Abstract
Mutual diffusion coefficients of chloroquine diphosphate (CDP) in aqueous solutions both without and with β-cyclodextrin (β-CD) were measured at concentrations from (0.0000 to 0.0100) mol dm−3 and 298.15 K, using the Taylor dispersion technique. Ternary mutual diffusion coefficients (Dik) measured by the same technique are reported for aqueous CDP + β-CD solutions at 298.15 K. The presence of β CD led to relevant changes in the diffusion process, as showed by nonzero values of the cross-diffusion coefficients, D12 and D21. β-CD concentration gradients produced significant co-current coupled flows of CDP. In addition, the effects of β-CD on the transport of CDP are assessed by comparing the binary diffusion coefficient of aqueous CDP solutions with the main diffusion coefficient (D11) measured for ternary {CDP(1) + β-CD(2)} solutions. These observations are supported by viscosity analysis. All data allow to have a better interpretation on the effect of cyclodextrin on the transport behavior of CDP.
1. Introduction
Chloroquine diphosphate (CDP) is a 4-aminoquinoline-based drug with a broad spectrum of applications, including all types of malaria infections and averse to cell growth and/or inducing cell death in human leukemia K562 cell [1,2,3]. CDP is also indicated for the treatment of inflammatory diseases and rheumatoid arthritis [4,5]. More recently, CDP has been highly cited as a consequence of its potential, though not confirmed, to treat severe acute respiratory syndrome coronavirus 2 [6].
CDP is soluble in water and characterized by having a high bioavailability when administrated orally [4]. However, this drug also shows some significant side effects [7]. These can be reduced by forming host–guest supramolecular compounds with cyclodextrins [2]. It is known that due to the amphiphilic behavior of cyclodextrins, supramolecular interactions mainly occur inside the hydrophobic CD’s cavity with hydrophobic guests [8,9]. However, interactions between guest molecules and cyclodextrins can also occur via, for example, H-bonding involving hydroxyl groups located outside the CD’s cavity [10,11]. Despite its solubility in aqueous solutions, the molecular structure of the CDP suggests the ability of the quinolone group to form host–guest supramolecular compounds with β-cyclodextrin. For example, Fan et al. have reported the complexation of 8-nitro-quinoline (at solid state) with β-cyclodextrin [12], whilst the equilibrium constant between quinolone and β-CD has been computed as equal to ca. 380 L mol−1 [13]. These values are in line with the binding constant of 1890 L mol−1 for a 1:1 CDP: β -CD complex obtained by Roy et al. [2]. Recently, it has been demonstrated by NMR and computational studies that chloroquine is able to protrude both α- and β–cyclodextrins, being that the stronger interaction occurs with the β-cyclodextrin [14].
Although much work has been done on the development of CDP-containing systems [1,15], its kinetics in aqueous solution are still poorly understood. In the present paper, transport properties (diffusion coefficients and viscosities) of CDP in water and in aqueous solutions containing β-cyclodextrin are reported.
Specifically, we have measured binary diffusion coefficients of this drug in water, and multicomponent chemical ternary diffusion coefficients (D11, D22, D12 and D21) for CDP(1) + β-CD(2) aqueous solutions, using the Taylor dispersion technique. The behavior diffusion of these systems (binary and ternary) and the coupled flows occurring in the solution were studied in order to better understand if there are interactions between CD–CDP by estimating its association constant, leading to better insight of these systems’ structure.
The comparison of Jones–Dole viscosity coefficients for CDP in water and in the {water + β-CD 0.0070 mol dm−3} mixture allowed to evaluate the solute–solvent interactions occurring in these solutions.
Additionally, interdiffusion coefficients correspond to the maximum limit value for the release kinetics of drugs (or complexes), in these systems.
2. Materials and Methods
2.1. Materials
Chloroquine diphosphate (CDP) (Merck) an β-cyclodextrin (β-CD) (Sigma-Aldrich) were used as received, without further purification (Table 1). All solutions were freshly prepared and degassed by sonication before each experiment.

Table 1.
Sample description.
2.2. Techniques
2.2.1. Viscosity Measurements
For viscosity measurements, a set of CDP aqueous solutions were prepared at concentrations 0.0010; 0.0020; 0.0050; 0.0070 and 0.0100 mol.dm−3 by dissolving the corresponding solid in pure water, under continuous stirring, at 298.15 K. Likewise, another set of CDP solutions were also prepared in a mixture {water + β-CD 0.0070 mol dm−3} as solvent at the same concentrations above indicated and following the same procedure.
Viscosity measurements were performed using a microviscometer (Lovis 2000 ME Anton Paar) at 298.15 ± 0.01 K. The average value of the viscosity data at each concentration was obtained from five independent measurements. The viscometer was calibrated with Milli-Q water (from A10 Millipore) before every set of experiments. The uncertainty of the values for this parameter was calculated as equal to 0.04 mPa s based on calibration data. The repeatability of the experiments was ± 0.05%.
2.2.2. Diffusion Measurements
Taylor dispersion technique for measuring diffusion in solutions has gained increasing popularity due to its fast and reliable analysis of multicomponent systems.
The theory of the Taylor dispersion technique is well described in the literature [16,17,18,19,20,21] and, consequently, only a summarized description of both the apparatus and the procedure used in our study is presented here.
At the begin of each experience, a 6-port Teflon injection valve (Rheodyne, model 5020) was used to introduce 63 mm3 of solution into a laminar carrier stream with a flow rate of 0.17 cm3 min−1 leading retention times ca. 1.1 × 104 s. The dispersion tube and the injection valve was kept at 298.15 ± 0.01 K in an air thermostat. The radius of the tube is equal to 0.32200 ± 0.00003 mm. The monitoring of the injected samples dispersion, at the dispersion tube outlet, was done using a differential refractometer (Waters model 2410).
Detector voltages, V(t), were measured by using a digital voltmeter (Agilent 34401 A).
The diffusion of CDP in aqueous solutions (binary system) is described by Fick’s equation
where C is the molar concentration of the solute and D the binary diffusion coefficient.
J = −D∇C
At the tube outlet, the distribution of the dispersed solute is followed by passing the carrier through a differential refractometer which gives a linear response to changes in V(t) composition dependent property. Combining this detector output signal V(t) and the equation derived by Taylor [22,23,24], that accurately describes the dispersion of the solutes, and that considers that the flow is laminar, the binary diffusion coefficients were evaluated by fitting the dispersion Equation (2) to the detected voltages. That is,
V(t) = V0 + V1t + Vmax (tR/t)1/2exp[−12D(t − tR)2/r2t]
V1 the baseline slope, V0 is the baseline voltage, Vmax the peak height, tR the mean sample retention time and r the inner radius of the dispersion tube.
Ternary mutual diffusion coefficients (Dik) of aqueous {CDP(C1) + β-CD(C2)} solutions were computed by using coupled Fick equations (Equations (3) and (4)) [20,21].
J1 = −D11∇C1 − D12∇C2
J2 = −D21∇C1 − D22∇C2
J1 and J2 represent the molar fluxes of CDP (1) and β-CD (2), respectively, driven by the concentration gradients ∇C1 and ∇C2 of each solute 1 and 2. Cross-diffusion coefficients D12 and D21 give the coupled flux of each solute, driven by a concentration gradient in the other solute. While a negative Dij coefficient indicates countercurrent coupled transport of solute i from regions of lower to higher concentration of solute j, a positive Dij cross-coefficient (i ≠ j) indicates co-current coupled transport of solute i from regions of higher to lower concentrations of solute j. Main diffusion coefficients D11 and D22 give the flux of each solute, driven by its own concentration gradient.
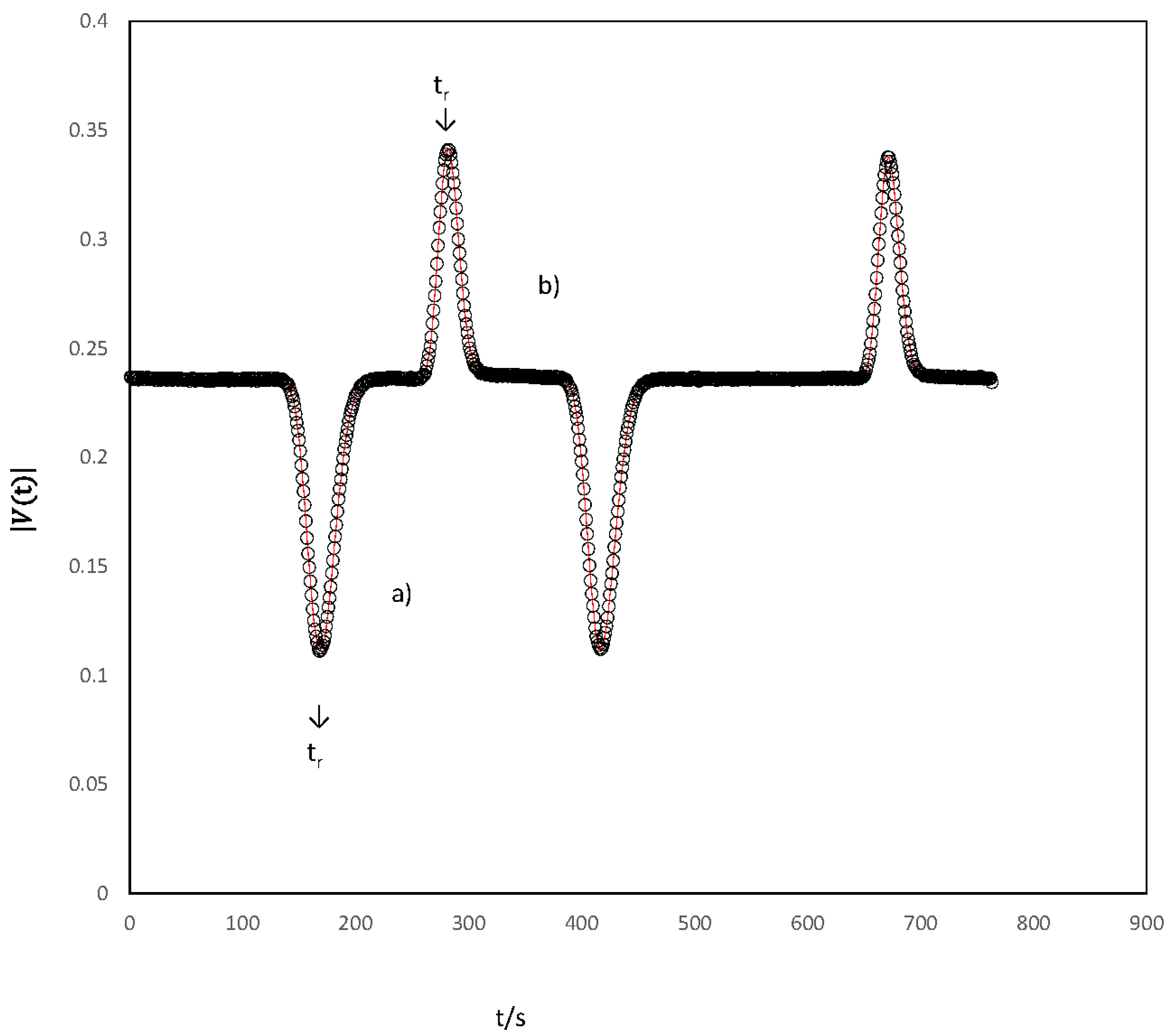
Ternary dispersion experiences were prepared by injecting {CDP(1) + β-CD(2)} solution samples of composition C1 + ΔC1, C2 + ΔC2 into carrier streams of composition C1, C2. In the tracer diffusion studies, the concentration of the component studied under trace conditions was zero; that is, in the carrier solutions, for tracer of CDP, = 0, C2 = C2, and for tracer of β-CD, C2 = 0 and C1 = C1. Considering the equation that describes ternary dispersion profiles provided and that the flow is also laminar, whose development is well reported in references [20,25,26]), the ternary Dij coefficients were calculated by fitting the Equation (5)) (Figure 1)
being:
Figure 1.
Ternary dispersion profiles relative to {chloroquine diphosphate (CDP) (C1) + β-cyclodextrin (β-CD) (C2)} solutions generated by injecting: (a) a 0.063 cm3 sample of chloroquine diphosphate 0.005 mol dm−3 into 0.007 mol dm−3 β-cyclodextrin (profile ∆C1 = 0.005 mol dm−3, ∆C2 = 0); (b) a 0.063 cm3 sample of 0.002 mol dm−3 β-cyclodextrin into 0.007 mol dm−3 β-cyclodextrin (Profile ∆C1 = 0 mol dm−3, ∆C2 =0.002 mol dm−3). Measured (ο) and fitted (−, Equation (5)). tR represents the mean sample retention time.
D1 and D2 are the eigenvalues of the matrix of Dik coefficients (Equations (7) and (8)) and α1 is the fraction of the initial refractive index difference due to CDP. R1 and R2 are the detector sensitivities for CDP and β-CD, respectively: R1 = ∂V/∂C1 and R2 = ∂V/∂C2.
Equation (5) was fitted to pairs of ternary profiles measured for α1 ≈ 0 (initial CDP concentration difference) and α1 ≈ 1 (initial β-CD concentration difference). Dik were determined from the relative detector sensitivity R2/R1 and the D1, D2, a, b fitting parameters, using
The parameters a and b, in these Equations (9)–(12) are defined by
3. Results
3.1. Viscosity Measurements
Viscosity values for CDP solutions both in pure water and in the mixture {water + β-CD 0.0070 mol dm−3} are reported in Table 2.

Table 2.
Viscosity values of aqueous solutions of chloroquine diphosphate (CDP) in pure water (ηw), and in a {water + β-CD 0.0070 mol dm−3} mixture (η(w+ β-CD)) as solvent, at P = 101.3 Pa and at T = 298.15 K.
3.2. Diffusion Measurements
Table 3 and Table 4 show the mean values of the diffusion coefficients for binary systems (CDP/H2O and β-CD/H2O) and for the aqueous ternary system: CDP(1)/β-CD (2), at 298.15 K.

Table 3.
Binary diffusion coefficients (D) measured in the present work of both aqueous solutions of chloroquine diphosphate(1) and of β-cyclodextrin(2), respectively, at P = 101.3 Pa and at T = 298.15 K.

Table 4.
Ternary mutual diffusion coefficients (D11, D12, D21, D22) of aqueous {chloroquine diphosphate (CDP) (C1) + β-cyclodextrin (β-CD) (C2)} solutions at P = 101.3 Pa and at T = 298.15 K.
Mutual binary diffusion coefficients, D, of CDP in aqueous solutions, in Table 3, denote the average ones from, at least, four independent experiments. Good reproducibility (within ±1%) was obtained.
Table 4 show the average (D11, D12, D21, D22) values of aqueous {chloroquine diphosphate (CDP) (C1) + β-cyclodextrin (β-CD) (C2)} solutions determined for each carrier solution composition by fitting Equation (5) to five replicate pairs of dispersion profiles. Main diffusion coefficients D11 and D22 were generally reproducible within ± 0.015 × 10−9 m2 s−1. Cross-diffusion coefficients D12 and D21, describing the coupled diffusion of CDP and β-CD; they were reproducible within ± 0.050 × 10−9 m2 s−1.
The main diffusion coefficients D11 and D22, which give the molar fluxes of the CDP (1) and β-CD (2) components, respectively, driven by their own concentration gradients, are compared with those obtained for the binary systems (CDP/water and β-CD/water [27]—Table 3).
It should be noted that D11 values are higher than the D22 ones, and, in general, lower than the binary D of CDP in pure water (deviations between 0.1 and 17%; Table 3). However, the values found for D22 are similar to those of the binary diffusion coefficients reported for β-CD in aqueous solution [27]. These results indicate that while the addition of CDP produces relatively small changes in the diffusion coefficient of β-CD (D22), the addition of β-CD leads to important changes in that of CDP (D11) (up to 13%). This effect of decrease in the diffusion of CDP, due to the presence of β-CD, is also highlighted by the positive values of the D12 cross-diffusion coefficients, from which it can be concluded that in solutions containing CDP at concentrations 0.0050, 0.0070 and 0.0100 mol dm−3, for which D12 >0 (Table 4), the gradient in the concentration of β-CD produces co-current coupled flows of CDP. Nevertheless, because the D21 values are almost zero, the CDP concentration gradient leads to weak countercurrent coupled flows of β-CD.
Considering that the D12/D22 ratio gives the number of moles of CDP transported per mole of β-CD, we may state that one mole of diffusing β-CD co-transports as a maximum 0.5 moles of CDP. Through the D21/D11 values, at the same concentrations, we can expect that one mole of diffusing CDP counter transports at most 0.04 moles of β-CD.
This behavior can be justified assuming the occurrence of CDP aggregates, with lower mobility and, consequently, being responsible for D11 decreasing. This effect is less relevant when we consider the effect of CDP on transport of β-CD, probably due to the resemblance of the mobilities of β-CD-free species and eventual aggregates of CDP and β-CD.
4. Discussion
The analysis of the dependence of the viscosity on the concentration was assessed by fitting the values of relative viscosity, ηr, to the Jones–Dole equation (Equation (15)) [28,29]
where A and B are empirical terms and C as the same meaning as before.
Being A and B coefficients related with the long-distance solute–solute interactions and to the solute–solvent interactions, respectively, through their values, it is possible to analyze the structure-making or structure-breaking character of the electrolyte in the solution. That is, when coefficient B is positive, we can say that the solute has an organizing capacity of the solvent structure (structure-making character). Contrariwise, a negative value of the B coefficient is related to a solute with the ability to break the structure of water (structure-breaking character) [30].
From the fitting of the viscosity values of the CDP aqueous solutions (Table 2) to the Jones–Dole equation [28,29], we have obtained A = 1.75 dm3/2 mol−1/2 and B = −10.6 dm3 mol−1. The small positive A value and the negative B value suggest that weak interactions between CDP-CDP entities are present, and CDP is a structure-breaking solute (chaotropic solute) [31].
With the purpose of evaluating the changes occurring in the structure and behavior of this solute when β-CD is present in the aqueous medium, viscosity measurements in a {water + β-CD 0.007 mol dm−3} mixture were carried out and the results also evaluated with the Jones–Dole equation. From this, slightly higher values for A and B parameters (that is, A = 2.39 dm3/2 mol−1/2 and B = −14.2 dm3 mol−1) were obtained, indicating that the situation is similar.
The interpretation of these data can be also achieved, by a more detailed treatment of the diffusion with supramolecular complexes, using some assumptions. That theory has been developed and well described in the literature [32,33,34,35]; In addition, the computation of ternary diffusion coefficients is also described in detail in the Supplementary Material; consequently, only some points are shown in this section.
Another approach to these data can be performed by assuming the occurrence of a 1:1 supramolecular complex between chloroquine diphosphate (CDP) and β-cyclodextrin (β-CD), (Equation (16))
where the association constant, K, that describes the stability of these complexes, is given by Equation (17).
CDP + β-CD ⇆ CDP–β-CD
CCDP, Cβ-CD and CCDP-β-CD represent the concentrations of free CDP and β-CD, and the concentration of the CDP–β-CD complex, respectively, which are correlated by the following mass balance equations,
C1 = CCDP + CCDP-β-CD
C2 = CCD + CCDP-β-CD
C2 = CCD + CCDP-β-CD
Identifying these solute species as CDP = 1, β-CD = 2, and CDP–β-CD complexes = 3, respectively, which are in equilibrium according to the equation (Equation (16)), the Equations (3) and (4)), should be replaced by
J1 = −D11∇C1 − D12∇C2 − D13∇C3
J2 = −D21∇C1 − D22∇C2 − D23∇C3
J3 = −D31∇C1 − D32∇C2 − D33∇C3
However, assuming that in diluted solutions that all cross-diffusion terms are negligible (D12, D13, D21, D23, D31, D32 = 0), and by noting that the total CDP flux (as well as β-CD flux) is the sum of the respective fluxes of free and CDP–β-CD complexes, by inserting this information in the Equations (3) and (4), after some mathematical rearrangement, it is possible to obtain the Equations (22)–(25). These equations supply the relations between the mutual diffusion coefficients D11, D12, D21, D22 for the total CDP(1) + β-CD(2) solute components, and the diffusion coefficients DCDP, Dβ-CD, DCDP–β-CD which indicate the diffusion coefficients of the free CDP, the free β-CD and the corresponding supramolecular complex, respectively.
where
The computed values for the limiting diffusion coefficients, Ds, of species CDP, β-CD and CDP–β-CD are reported in Table 5.

Table 5.
Diffusion coefficients, Ds, of species at infinitesimal concentration and T = 298.15 K and P = 101.3 kPa.
From D11 at X1 = 1 and from D22 at X1 = 0, the diffusion coefficients of free CDP (DCDP = 0.670 × 10−9 m2 s−1) and free β-cyclodextrin (Dβ-CD = 0.380 × 10−9 m2 s−1) are obtained, respectively.
By using the equation of Stokes–Einstein (Equations (26) and (27)):
where kB is the Boltzmann constant, T is the absolute temperature and η is the viscosity of the solvent, the diffusion coefficient of the CDP–β-CD complex can be estimated as equal to DCDP–β-CD = 0.360 ×10−9 m2 s−1.
K, was chosen in order to obtain the best agreement between these theoretical values (Equations (22)–25)) and the experimental Dij data. In the present case, for CDP molar factions X1 ≤ 0.5, a complexation constant K equal to 30.0 (±0.8) mol−1 dm3 was found.
This value demonstrates that the interaction between β-CD and CDP is weak, which can easily be justified by the high solubility of CDP in water, suggesting that the H-bonding plays an important role in the interactions between CDP and water and, probably, CDP and β-CD [9,36]. This is in line with similar systems involving other drugs such as, for example, L-dopa and paracetamol [37,38]. However, for X1 > 0.5, the model is not applicable. In fact, for these concentrations, the gradient in the concentration of β-CD produces significant co-current coupled flows of CDP and, therefore, leads to disadvantageous conditions for the formation of supramolecular complexes with this sterically hindered cyclodextrin in solution. This fact is in complete agreement with the viscosity results. That is, CDP is a structure-breaking solute, suggesting that, indeed, there is no complexation between β-CD and CDP.
5. Conclusions
We can conclude that the diffusion of the CDP is influenced by the presence of this macromolecular cyclodextrin (β-CD), suggesting that at low concentrations of this drug there is a very weak interaction between these solutes (which is supported by the small value that can be estimated for the equilibrium constant of the complexation between both solutes, CDP and β-CD, K = (30 ± 0.8) mol−1 dm3). This result is consistent with weak chloroquine binding to cyclodextrin, in contrast to a recent report of anomalously large-chloroquine binding constants. For more concentrated solutions, for which it is obtained that D12 > 0, a coflow of CDP is observed, showing thus, there is no predisposition of inclusion of CDP in the cavity of the sterically hindered β-CD. Support for this evidence is given by the viscosity data measured.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pr9081433/s1, The theoretical computation of the ternary diffusion coefficients, contemplating the for-mation of supramolecular complexes, is described in detail in the Supplementary Material.
Author Contributions
Conceptualization, A.C.F.R. and A.M.; methodology, A.C.F.R. and A.M.; software, A.C.F.R., L.M., A.M., E.F.G.A.; M.M.R., M.A.E. and A.J.M.V.; validation, A.C.F.R., L.M., A.M., A.J.M.V. and M.A.E.; formal analysis, A.C.F.R., A.M., M.A.E. and A.J.M.V.; investigation, A.C.F.R., L.M., A.M., E.F.G.A.; M.M.R.; A.J.M.V. and M.A.E.; resources, A.C.F.R. and A.M.; data curation, L.M.; A.C.F.R. and A.M.; writing—original draft preparation, A.C.F.R. and A.M.; writing–review and editing, A.C.F.R., A.M.; M.A.E. and A.J.M.V.; visualization, A.C.F.R., A.M., M.A.E. and A.J.M.V.; supervision, A.C.F.R. and A.M.; project administration, A.C.F.R. and A.M.; funding acquisition, A.C.F.R. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors in Coimbra are grateful for funding from “The Coimbra Chemistry Centre” which is supported by the Fundação para a Ciência e a Tecnologia (FCT), Portuguese Agency for Scientific Research, through the programs UID/QUI/UI0313/2019 and COMPETE. The authors in Zlín are grateful for research funding by the Ministry of Education, Youth and Sports of the Czech Republic DKRVO (RP/CPS/2020/003). MMR is thankful to the University of Alcalá (Spain) for the financial assistance (Mobility Grants for Researchers-2021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are thankful to António M.C. Ferreira for the technical support for diffusion technique maintenance and to Master L.M.P. Verissimo for discussions about the drug.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare no conflict of interest.
References
- Daneshfar, A.; Vafafard, S. Solubility of Chloroquine Diphosphate and 4,7-Dichloroquinoline in Water, Ethanol, Tetrahydrofuran, Acetonitrile, and Acetone from (298.2 to 333.2) K. J. Chem. Eng. Data 2009, 54, 2170–2173. [Google Scholar] [CrossRef]
- Roy, A.; Saha, S.; Roy, D.; Bhattacharyya, S.; Roy, M.N. Formation & specification of host–guest inclusion complexes of an anti-malarial drug inside into cyclic oligosaccharides for enhancing bioavailability. J. Incl. Phenom. Macrocycl. Chem. 2020, 97, 65–76. [Google Scholar] [CrossRef]
- Kamitsuji, Y.; Kuroda, J.; Kimura, S.; Toyokuni, S.; Watanabe, K.; Ashihara, E.; Tanaka, H.; Yui, Y.; Watanabe, M.; Matsubara, H.; et al. The Bcr-Abl kinase inhibitor INNO-406 induces autophagy and different modes of cell death execution in Bcr-Abl-positive leukemias. Cell Death Differ. 2008, 15, 1712–1722. [Google Scholar] [CrossRef][Green Version]
- Furst, D.E. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus 1996, 5, 11–15. [Google Scholar] [CrossRef]
- Verbeeck, R.K.; Junginger, H.E.; Midha, K.K.; Shah, V.P.; Barends, D.M. Biowaiver monographs for immediate release solid oral dosage forms based on biopharmaceutics classification system (BCS) literature data: Chloroquine phosphate, chloroquine sulfate, and chloroquine hydrochloride.This study reflects the scientific opinion. J. Pharm. Sci. 2005, 1389–1395. [Google Scholar] [CrossRef]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 71, 732–739. [Google Scholar] [CrossRef]
- Braga, C.B.; Martins, A.C.; Cayotopa, A.D.E.; Klein, W.W.; Schlosser, A.R.; Silva, A.F.; Sousa, M.N.; Andrade, B.W.B.; Junior, J.A.F.; Pinto, W.J.; et al. Side Effects of Chloroquine and Primaquine and Symptom Reduction in Malaria Endemic Area (Mâncio Lima, Acre, Brazil). Interdiscip. Perspect. Infect. Dis. 2015, 2015, 346853. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Duarte, F.I.C.; Heimfarth, L.; Quintans, J.S.S.; Quintans-Junior, L.J.; Junior, V.F.V.; Lima, A.A.N. Cyclodextrin–Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef]
- Valente, A.J.; Soderman, O. The formation of host-guest complexes between surfactants and cyclodextrins. Adv. Colloid Interface Sci. 2014, 205, 156. [Google Scholar] [CrossRef] [PubMed]
- Niether, D.; Kawaguchi, T.; Hovancova, J.; Eguchi, K.; Dhont, J.K.G.; Kita, R.; Wiegand, S. Role of Hydrogen Bonding of Cyclodextrin–Drug Complexes Probed by Thermodiffusion. Langmuir 2017, 33, 8483. [Google Scholar] [CrossRef] [PubMed]
- Ryzhakov, A.; Thi, T.D.; Stappaerts, J.; Bertoletti, L.; Kimpe, K.; Couto, A.R.S.; Saokham, P.; Mooter, G.V.; Augustijns, P.; Somsen, G.W.; et al. Self-Assembly of Cyclodextrins and Their Complexes in Aqueous Solutions. J. Pharm. Sci. 2016, 105, 2556. [Google Scholar] [CrossRef]
- Fan, Z.; Diao, C.-H.; Yu, M.; Jing, Z.-L.; Chen, X.; Deng, Q.-L. An Investigation of the Inclusion Complex of β-Cyclodextrin with 8-Nitro-Quinoline in the Solid State. Supramol. Chem. 2006, 18, 7–11. [Google Scholar] [CrossRef]
- Duan, Z.; Bu, T.; Bian, H.; Zhu, L.; Xiang, Y.; Xia, D. Effective Removal of Phenylamine, Quinoline, and Indole from Light Oil by β-Cyclodextrin Aqueous Solution through Molecular Inclusion. Energy Fuels 2018, 32, 9280–9288. [Google Scholar] [CrossRef]
- Assaba, I.M.; Rahali, S.; Belhocine, Y.; Allal, H. Inclusion complexation of chloroquine with α and β-cyclodextrin: Theoretical insights from the new B97-3c composite method. J. Mol. Struct. 2021, 1227, 129696. [Google Scholar] [CrossRef]
- Guoquan, Z.; Tinggong, W.; Danfeng, S.; Jian, S.; Zehui, Y. The solubility and dissolution thermodynamic properties of chloroquine diphosphate in different organic solvents. J. Chem. Thermodyn. 2021, 156, 106368. [Google Scholar] [CrossRef]
- Callendar, R.; Leaist, D.G. Diffusion Coefficients for Binary, Ternary, and Polydisperse Solutions from Peak-Width Analysis of Taylor Dispersion Profiles. J. Solut. Chem. 2006, 35, 353–379. [Google Scholar] [CrossRef]
- Barthel, J.; Gores, H.J.; Lohr, C.M.; Seidl, J.J. Taylor dispersion measurements at low electrolyte concentrations. I. Tetraalkylammonium perchlorate aqueous solutions. J. Solut. Chem. 1996, 25, 921–935. [Google Scholar] [CrossRef]
- Loh, W. A técnica de dispersão de taylor para estudos de difusão em líquidos e suas aplicações. Quim. Nova 1997, 20, 541–545. [Google Scholar] [CrossRef]
- Alizadeh, A.; Nieto de Castro, C.A.; Wakeham, W.A. The theory of the Taylor dispersion technique for liquid diffusivity measurements. Int. J. Thermophys. 1980, 1, 243–284. [Google Scholar] [CrossRef]
- Price, W.E. Theory of the taylor dispersion technique for three-component-system diffusion measurements. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1988, 84, 2431–2439. [Google Scholar] [CrossRef]
- Deng, Z.; Leaist, D.G. Ternary mutual diffusion coefficients of MgCl2 + MgSO4 + H2O and Na2SO 4 + MgSO4 + H2O from Taylor dispersion profiles. Can. J. Chem. 1991, 69, 1548–1553. [Google Scholar] [CrossRef]
- Taylor, G. Dispersion of Soluble Matter in Solvent Flowing Slowly through a Tube. Proc. R. Soc. Lond. A 1953, 219, 186–203. [Google Scholar] [CrossRef]
- Taylor, G. The dispersion of matter in turbulent flow through a pipe. Proc. R. Soc. Lond. A 1954, 223, 446–468. [Google Scholar] [CrossRef]
- Taylor, G. Conditions under which dispersion of a solute in a stream of solvent can be used to measure molecular diffusion. Proc. R. Soc. Lond. A. 1954, 225, 473–477. [Google Scholar] [CrossRef]
- Leaist, D.G. Determination of ternary diffusion coefficients by the Taylor dispersion method. J. Phys. Chem. 1990, 94, 5180–5183. [Google Scholar] [CrossRef]
- Leaist, D.G. Ternary diffusion coefficients of 18-crown-6 ether–KCl–water by direct least-squares analysis of Taylor dispersion measurements. J. Chem. Soc. Faraday Trans. 1991, 87, 597–601. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Leaist, D.G.; Esteso, M.A.; Lobo, V.M.; Valente, A.J.; Santos, C.I.; Cabral, A.M.; Veiga, F.J. Binary Mutual Diffusion Coefficients of Aqueous Solutions of β-Cyclodextrin at Temperatures from 298.15 to 312.15 K. J. Chem. Eng. Data 2006, 51, 1368–1371. [Google Scholar] [CrossRef]
- Jones, G.; Christian, S.M. The Viscosity of Aqueous Solutions of Electrolytes as a Function of the Concentration. V. Sodium Chloride. J. Am. Chem. Soc. 1937, 59, 484–486. [Google Scholar] [CrossRef]
- Jones, G.; Dole, M. The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride. J. Am. Chem. Soc. 1929, 2950–2964. [Google Scholar] [CrossRef]
- Marcus, Y. Effect of Ions on the Structure of Water: Structure Making and Breaking. Chem. Rev. 2009, 109, 1346–1370. [Google Scholar] [CrossRef]
- Musilová, L.; Mráček, A.; Kašpárková, V.; Minařík, A.; Valente, A.J.; Azevedo, E.F.; Verissimo, L.M.; Rodrigo, M.M.; Esteso, M.A.; Ribeiro, A.C. Effect of Hofmeister Ions on Transport Properties of Aqueous Solutions of Sodium Hyaluronate. Int. J. Mol. Sci. 2021, 22, 1932. [Google Scholar] [CrossRef]
- Paduano, L.; Sartorio, R.; Vitagliano, V.; Castronuovo, G. Calorimetric and diffusional behaviour of the system α-cyclodextrin-L-phenylalanine in aqueous solution. Thermochim. Acta 1990, 162, 155–161. [Google Scholar] [CrossRef]
- Paduano, L.; Sartorio, R.; Vitagliano, V.; Costantino, L. Diffusion coefficients in systems with inclusion compounds. Part 2. α-Cyclodextrin-(DL)norleucine-water at 25 °C. Ber. Bunsenges. Phys. Chem. 1990, 94, 741–745. [Google Scholar] [CrossRef]
- Paduano, L.; Vergara, A.; Corradino, M.R.; Vitagliano, V.; Sartorio, R. Equilibrium properties of the system (dibutyl L-tartrate)–(α-cyclodextrin)–(water) at 25 °C. A 1H NMR and UV study. Phys. Chem. Chem. Phys. 1999, 1, 3627–3631. [Google Scholar] [CrossRef]
- Paduano, L.; Sartorio, R.; Vitagliano, V. Diffusion coefficients of the ternary system α-cyclodextrin−sodium benzenesulfonate−water at 25 °C: The effect of chemical equilibrium and complex formation on the diffusion coefficients of a ternary system. J. Phys. Chem. B 1998, 102, 5023–5028. [Google Scholar] [CrossRef]
- Saenger, W.; Steiner, T. Cyclodextrin Inclusion Complexes: Host–Guest Interactions and Hydrogen-Bonding Networks. Acta Crystallogr. Sect. A Found. Crystallogr. 1998, 54, 798–805. [Google Scholar] [CrossRef]
- Barros, M.C.; Ramos, M.L.; Burrows, H.D.; Esteso, M.A.; Leaist, D.G.; Ribeiro, A.C. Ternary mutual diffusion coefficients of aqueous {l-dopa (1)+β-CD (2)} solutions at T = 298.15 K. J. Chem. Thermodyn. 2015, 90, 169–173. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Musilová, L.; Mráček, A.; Cabral, A.M.; Santos, M.A.; Cabral, I.; Esteso, M.A.; Valente, A.J.M.; Leaist, D. Host-guest paracetamol/cyclodextrin complex formation evaluated from coupled diffusion measurements. J. Chem. Thermodyn. 2021, 161, 106551. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).