Preparation and Evaluation of Epoxy Resin Prepared from the Liquefied Product of Cotton Stalk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
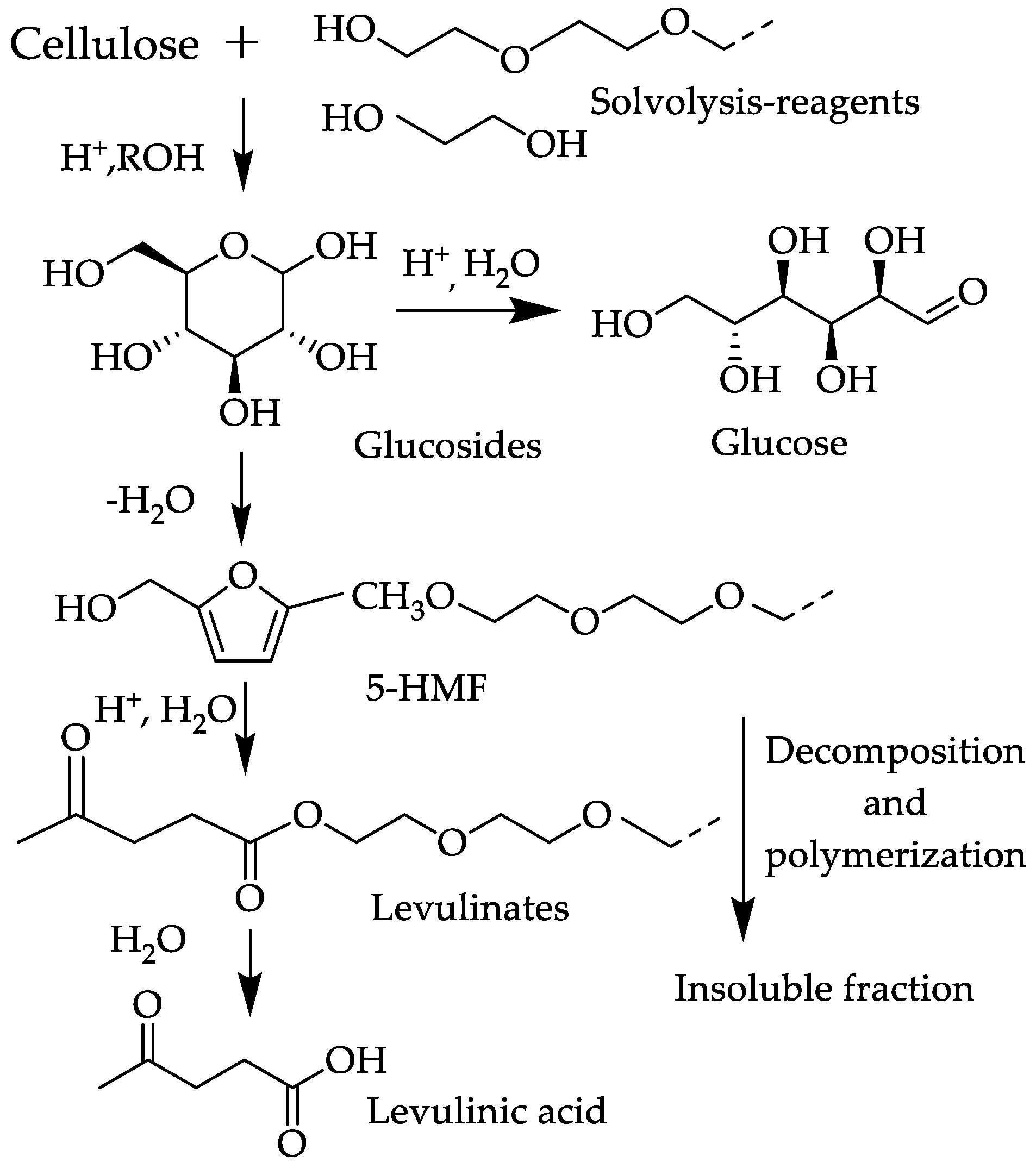
2.2. Preparation of Liquefied Cotton Stalk
2.3. Characterization of Liquefaction Products
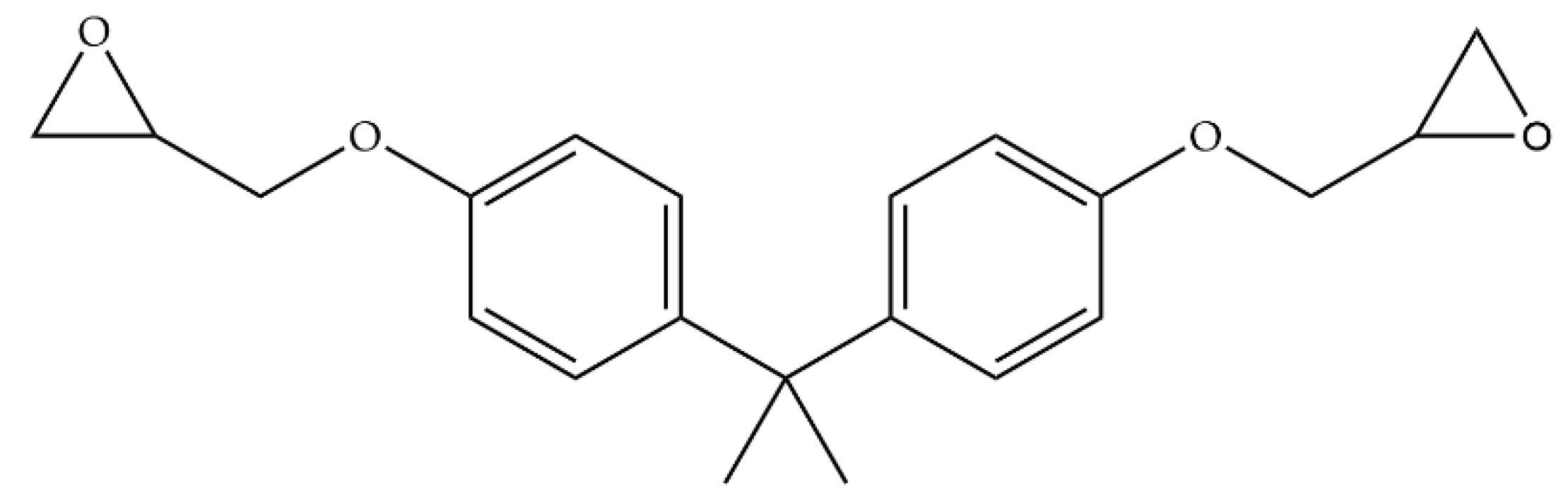
2.4. Preparation of Liquefied Cotton-Stalk-Based Epoxy Resins
2.5. Fourier-Transform Infrared (FTIR) Spectroscopy
2.6. Molecular Weight, Epoxy Equivalent Weight, and Viscosity
2.7. Preparation of Cured Resins Using the Cotton-Stalk-Based Epoxy Resins
2.8. Tensile Test
2.9. DSC and TGA
3. Results and Discussion
3.1. Optimization of the Cotton Stalk Liquefaction Process
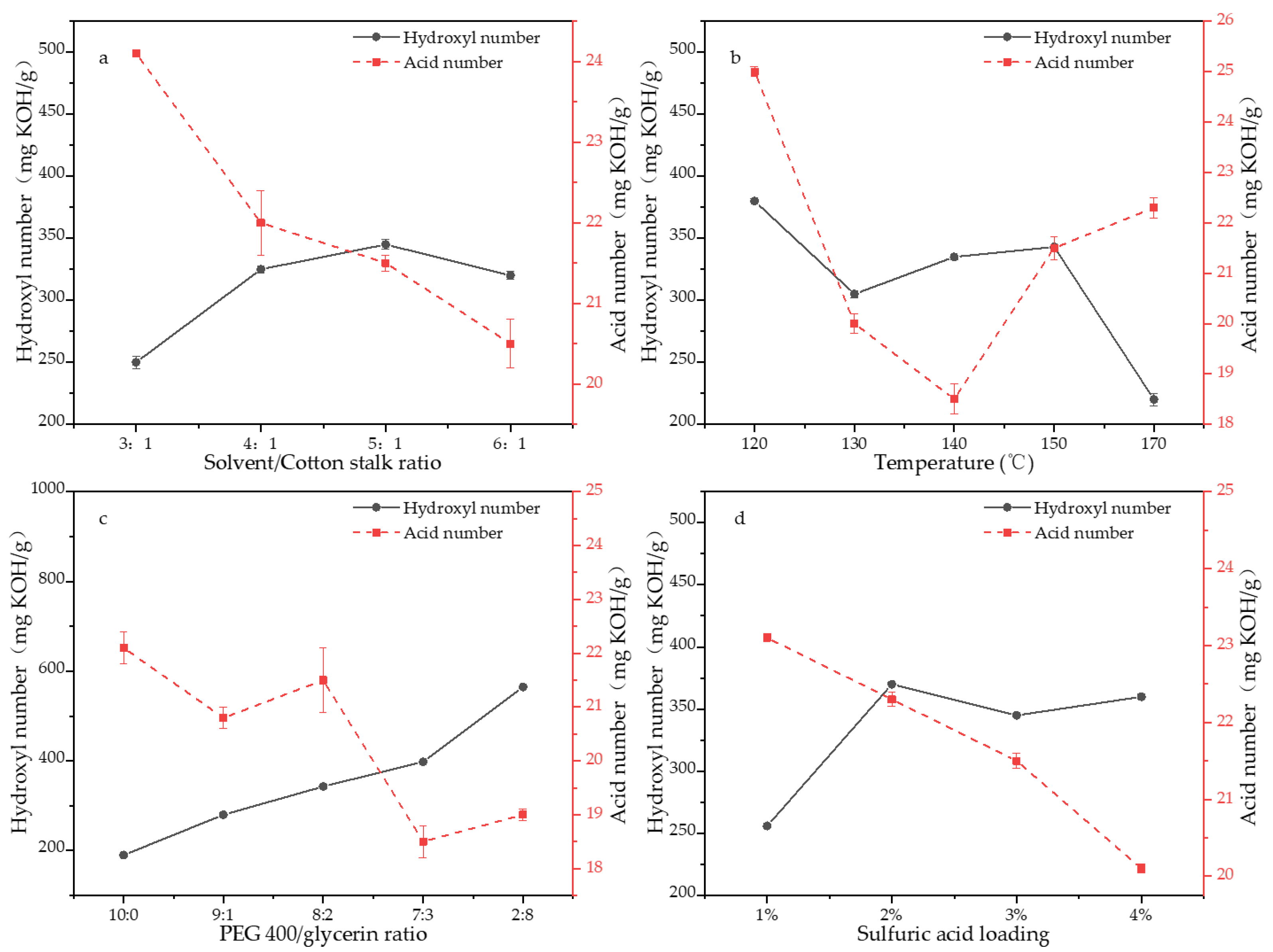
3.1.1. Effects of Reaction Parameters on the Hydroxyl Value
3.1.2. Effects of Reaction Parameters on the Residue Content
3.1.3. Structural Characteristics of the Liquefied Cotton Stalk and Liquefied Cotton-Stalk-Based Epoxy Resin
3.2. Properties of the Liquefied Cotton-Stalk-Based Epoxy Resin
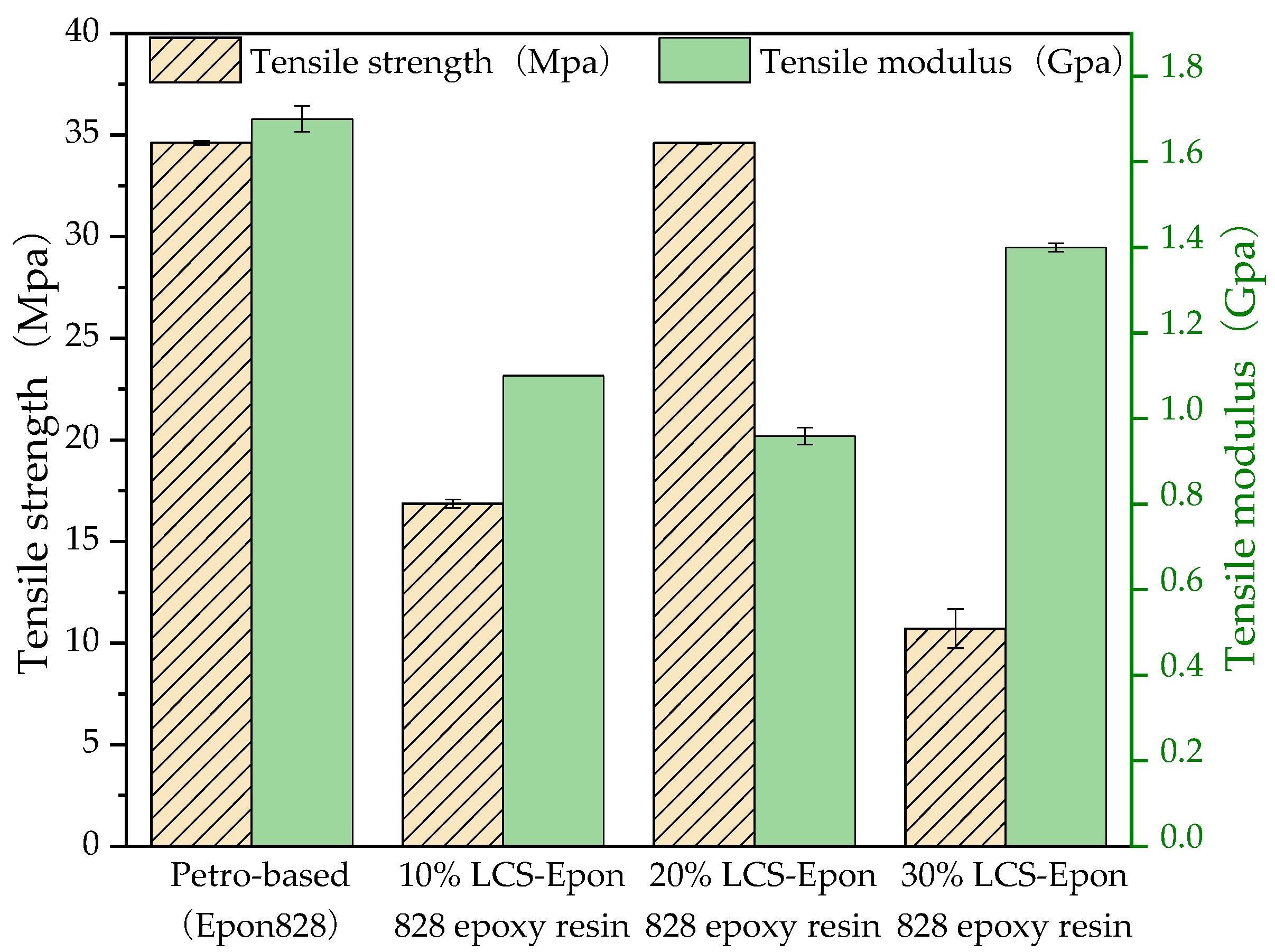
3.3. Properties of the Cured Liquefied Cotton-Stalk-Based Epoxy Resin
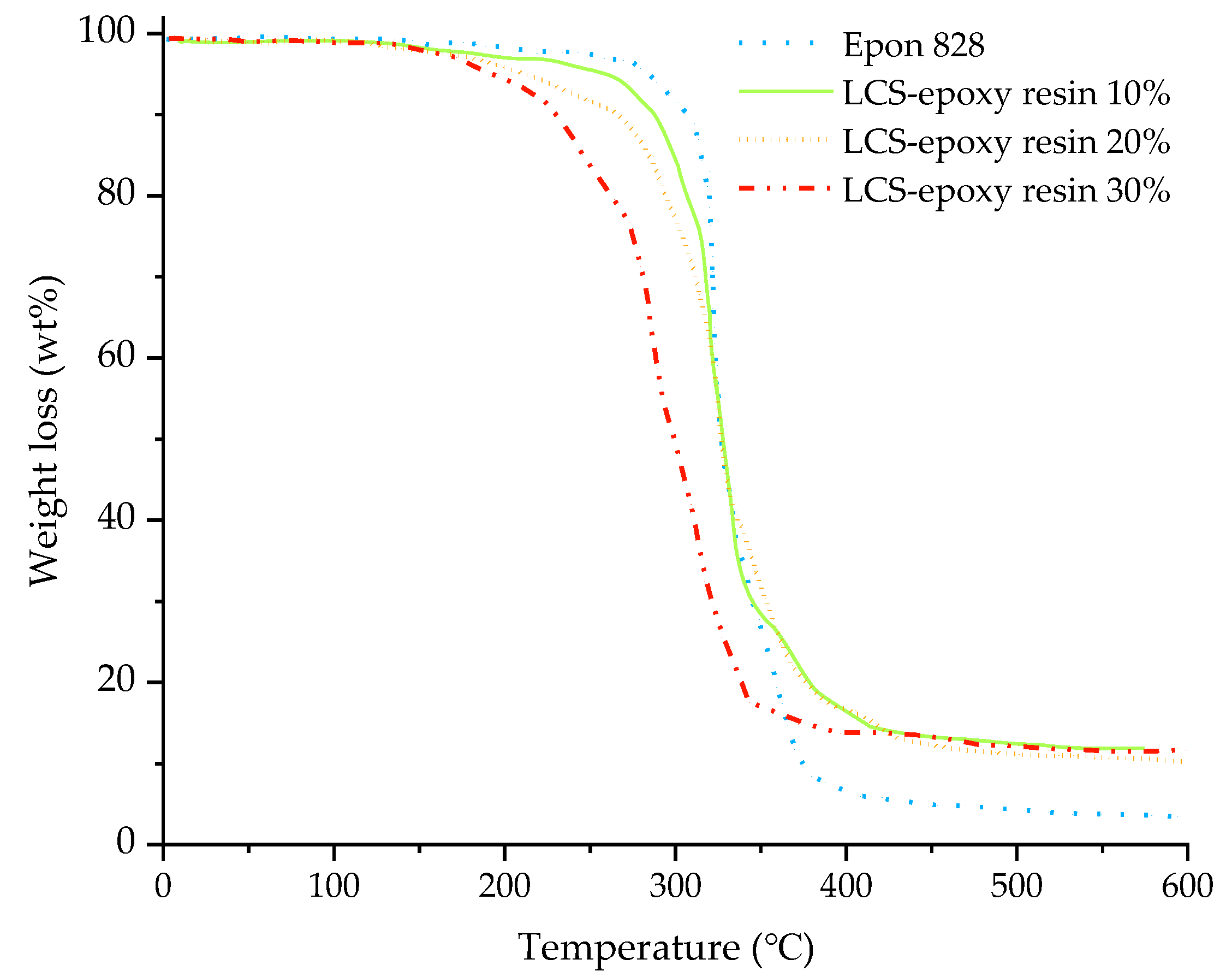
3.4. Thermal Stability of the Epoxy Resins
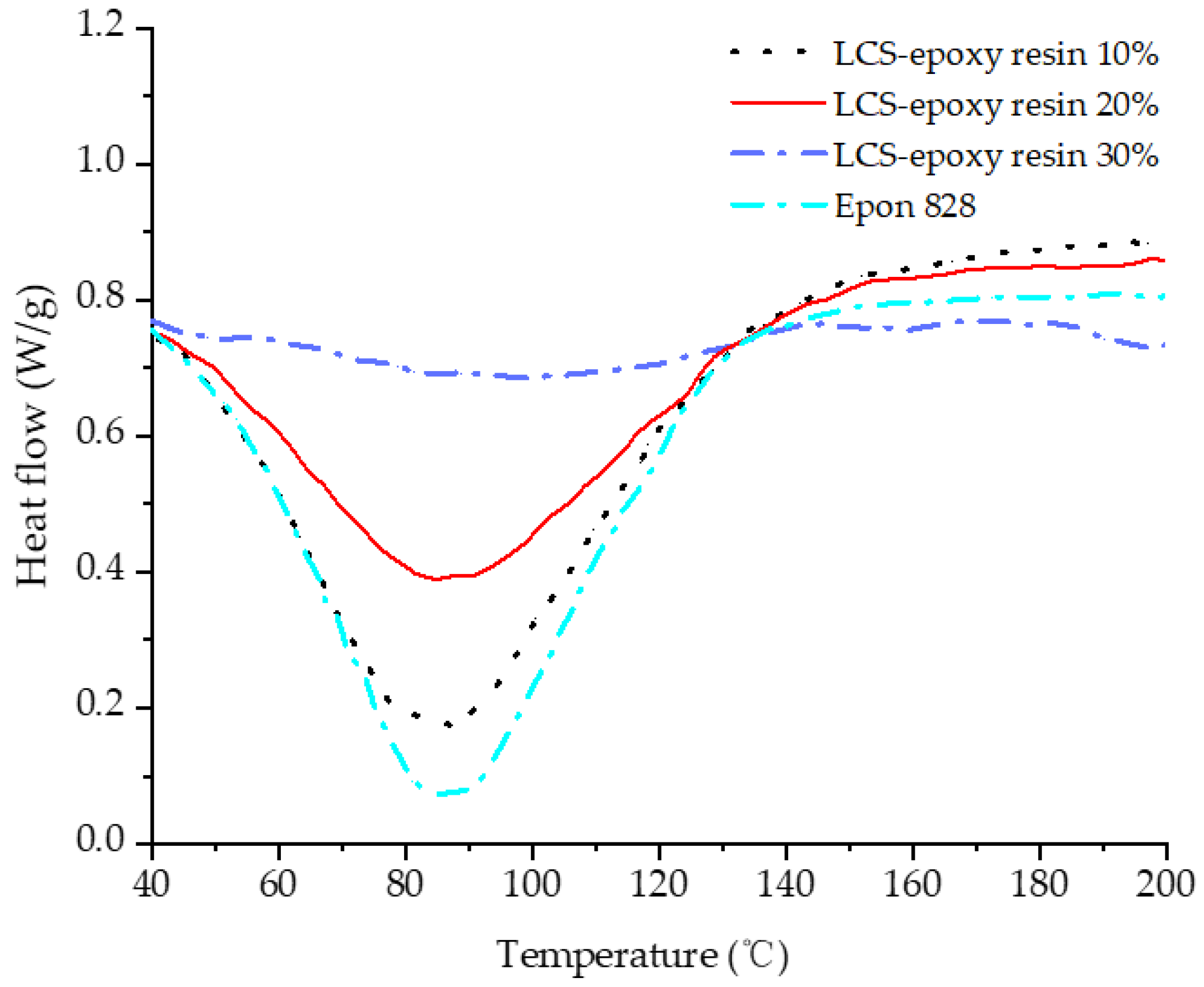
3.5. Thermal Properties of Cured Liquefied Cotton-Stalk-Based Epoxy Resins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, H. Wood Liquefaction in the Presence of Phenol with a Weak Acid Catalyst and Its Potential for Novolac Type Wood Adhesives. Ph.D. Thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2007; p. 525. [Google Scholar]
- Ramon, E.; Sguazzo, C.; Moreira, P.M.G.P. A review of recent research on bio-based epoxy systems for engineering applications and potentialities in the aviation sector. Aerospace 2018, 5, 110. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Kumar, A.; Adamopoulos, S. Liquefaction of lignocellulosic materials and its applications in wood adhesives—A review. Ind. Crops Prod. 2018, 124, 325–342. [Google Scholar] [CrossRef]
- Rastegarfar, N.; Behrooz, R.; Auad, M.L. Characterization of polyurethane foams prepared from liquefied sawdust by crude glycerol and polyethylene glycol. J. Polym. Res. 2018, 25. [Google Scholar] [CrossRef] [Green Version]
- Kishi, H.; Fujita, A.; Miyazaki, H.; Matsuda, S.; Murakami, A. Synthesis of wood-based epoxy resins and their mechanical and adhesive properties. J. Appl. Polym. Sci. 2006, 102, 2285–2292. [Google Scholar] [CrossRef]
- Wang, Q.; Tuohedi, N. Polyurethane foams and bio-polyols from liquefied cotton stalk agricultural waste. Sustainability 2020, 12, 4214. [Google Scholar] [CrossRef]
- Hernandez, E.D.; Bassett, A.W.; Sadler, J.M.; La Scala, J.J.; Stanzione, J.F. Synthesis and Characterization of Bio-based Epoxy Resins Derived from Vanillyl Alcohol. ACS Sustain. Chem. Eng. 2016, 4, 4328–4339. [Google Scholar] [CrossRef]
- Rao, B.S.; Palanisamy, A. Synthesis of bio based low temperature curable liquid epoxy, benzoxazine monomer system from cardanol: Thermal and viscoelastic properties. Eur. Polym. J. 2013, 49, 2365–2376. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Q.; Mitsumura, N.; Animesh, S. Behavior of cellulose liquefaction after pretreatment using ionic liquids with water mixtures. J. Appl. Polym. Sci. 2014, 131, 40255. [Google Scholar] [CrossRef]
- Hu, S.; Wan, C.; Li, Y. Production and characterization of biopolyols and polyurethane foams from crude glycerol based liquefaction of soybean straw. Bioresour. Technol. 2012, 103, 227–233. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Zhang, Y.; Cheng, X.; Yu, Y.; Jin, Y. Preparation of bio-polyols by liquefaction of hardwood residue and their application in the modification of polyurethane foams. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2016, 31, 918–924. [Google Scholar] [CrossRef]
- Kuo, P.Y.; De Assis Barros, L.; Sain, M.; Tjong, J.S.Y.; Yan, N. Effects of Reaction Parameters on the Glycidyl Etherification of Bark Extractives during Bioepoxy Resin Synthesis. ACS Sustain. Chem. Eng. 2016, 4, 1016–1024. [Google Scholar] [CrossRef]
- Li, H.; Feng, S.; Yuan, Z.; Wei, Q.; Xu, C.C. Highly efficient liquefaction of wheat straw for the production of bio-polyols and bio-based polyurethane foams. Ind. Crop. Prod. 2017, 109, 426–433. [Google Scholar] [CrossRef]
- Liu, Y.; Via, B.K.; Pan, Y.; Cheng, Q.; Guo, H.; Auad, M.L.; Taylor, S. Preparation and characterization of epoxy resin cross-linked with high wood pyrolysis bio-oil substitution by acetone pretreatment. Polymers 2017, 9, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dísouza, J.; Wong, S.Z.; Camargo, R.; Yan, N. Solvolytic Liquefaction of Bark: Understanding the Role of Polyhydric Alcohols and Organic Solvents on Polyol Characteristics. ACS Sustain. Chem. Eng. 2016, 4, 851–861. [Google Scholar] [CrossRef]
- Kishi, H.; Fujita, A. Wood-based epoxy resins and the ramie fiber reinforced composites. Environ. Eng. Manag. J. 2008, 7, 517–523. [Google Scholar] [CrossRef]
- Daneshvar, S.; Behrooz, R.; Najafi, S.K.; Mir, G.; Sadeghi, M. Characterization of Polyurethane Wood Adhesive Prepared from Liquefied Sawdust by Ethylene Carbonate. BioResources 2019, 14, 796–815. [Google Scholar]
- Li, Y.; Yang, L.; Zhang, H.; Tang, Z. Synthesis and curing performance of a novel bio-based epoxy monomer from soybean oil. Eur. J. Lipid Sci. Technol. 2017, 119, 1600429. [Google Scholar] [CrossRef]
- Lee, H.; Neville, K.; Henry, L.; Neville, K. Handbook of Epoxy Resins, 2nd ed.McGraw-Hill: New York, NY, USA, 1982. [Google Scholar]
- Li, R.; Li, W.; Zheng, F.; Zhang, Y.; Hu, J. Versatile bio-based epoxy resin: From banana waste to applied materials. J. Appl. Polym. Sci. 2019, 136, 1–8. [Google Scholar] [CrossRef]
- Weng, Z.; Li, J.; Qi, Y.; Wang, J.; Zhang, S.; Jian, X. Progress on High Performance and Functionalization of Bio-Based Epoxy Resins. Mater. China 2019, 38, 999–1008. [Google Scholar]
- Cela-Dablanca, R.; Nebot, C.; Rodríguez López, L.; Fernández-Calviño, D.; Arias-Estévez, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E. Efficacy of Different Waste and By-Products from Forest and Food Industries in the Removal/Retention of the Antibiotic Cefuroxime. Processes 2021, 9, 1151. [Google Scholar] [CrossRef]
- Wang, Q.; Kawamura, S. Decayed woody material from mushroom cultivation: Characterization of liquefaction. WIT Trans. Ecol. Environ. 2018, 217, 481–492. [Google Scholar]
- Yamada, T.; Aratani, M.; Kubo, S.; Ono, H. Chemical analysis of the product in acid-catalyzed solvolysis of cellulose using polyethylene glycol and ethylene carbonate. J. Wood Sci. 2007, 53, 487–493. [Google Scholar] [CrossRef]
- Guo, K.; Guan, Q.; Xu, J.; Tan, W. Mechanism of Preparation of Platform Compounds from Lignocellulosic Biomass Liquefaction Catalyzed by Bronsted Acid: A Review. J. Bioresour. Bioprod. 2019, 4, 202–213. [Google Scholar]
- Yao, Y.; Yoshioka, M.; Shiraishi, N. Water-absorbing polyurethane foams from liquefied starch. J. Appl. Polym. Sci. 1996, 60, 1939–1949. [Google Scholar] [CrossRef]
- Hassan, E.; Barbary, M.; Shukry, N. Polyhydric alcohol liquefaction of some lignocellulosic agricultural residues. Ind. Crop. Prod. 2008, 27, 33–38. [Google Scholar] [CrossRef]
- Cinelli, P.; Chiellini, E.; Imam, S.H. Hybrid composite based on poly (vinyl alcohol) and fillers from renewable resources. J. Appl. Polym. Sci. 2008, 109, 1684–1691. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Feng, Q.; Wei, N.; Li, S.; Zhang, W.; Yang, K.; Zhang, Y. Investigation of the Relationship Between Reed Liquefaction Behavior and Thermodynamic Properties of Epoxy Resin Based on Liquefied Reed. Waste Biomass Valorization 2017, 8, 1285–1294. [Google Scholar] [CrossRef]
- D’Souza, J.; Yan, N. Producing bark-based polyols through liquefaction: Effect of liquefaction temperature. ACS Sustain. Chem. Eng. 2013, 1, 534–540. [Google Scholar] [CrossRef]
- Wu, C.C.; Lee, W.J. Curing behavior and adhesion properties of epoxy resin blended with polyhydric alcohol-liquefied Cryptomeria japonica wood. Wood Sci. Technol. 2011, 45, 559–571. [Google Scholar] [CrossRef]
- Chen, J.; Wu, G.; Huo, S.; Kong, Z. Synthesis and Characterization of Curing Reaction of Epoxy Resin Obtained from Anacardic Acids. Chem. Ind. For. Prod. 2020, 40, 41–46. [Google Scholar]
- Benyahya, S.; Aouf, C.; Caillol, S.; Boutevin, B.; Pascault, J.P.; Fulcrand, H. Functionalized green tea tannins as phenolic prepolymers for bio-based epoxy resins. Ind. Crop. Prod. 2014, 53, 296–307. [Google Scholar] [CrossRef]
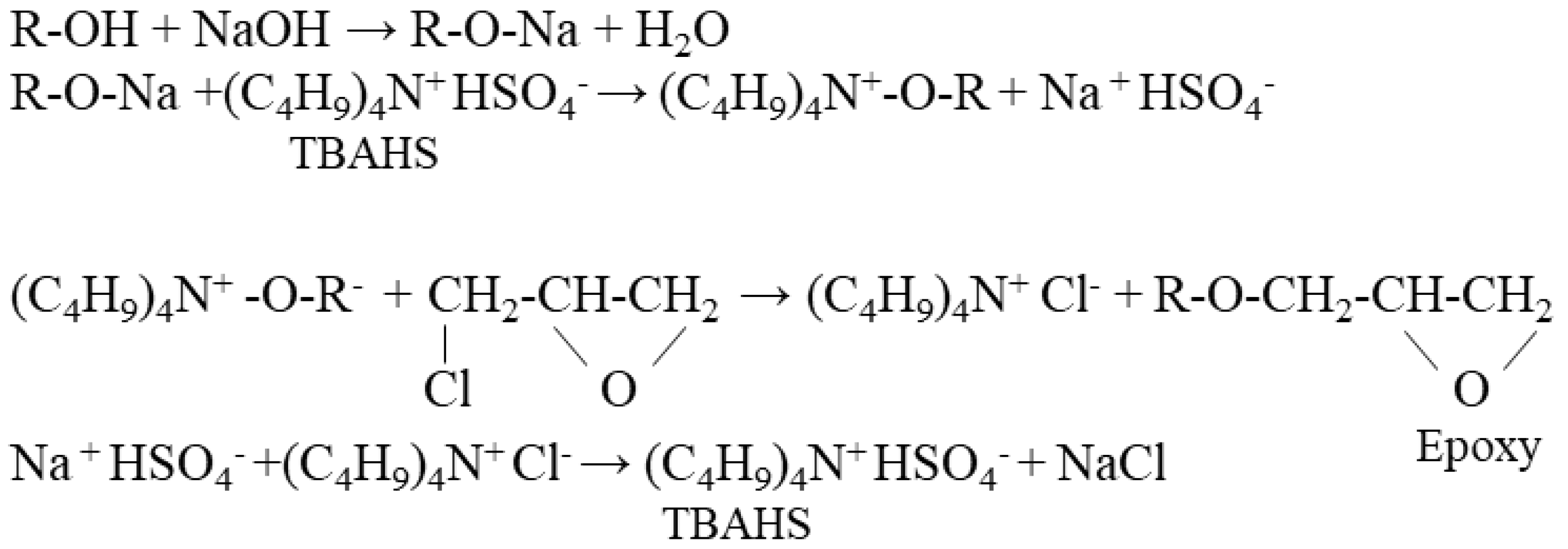

| Parameter | Numerical Value | Minimal Residue (%) | Reference |
|---|---|---|---|
| Temperature (°C) | 130 | 30.0 | Wang et al. (2020) |
| 140 | 20.2 | ||
| 150 | 13.5 | ||
| 160 | 16.6 | ||
| Catalyst content (%) | 1 | 22.4 | |
| 2 | 20.0 | ||
| 3 | 13.2 | ||
| 4 | 12.5 | ||
| Solvent/cotton stalk (w/w%) | 3 | 32.4 | |
| 4 | 28.3 | ||
| 5 | 12.4 | ||
| 6 | 10.2 | ||
| ※ PEG 400/Glycerin (w/w%) | 10:0 | 43.1 | This study |
| 9:1 | 16.2 | ||
| 8:2 | 13.2 | ||
| 7:3 | 16.8 |
| Epoxy Resin | Properties of Liquefied Cotton Stalk | Properties of Epoxy Resins | ||||
|---|---|---|---|---|---|---|
| PEG 400/glycerin | Hydroxyl Value (mg KOH/g) | Viscosity (m Pa·s) | Epoxy Equivalent Weight (g/eq) | Molecular Weight | Viscosity (m Pa·s) | |
| LCS-based epoxy resin | 8/2 | 343 | 1020 | 250 | 726 | 540 |
| PEG 400/glycerin-based epoxy resin | 8/2 | 321 | 320 | 440 | 690 | 577 |
| Epon 828 | -- | --- | --- | 190 | 370 | 12,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuohedi, N.; Wang, Q. Preparation and Evaluation of Epoxy Resin Prepared from the Liquefied Product of Cotton Stalk. Processes 2021, 9, 1417. https://doi.org/10.3390/pr9081417
Tuohedi N, Wang Q. Preparation and Evaluation of Epoxy Resin Prepared from the Liquefied Product of Cotton Stalk. Processes. 2021; 9(8):1417. https://doi.org/10.3390/pr9081417
Chicago/Turabian StyleTuohedi, Nuerjiamali, and Qingyue Wang. 2021. "Preparation and Evaluation of Epoxy Resin Prepared from the Liquefied Product of Cotton Stalk" Processes 9, no. 8: 1417. https://doi.org/10.3390/pr9081417







