 2013, 48, 61–78. [Google Scholar] [CrossRef]
- Rahman, N.A.; Khatoon, H.; Yusuf, N.; Banerjee, S.; Haris, N.A.; Lananan, F.; Tomoyo, K. Tetraselmis chuii biomass as a potential feed additive to improve survival and oxidative stress status of pacific white-leg shrimp litopenaeus vannamei postlarvae. Int. Aquat. Res. 2017, 9, 235–247. [Google Scholar] [CrossRef] [Green Version]
- Zittelli, G.C.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Productivity and photosynthetic efficiency of outdoor cultures of tetraselmis suecica in annular columns. Aquaculture 2006, 261, 932–943. [Google Scholar] [CrossRef]
- Schüler, L.M.; Santos, T.; Pereira, H.; Duarte, P.; Katkam, N.G.; Florindo, C.; Schulze, P.S.C.; Barreira, L.; Varela, J.C.S. Improved production of lutein and β-carotene by thermal and light intensity upshifts in the marine microalga tetraselmis sp. CTP4. Algal Res. 2020, 45, 101732. [Google Scholar] [CrossRef]
- EU Regulation, 2017/2470 Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 Establishing the Union List of Novel Foods in Accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods. 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R2470 (accessed on 25 July 2021).
- Borowitzka, M. Culturing microalgae in outdoor ponds. In Algal Culturing Techniques; Andersen, R., Ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 205–218. ISBN 978-0-08-045650-8. [Google Scholar]
- Dammak, M.; Hadrich, B.; Miladi, R.; Barkallah, M.; Hentati, F.; Hachicha, R.; Laroche, C.; Michaud, P.; Fendri, I.; Abdelkafi, S. Effects of nutritional conditions on growth and biochemical composition of tetraselmis sp. Lipids Health Dis. 2017, 16, 41. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Mujtaba, G.; Lee, K. Effects of nitrogen sources on cell growth and biochemical composition of marine chlorophyte tetraselmis sp. for lipid production. ALGAE 2016, 31, 257–266. [Google Scholar] [CrossRef]
- Biondi, N.; Cheloni, G.; Rodolfi, L.; Viti, C.; Giovannetti, L.; Tredici, M.R. Tetraselmis suecica F&M-M33 growth is influenced by its associated bacteria. Microb. Biotechnol. 2018, 11, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms: I. cyclotella nana hustedt, and detonula confervacea (cleve) gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Mendez, C.; Uribe, E. Control of branchionus sp. and amoeba sp. in cultures of arthrospira sp. Lat. Am. J. Aquat. Res. 2012, 40, 553–561. [Google Scholar] [CrossRef]
- Cuaresma, M.; Janssen, M.; Vílchez, C.; Wijffels, R.H. Productivity of chlorella sorokiniana in a short light-path (slp) panel photobioreactor under high irradiance. Biotechnol. Bioeng. 2009, 104, 352–359. [Google Scholar] [CrossRef]
- Duboc, P.; Marison, I.; von Stockar, U. Quantitative calorimetry and biochemical engineering. In Handbook of Thermal Analysis and Calorimetry; Kemp, R., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 4, pp. 267–365. ISBN 978-0-08-053569-2. [Google Scholar]
- Gojkovic, Z.; Lu, Y.; Ferro, L.; Toffolo, A.; Funk, C. Modeling biomass production during progressive nitrogen starvation by north swedish green microalgae. Algal Res. 2020, 47, 101835. [Google Scholar] [CrossRef]
- Gojkovic, Ž.; Vílchez, C.; Torronteras, R.; Vigara, J.; Gómez-Jacinto, V.; Janzer, N.; Gómez-Ariza, J.-L.; Márová, I.; Garbayo, I. Effect of Selenate on viability and selenomethionine accumulation of chlorella sorokiniana grown in batch culture. Sci. World J. 2014, 2014, 13. [Google Scholar] [CrossRef] [Green Version]
- Doan, T.T.Y.; Sivaloganathan, B.; Obbard, J.P. Screening of marine microalgae for biodiesel feedstock. Biomass Bioenergy 2011, 35, 2534–2544. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Abou-Shanab, R.A.I.; Ji, M.-K.; Choi, J.; Kim, J.O.; Jeon, B.-H. Biodegradation of carbamazepine using freshwater microalgae chlamydomonas mexicana and scenedesmus obliquus and the determination of its metabolic fate. Bioresour. Technol. 2016, 205, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Scherholz, M.L.; Curtis, W.R. Achieving PH control in microalgal cultures through fed-batch addition of stoichiometrically-balanced growth media. BMC Biotechnol. 2013, 13, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Curtis, W.R. Proton stoichiometric imbalance during algae photosynthetic growth on various nitrogen sources: Toward metabolic ph control. J. Appl. Phycol. 2016, 28, 43–52. [Google Scholar] [CrossRef]
- Tredici, M.R. Photobiology of microalgae mass cultures: Understanding the tools for the next green revolution. Biofuels 2010, 1, 143–162. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Kirk, P.L. Kjeldahl method for total nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- James, C.S. Analytical Chemistry of Foods; Springer: Boston, MA, USA, 1995; ISBN 978-1-4613-5905-0. [Google Scholar]
- Hammer, Ø.; Harper, D.; Ryan, P. Past: Paleontological Statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Biondi, N.; Bassi, N.; Chini Zittelli, G.; De Faveri, D.; Giovannini, A.; Rodolfi, L.; Allevi, C.; Macrì, C.; Tredici, M.R. Nannochloropsis Sp. F&M-M24: Oil production, effect of mixing on productivity and growth in an industrial wastewater. Environ. Prog. Sustain. Energy 2013, 32, 846–853. [Google Scholar] [CrossRef]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Su, C.-H.; Chien, L.-J.; Gomes, J.; Lin, Y.-S.; Yu, Y.-K.; Liou, J.-S.; Syu, R.-J. Factors affecting lipid accumulation by nannochloropsis oculata in a two-stage cultivation process. J. Appl. Phycol. 2011, 23, 903–908. [Google Scholar] [CrossRef]
- Carney, L.T.; Lane, T.W. Parasites in algae mass culture. Front. Microbiol. 2014, 5, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, J.G.; Gong, Y.; Hu, Q. Microzooplanktonic grazers–a potentially devastating threat to the commercial success of microalgal mass culture. Algal Res. 2017, 27, 356–365. [Google Scholar] [CrossRef]
- Huang, Y.; Li, L.; Liu, J.; Lin, W. Botanical pesticides as potential rotifer-control agents in microalgal mass culture. Algal Res. 2013, 4, 62–69. [Google Scholar] [CrossRef]
- Jiménez, C.; Cossío, B.R.; Niell, F.X. Relationship between physicochemical variables and productivity in open ponds for the production of spirulina: A predictive model of algal yield. Aquaculture 2003, 221, 331–345. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, and fermenters. In Progress in Industrial Microbiology; Osinga, R., Tramper, J., Burgess, J.G., Wijffels, R.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 35, pp. 313–321. ISBN 0079-6352. [Google Scholar]
- Jorquera, O.; Kiperstok, A.; Sales, E.A.; Embiruçu, M.; Ghirardi, M.L. Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresour. Technol. 2010, 101, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Tredici, M.R.; Materassi, R. From open ponds to vertical alveolar panels: The italian experience in the development of reactors for the mass cultivation of phototrophic microorganisms. J. Appl. Phycol. 1992, 4, 221–231. [Google Scholar] [CrossRef]
- Tredici, M.R.; Bassi, N.; Prussi, M.; Biondi, N.; Rodolfi, L.; Zittelli, G.C.; Sampietro, G. Energy balance of algal biomass production in a 1-Ha “Green Wall Panel” plant: How to produce algal biomass in a closed reactor achieving a high net energy ratio. Appl. Energy 2015, 154, 1103–1111. [Google Scholar] [CrossRef] [Green Version]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Kujawska, N.; Talbierz, S. Microalgae cultivation technologies as an opportunity for bioenergetic system development—Advantages and limitations. Sustainability 2020, 12, 9980. [Google Scholar] [CrossRef]
- Slegers, P.M.; Lösing, M.B.; Wijffels, R.H.; Van Straten, G.; Van Boxtel, A.J.B. Scenario evaluation of open pond microalgae production. Algal Res. 2013, 2, 358–368. [Google Scholar] [CrossRef]
- Trovão, M.; Pereira, H.; Silva, J.; Páramo, J.; Quelhas, P.; Santos, T.; Silva, J.T.; Machado, A.; Gouveia, L.; Barreira, L.; et al. Growth performance, biochemical composition and sedimentation velocity of tetraselmis sp. ctp4 under different salinities using low-cost lab- and pilot-scale systems. Heliyon 2019, 5, e01553. [Google Scholar] [CrossRef] [Green Version]
- Pereira, H.; Gangadhar, K.N.; Schulze, P.S.C.; Santos, T.; de Sousa, C.B.; Schueler, L.M.; Custódio, L.; Malcata, F.X.; Gouveia, L.; Varela, J.C.S.; et al. Isolation of a Euryhaline microalgal strain, tetraselmis sp. ctp4, as a robust feedstock for biodiesel production. Sci. Rep. 2016, 6, 35663. [Google Scholar] [CrossRef] [Green Version]
- Norsker, N.-H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina–from growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Masojídek, J.; Torzillo, G.; Koblížek, M. Photosynthesis in microalgae. In Handbook of Microalgal Culture; Richmond, A., Hu, Q., Eds.; Wiley-Blackwell: Hoboken, HY, USA, 2005; ISBN 0-632-05953-2. [Google Scholar]
- Pereira, H.; Silva, J.; Santos, T.; Gangadhar, N.K.; Raposo, A.; Nunes, C.; Coimbra, A.M.; Gouveia, L.; Barreira, L.; Varela, J. Nutritional potential and toxicological evaluation of tetraselmis sp. ctp4 microalgal biomass produced in industrial photobioreactors. Molecules 2019, 24, 3192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondioli, P.; Della Bella, L.; Rivolta, G.; Chini Zittelli, G.; Bassi, N.; Rodolfi, L.; Casini, D.; Prussi, M.; Chiaramonti, D.; Tredici, M.R. Oil production by the marine microalgae nannochloropsis sp. f&m-m24 and tetraselmis suecica F&M-M33. Bioresour. Technol. 2012, 114, 567–572. [Google Scholar] [CrossRef]
- Karemore, A.; Pal, R.; Sen, R. Strategic enhancement of algal biomass and lipid in chlorococcum infusionum as bioenergy feedstock. Algal Res. 2013, 2, 113–121. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Y.; Xu, H.; Liu, Y.; Sun, J.; Qiao, D.; Cao, Y. Effect of nitrogen-starvation, light intensity and iron on triacylglyceride/carbohydrate production and fatty acid profile of neochloris oleoabundans HK-129 by a two-stage process. Bioresour. Technol. 2014, 155, 204–212. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Gheda, S.F.; Khairy, H.M.; El-Shenody, R.A. Optimization of Medium Components Using Plackett-Burman Design for High Production of Protein, Carbohydrates and Lipids in the Microalga Tetraselmis Chuii. Egypt. J. Exp. Biol. 2015, 11, 77–88. [Google Scholar]
- El-Sheekh, M.; Abomohra, A.E.F.; El Azim, M.; Abou-Shanab, R. Effect of temperature on growth and fatty acids profile of the biodiesel producing microalga scenedesmus acutus. BASE 2017, 21, 233–239. [Google Scholar]
- Robert, R.; Parisi, G.; Rodolfi, L.; Poli, B.M.; Tredici, M.R. Use of fresh and preserved tetraselmis suecica for feeding crassostrea gigas larvae. Aquaculture 2001, 192, 333–346. [Google Scholar] [CrossRef]
- EU Regulation, 629/2008 COMMISSION REGULATION (EC) No 629/2008 of Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs (Text with EEA Relevance). 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32008R0629 (accessed on 25 July 2021).
- EU Regulation, 32/2002 DIRECTIVE 2002/32/EC of the EUROPEAN PARLIAMENT and of the COUNCIL of 7 May 2002 on Undesirable Substances in Animal Feed. 2002. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32002L0032 (accessed on 25 July 2021).
- EU Regulation, 1869/2019 COMMISSION REGULATION (EU) 2019/1869 of 7 November 2019 Amending and Correcting Annex I to Directive 2002/32/EC of the European Parliament and of the Council as Regards Maximum Levels for Certain Undesirable Substances in Animal Feed. 2019. Available online: https://eur-lex.europa.eu/eli/reg/2019/1869/oj/eng (accessed on 25 July 2021).
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Kazimierowicz, J.; Dudek, M.; Świca, I.; Rudnicka, A. The cultivation of lipid-rich microalgae biomass as anaerobic digestate valorization technology—A pilot-scale study. Processes 2020, 8, 517. [Google Scholar] [CrossRef]
- Ivanov, I.N.; Zachleder, V.; Vítová, M.; Barbosa, M.J.; Bišová, K. Starch production in chlamydomonas reinhardtii through supraoptimal temperature in a pilot-scale photobioreactor. Cells 2021, 10, 1084. [Google Scholar] [CrossRef] [PubMed]


| RW80in (8000 L–80 m2) | RW76out (8000 L–76 m2) | RW300out (45,000 L–300 m2) | ||||
|---|---|---|---|---|---|---|
| Harvest | PDW, max (kgDW) | Harvest | PDW, max (kgDW) | Harvest | PDW, max (kgDW) | Paste (kg) |
| 26-Aug | 2.61 ± 0.13 | 21-Oct | 3.72 ± 0.28 | 28-Nov | 5.24 ± 0.09 | 18.0 |
| 30-Aug | 2.71 ± 0.01 | 25-Oct | 3.47 ± 0.17 | 2-Dec | 12.56 ± 0.13 | 51.0 |
| 3-Sep | 3.69 ± 0.20 | 28-Oct | 3.81 ± 0.50 | 5-Dec | 4.04 ± 0.02 | 11.4 |
| 6-Sep | 4.38 ± 0.45 | 31-Oct | 4.13 ± 0.01 | 9-Dec | 6.15 ± 0.16 | 17.3 |
| 10-Sep | 2.89 ± 0.05 | 4-Nov | 4.03 ± 0.14 | 12-Dec | 6.96 ± 0.02 | 21.4 |
| 13-Sep | 3.95 ± 0.02 | 8-Nov | 3.88 ± 0.06 | 16-Dec | 6.42 ± 0.14 | 18.0 |
| 20-Sep | 4.44 ± 0.13 | 12-Nov | 3.93 ± 0.37 | 18-Dec | 7.03 ± 0.46 | 31.2 |
| Total (kgDW) | 24.66 ± 0.53 | 15-Nov | 4.30 ± 0.28 | 20-Dec | 6.53 ± 0.25 | 24.8 |
| 19-Nov | 3.99 ± 0.06 | Total (kgDW) | 54.9 ± 0.58 | 193.1 | ||
| 22-Nov | 3.07 ± 0.13 | |||||
| 26-Nov | 3.99 ± 0.20 | |||||
| Total (kgDW) | 42.32 ± 0.81 | |||||
| RW80in (8000 L–80 m2) | RW76out (8000 L–76 m2) | RW300out (45,000 L–300 m2) | |||
|---|---|---|---|---|---|
| Harvest | PE (%) | Harvest | PE (%) | Harvest | PE (%) |
| 26-Aug | 0.65 ± 0.03 | 21-Oct | 0.84 ± 0.06 | 28-Nov | 0.41 ± 0.01 |
| 30-Aug | 1.36 ± 0.00 | 25-Oct | 1.82 ± 0.09 | 2-Dec | 1.83 ± 0.02 |
| 3-Sep | 1.66 ± 0.09 | 28-Oct | 3.18 ± 0.42 | 5-Dec | 0.84 ± 0.00 |
| 6-Sep | 3.52 ± 0.36 | 31-Oct | 2.51 ± 0.00 | 9-Dec | 0.91 ± 0.02 |
| 10-Sep | 1.36 ± 0.02 | 4-Nov | 1.76 ± 0.06 | 12-Dec | 1.29 ± 0.00 |
| 13-Sep | 2.33 ± 0.01 | 8-Nov | 1.88 ± 0.03 | 16-Dec | 1.12 ± 0.02 |
| 20-Sep | 1.20 ± 0.04 | 12-Nov | 2.01 ± 0.19 | 18-Dec | 1.84 ± 0.12 |
| Cumulative PE, % | 1.45 ± 0.03 | 15-Nov | 3.49 ± 0.23 | 20-Dec | 1.76 ± 0.07 |
| 19-Nov | 2.34 ± 0.04 | Cumulative PE, % | 1.10 ± 0.01 | ||
| 22-Nov | 2.06 ± 0.09 | ||||
| 26-Nov | 2.20 ± 0.11 | ||||
| Cumulative PE, % | 1.95 ± 0.04 | ||||
| Harvest Date | 10-Sep | 21-Oct | 28-Nov | 2-Dec | 9-Dec | |
|---|---|---|---|---|---|---|
| Fatty Acid (% of Total FA) | RW80in | RW76out | RW300out | RW300out | RW300out | Average |
| 14:0 | 1.9 | 0.2 | 0.9 | 0.9 | 0.8 | 0.9 ± 0.6 |
| 16:0 (Palmitic) | 20.4 | 16.8 | 18.4 | 19.6 | 23.2 | 19.7 ± 2.4 |
| 16:1n-7 | 2.0 | 2.3 | 1.2 | 1.2 | 1.0 | 1.6 ± 0.6 |
| 16:3n-1 | 3.6 | 12.7 | 8.7 | 7.3 | 8.0 | 8.1 ± 3.3 |
| 18:0 | 2.7 | 0.6 | 1.9 | 1.5 | 1.4 | 1.6 ± 0.8 |
| 18:1n-9 (Oleic) | 21.1 | 18.8 | 12.1 | 13.3 | 12.3 | 15.5 ± 4.1 |
| 18:2n-6 (Linoleic) | 8.2 | 6.1 | 3.6 | 4.3 | 3.7 | 5.2 ± 2.0 |
| 18:3n-3 (ALA) | 5.9 | 22.1 | 14.7 | 15.1 | 14.5 | 14.4 ± 5.7 |
| 18:4n-3 | 2.3 | 4.0 | 5.8 | 4.4 | 4.5 | 4.2 ± 1.3 |
| 20:5n-3 (EPA) | 5.9 | 5.3 | 8.7 | 6.8 | 4.8 | 6.3 ±1.5 |
| 22:6n-3 (DHA) | 3.1 | - | 3.5 | 4.3 | 1.6 | 3.1 ± 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gojkovic, Z.; Guidi, F.; Bustamante, B.; Venuleo, M.; Assunçao, P.A.C.J.d.; Portillo, E. Scaling-Up and Semi-Continuous Cultivation of Locally Isolated Marine Microalgae Tetraselmis striata in the Subtropical Island of Gran Canaria (Canary Islands, Spain). Processes 2021, 9, 1326. https://doi.org/10.3390/pr9081326
Gojkovic Z, Guidi F, Bustamante B, Venuleo M, Assunçao PACJd, Portillo E. Scaling-Up and Semi-Continuous Cultivation of Locally Isolated Marine Microalgae Tetraselmis striata in the Subtropical Island of Gran Canaria (Canary Islands, Spain). Processes. 2021; 9(8):1326. https://doi.org/10.3390/pr9081326
Chicago/Turabian StyleGojkovic, Zivan, Flavio Guidi, Begoña Bustamante, Marianna Venuleo, Patrícia Alexandra Clemente Janeiro de Assunçao, and Eduardo Portillo. 2021. "Scaling-Up and Semi-Continuous Cultivation of Locally Isolated Marine Microalgae Tetraselmis striata in the Subtropical Island of Gran Canaria (Canary Islands, Spain)" Processes 9, no. 8: 1326. https://doi.org/10.3390/pr9081326
APA StyleGojkovic, Z., Guidi, F., Bustamante, B., Venuleo, M., Assunçao, P. A. C. J. d., & Portillo, E. (2021). Scaling-Up and Semi-Continuous Cultivation of Locally Isolated Marine Microalgae Tetraselmis striata in the Subtropical Island of Gran Canaria (Canary Islands, Spain). Processes, 9(8), 1326. https://doi.org/10.3390/pr9081326







