Thermogravimetric Analysis of Coal Semi-Char Co-Firing with Straw in O2/CO2 Mixtures
Abstract
:1. Introduction
2. Experiments
2.1. Samples Description
2.2. Experimental Apparatus, Methods and Kinetic Method
3. Results and Discussion
3.1. Co-Firing Behavior of Semi-Char and Straw under Different Blending Ratios in 21% O2/79% CO2 Mixtures
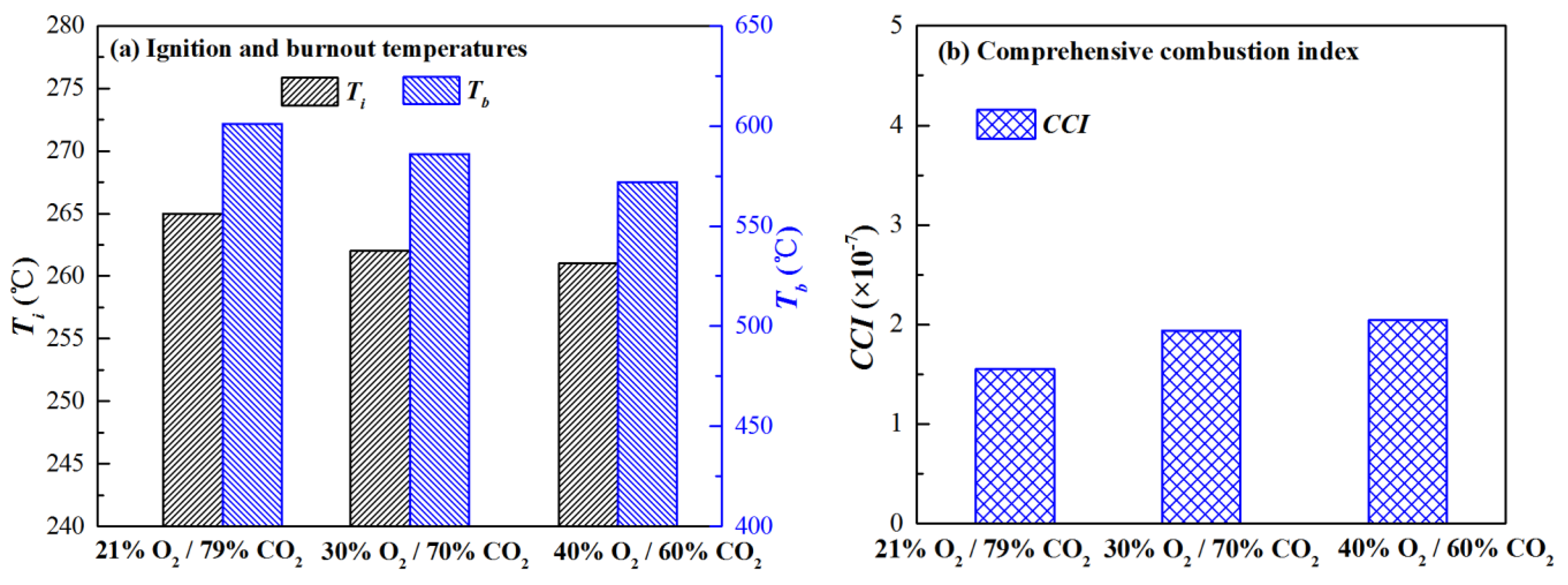
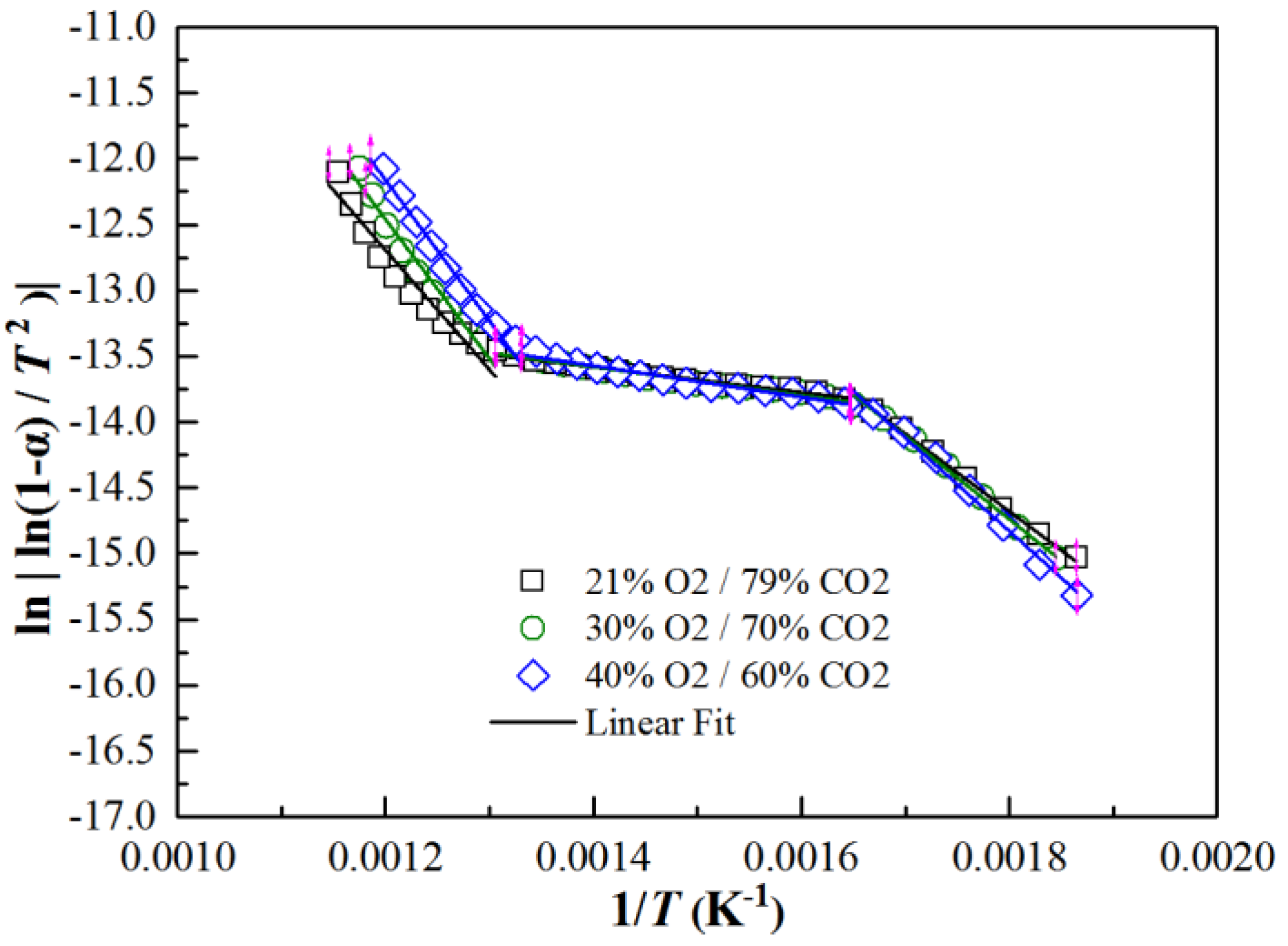
3.2. Co-Firing Behavior of Semi-Char and Straw under Different O2 Concentrations in O2/CO2 Mixture
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Hu, S.; Xu, K.; Jiang, L.; Wang, Y.; Su, S.; Xiao, Y.; Shan, L.; Shen, W.; Li, H. Study on the structural evolution of semi-chars and their solvent extracted materials during pyrolysis process of a Chinese low-rank coal. Fuel 2018, 214, 363–368. [Google Scholar] [CrossRef]
- Li, C.Z. Importance of volatile–char interactions during the pyrolysis and gasification of low-rank fuels—A review. Fuel 2013, 112, 609–623. [Google Scholar] [CrossRef]
- Katalambula, H.; Gupta, R. Low-grade coals: A review of some prospective upgrading technologies. Energy Fuels 2009, 23, 3392–3405. [Google Scholar] [CrossRef]
- Meng, F.; Yu, J.; Tahmasebi, A.; Han, Y.; Zhao, H.; Lucas, J.; Wall, T. Characteristics of chars from low-temperature pyrolysis of lignite. Energy Fuels 2013, 28, 5612–5622. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, J.; Qinggang, L.; Zhou, Z. Experimental study on preheated combustion of pulverized semi-coke. J. Therm. Sci. 2015, 24, 370–377. [Google Scholar] [CrossRef]
- Huang, Q.; Li, S.; Li, G.; Zhao, Y.; Yao, Q. Reduction of fine particulate matter by blending lignite with semi-char in a down-fired pulverized coal combustor. Fuel 2016, 181, 1162–1169. [Google Scholar] [CrossRef]
- Yan, L.; He, B. On a clean power generation system with the co-gasification of biomass and coal in a quadruple fluidized bed gasifier. Bioresour. Technol. 2017, 235, 113–121. [Google Scholar] [CrossRef]
- Gong, Z.Q.; Liu, Z.C.; Zhu, Z.P.; Yu, K.S.; Meng, G.J.; Liu, J.P.; Ouyang, Z.Q.; Sun, Y.K.; Liu, Q.G. Experimental study on semi-coke combustion and coal pyrolysis and combustion coupling. J. China Coal Soc. 2014, 39, 519–525. (In Chinese) [Google Scholar]
- Jun, W.; Zhu, J.G.; Lu, Q.G. Experimental study on combustion characteristics and nox emissions of pulverized anthracite preheated by circulating fluidized bed. J. Therm. Sci. 2011, 20, 355–361. [Google Scholar]
- Ouyang, Z.; Zhu, J.; Lu, Q. Experimental study on preheating and combustion characteristics of pulverized anthracite coal. Fuel 2013, 113, 122–127. [Google Scholar] [CrossRef]
- Zheng, S.; Hu, Y.; Wang, Z.; Cheng, X. Experimental investigation on ignition and burnout characteristics of semi-coke and bituminous coal blends. J. Energy Inst. 2020, 93, 1373–1381. [Google Scholar] [CrossRef]
- Zhang, J.P.; Jia, X.; Wang, C.A.; Zhao, N.; Wang, P.Q.; Che, D.F. Experimental investigation on combustion and NO formation characteristics of semi-coke and bituminous coal blends. Fuel 2019, 247, 87–96. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, W.; Liu, H.; Jia, C.; Li, S. Interactions and kinetic analysis of oil shale semi-coke with cornstalk during co-combustion. Appl. Energy 2011, 88, 2080–2087. [Google Scholar] [CrossRef]
- Liu, H.P.; Liang, W.X.; Qin, H.; Wang, Q. Thermal behavior of co-combustion of oil shale semi-coke with torrefied cornstalk. Appl. Therm. Eng. 2016, 109, 413–422. [Google Scholar] [CrossRef]
- Liu, H.P.; Liang, W.X.; Qin, H.; Wang, Q. Synergy in co-combustion of oil shale semi-coke with torrefied cornstalk. Appl. Therm. Eng. 2016, 109, 653–662. [Google Scholar] [CrossRef]
- Wall, T.; Liu, Y.; Spero, C.; Elliott, L.; Khare, S.; Rathnam, R.; Zeenathal, F.; Moghtaderi, B.; Buhre, B.; Cheng, S. An overview on oxyfuel coal combustion—State of the art research and technology development. Chem. Eng. Res. Des. 2009, 87, 1003–1016. [Google Scholar] [CrossRef]
- Chen, L.; Yong, S.Z.; Ghoniem, A.F. Oxy-fuel combustion of pulverized coal: Characterization, fundamentals, stabilization and CFD modeling. Prog. Energy Combust. Sci. 2012, 38, 156–214. [Google Scholar] [CrossRef]
- Buhre, B.J.P.; Elliott, L.K.; Sheng, C.D.; Gupta, R.P.; Wall, T.F. Oxy-fuel combustion technology for coal-fired power generation. Prog. Energy Combust. Sci. 2005, 31, 283–307. [Google Scholar] [CrossRef]
- Wall, T.F. Combustion processes for carbon capture. Proc. Combust. Inst. 2007, 31, 31–47. [Google Scholar] [CrossRef]
- Ma, L.; Wang, T.X.; Liu, J.C.; Fang, Q.Y.; Zhang, C.; Chen, G. Effect of different conditions on the combustion interactions of blended coals in O2/CO2 mixtures. J. Energy Inst. 2019, 92, 413–427. [Google Scholar] [CrossRef]
- Tong, C.; Yang, X.; Chen, G.; Zhang, Y.; Chen, L.; Zhou, Y.; He, T.; Jin, B. Experimental investigation for the combustion characteristics of blends of three kinds of coal. Fuel 2021, 300, 120937. [Google Scholar] [CrossRef]
- Sun, X.X. The Experiment Technology and Method of Boiler Combustion; Chinese Electrical Power Press: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Sutcu, H.; Piskin, S. Characterization and combustion kinetics of chars obtained from loquat stones. Combust. Sci. Technol. 2009, 181, 264–273. [Google Scholar] [CrossRef]
- Yorulmaz, S.Y.; Atimtay, A.T. Investigation of combustion kinetics of treated and untreated waste wood samples with thermogravimetric analysis. Fuel Process. Technol. 2009, 90, 939–946. [Google Scholar] [CrossRef]
- Wang, C.A.; Liu, Y.; Zhang, X.; Che, D.F. A study on coal properties and combustion characteristics of blended coals in northwestern China. Energy Fuels 2011, 25, 3634–3645. [Google Scholar] [CrossRef]
- Shen, G.D.; Wang, Z.Q.; Wu, J.L.; He, T.; Li, J.Q.; Yang, J.; Wu, J.H. Combustion characteristics of low-rank coal chars in O2/CO2, O2/N2 and O2/Ar by TGA. J. Fuel Chem. Technol. 2016, 44, 1066–1073. [Google Scholar] [CrossRef]
- Masel, R.I. Chemical Kinetics, Catalysis; Wiley-Interscience: New York, NY, USA, 2001. [Google Scholar]
- Chen, C.X.; Ma, X.Q.; Liu, K. Thermogravimetric analysis of microalgae combustion under different oxygen supply concentrations. J. Appl. Energy 2011, 88, 3189–3196. [Google Scholar] [CrossRef]
- Liu, G.H.; Ma, X.Q.; Yu, Z.S. Experimental and kinetic modeling of oxygen-enriched air combustion of municipal solid waste. J. Waste Manag. 2011, 29, 792–796. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Z.; Ma, X.; Long, J.; Peng, Y.; Hu, L.; Lu, Q. Oxy-fuel combustion characteristics and kinetics of microalgae Chlorella vulgaris by thermogravimetric analysis. Bioresour. Technol. 2013, 144, 563–571. [Google Scholar] [CrossRef]
- Yao, H.; He, B.; Ding, G.; Tong, W.; Kuang, Y. Thermogravimetric analyses of oxy-fuel co-combustion of semi-coke and bituminous coal. Appl. Therm. Eng. 2019, 156, 708–721. [Google Scholar] [CrossRef]
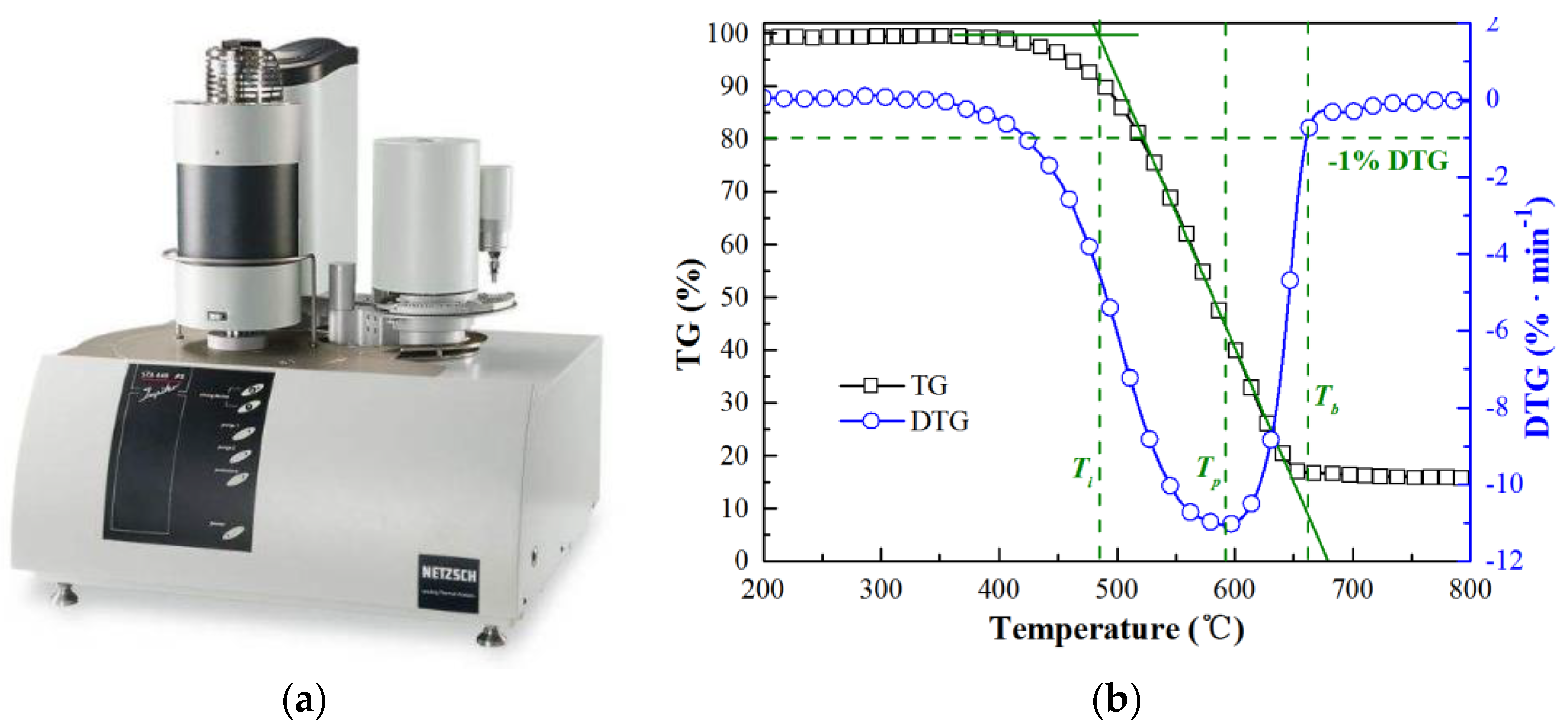
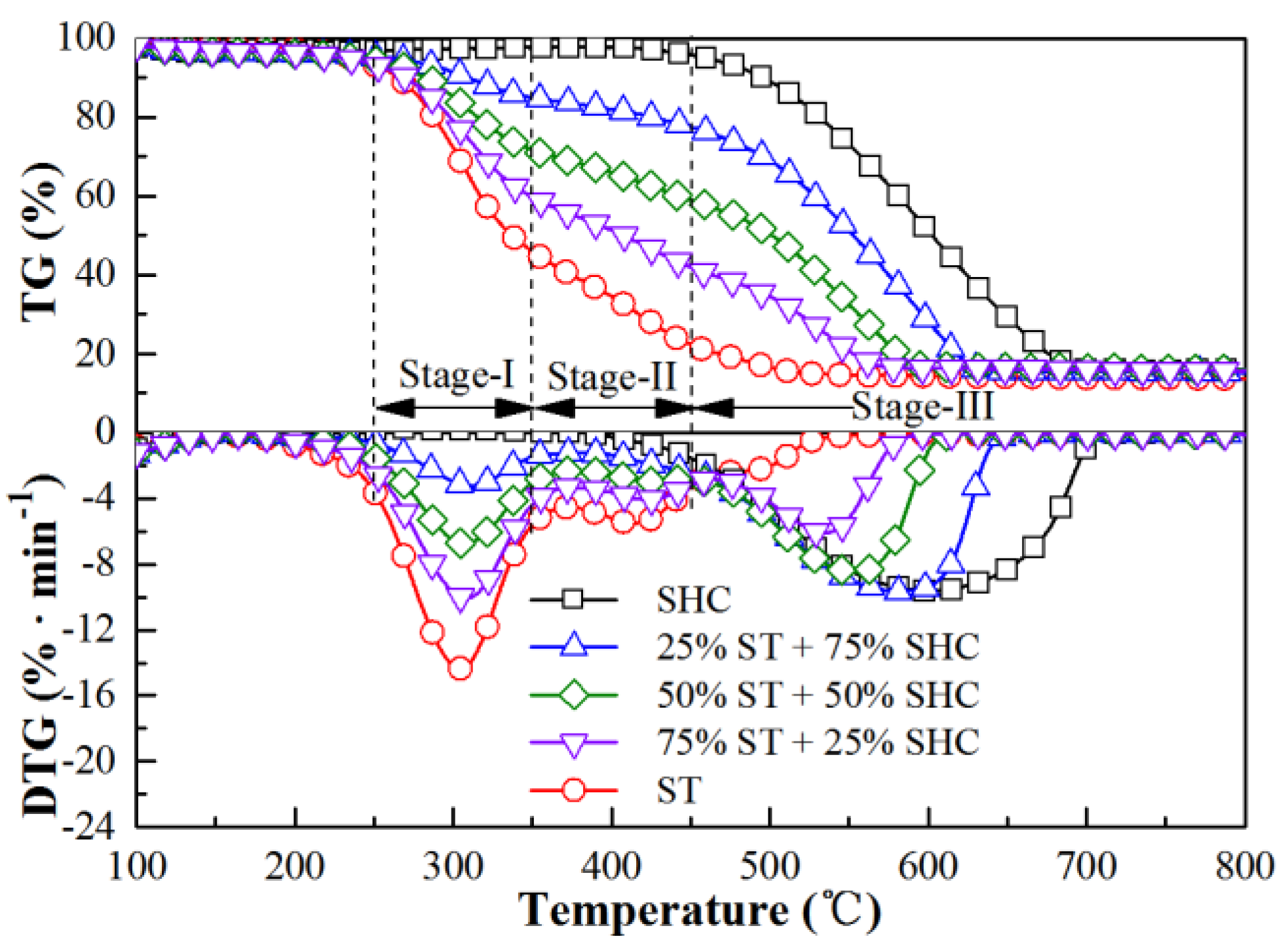
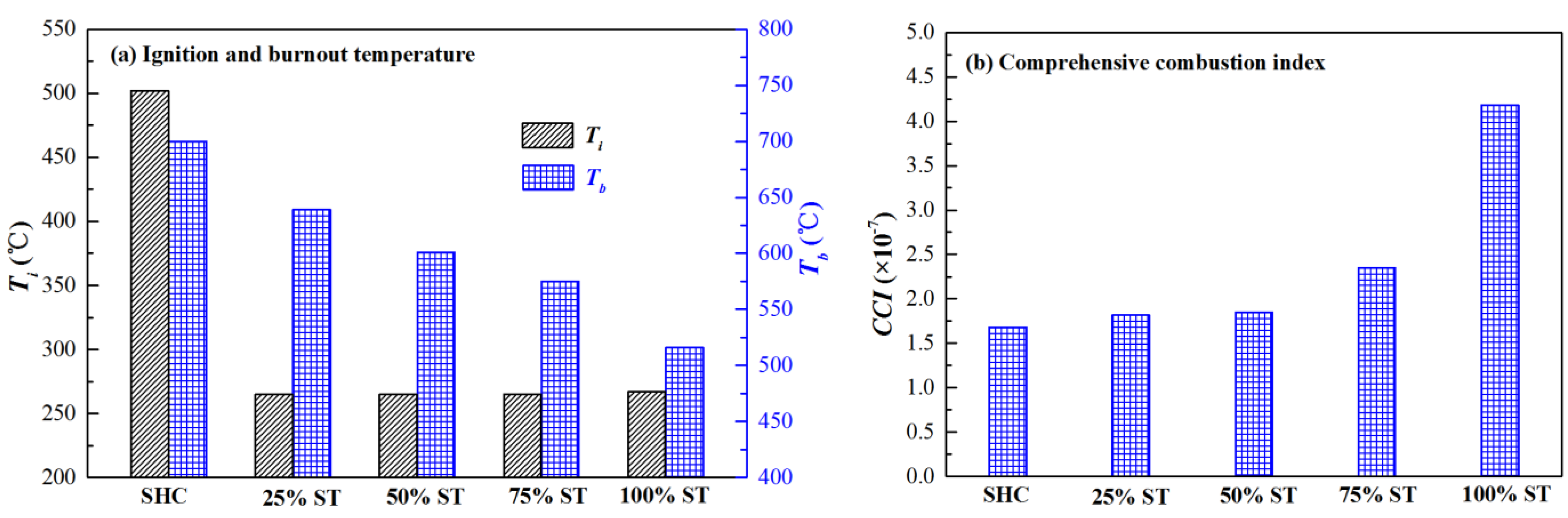
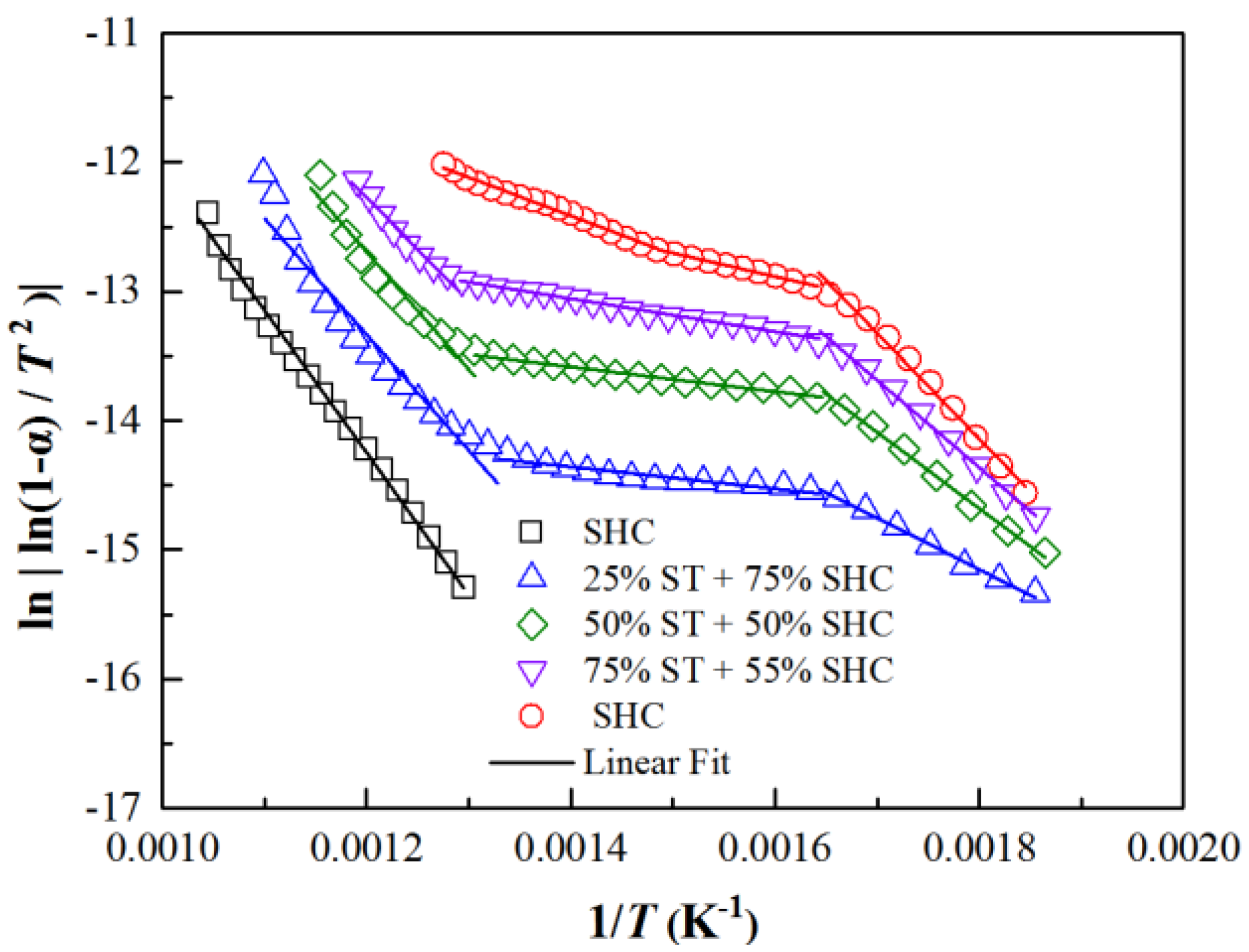

| Samples | Proximate Analysis (Dry, wt%) | Ultimate Analysis (Daf, wt%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Volatile Matter | Ash | Fixed Carbon | C | H | O | N | S | |
| Shenhua coal | 32.32 | 10.27 | 57.41 | 81.52 | 3.33 | 13.65 | 1.18 | 0.31 |
| SHC | 6.45 | 14.93 | 78.62 | 72.25 | 0.43 | 11.21 | 0.89 | 0.29 |
| ST | 75.90 | 10.75 | 13.35 | 48.22 | 6.62 | 33.05 | 1.06 | 0.31 |
| Item | Stages | Fi (%) | Ei (kJ/mol) | E (kJ/mol) | lnAi (s−1) | lnA (s−1) | R2 |
|---|---|---|---|---|---|---|---|
| SHC | 502–700 | - | - | 91.51 | - | 17.35 | 0.99117 |
| 25% ST + 75% SHC | Stage I | 11 | 43.09 | 56.43 | 21.96 | 19.66 | 0.99550 |
| Stage II | 14 | 5.82 | 25.63 | 0.91626 | |||
| Stage III | 75 | 67.84 | 18.21 | 0.93601 | |||
| 50% ST + 50% SHC | Stage I | 27 | 49.03 | 47.44 | 18.43 | 19.62 | 0.99590 |
| Stage II | 31 | 7.94 | 24.79 | 0.98230 | |||
| Stage III | 42 | 75.58 | 16.58 | 0.94467 | |||
| 75% ST + 25% SHC | Stage I | 39 | 56.20 | 42.38 | 16.69 | 19.48 | 0.96747 |
| Stage II | 36 | 10.64 | 23.99 | 0.99330 | |||
| Stage III | 25 | 66.52 | 17.35 | 0.99529 | |||
| 100% ST | Stage I | 54 | 67.93 | 46.18 | 14.13 | 17.96 | 0.99101 |
| Stage II | 20 | 15.18 | 23.16 | 0.99212 | |||
| Stage III | 26 | 24.833 | 21.92 | 0.99286 |
| Item | Stages | Fi (%) | Ei (kJ/mol) | E (kJ/mol) | lnAi (s−1) | lnA (s−1) | R2 |
|---|---|---|---|---|---|---|---|
| 21% O2/79% CO2 | Stage I | 27 | 49.03 | 47.44 | 18.43 | 19.62 | 0.99590 |
| Stage II | 31 | 7.94 | 24.79 | 0.98230 | |||
| Stage III | 42 | 75.58 | 16.58 | 0.94467 | |||
| 30% O2/70% CO2 | Stage I | 25 | 52.47 | 56.29 | 17.81 | 18.76 | 0.99377 |
| Stage II | 29 | 9.03 | 24.74 | 0.96212 | |||
| Stage III | 46 | 88.15 | 15.52 | 0.96876 | |||
| 40% O2/60% CO2 | Stage I | 24 | 58.92 | 57.33 | 16.62 | 18.61 | 0.99045 |
| Stage II | 31 | 9.924 | 24.67 | 0.95589 | |||
| Stage III | 45 | 89.14 | 15.50 | 0.98724 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Zhao, N.; Feng, Y.; Xie, Z. Thermogravimetric Analysis of Coal Semi-Char Co-Firing with Straw in O2/CO2 Mixtures. Processes 2021, 9, 1421. https://doi.org/10.3390/pr9081421
Li D, Zhao N, Feng Y, Xie Z. Thermogravimetric Analysis of Coal Semi-Char Co-Firing with Straw in O2/CO2 Mixtures. Processes. 2021; 9(8):1421. https://doi.org/10.3390/pr9081421
Chicago/Turabian StyleLi, Debo, Ning Zhao, Yongxin Feng, and Zhiwen Xie. 2021. "Thermogravimetric Analysis of Coal Semi-Char Co-Firing with Straw in O2/CO2 Mixtures" Processes 9, no. 8: 1421. https://doi.org/10.3390/pr9081421
APA StyleLi, D., Zhao, N., Feng, Y., & Xie, Z. (2021). Thermogravimetric Analysis of Coal Semi-Char Co-Firing with Straw in O2/CO2 Mixtures. Processes, 9(8), 1421. https://doi.org/10.3390/pr9081421





