Optimization of Process Parameters and Kinetics Analysis of Cd Removal in ZnSO4 Production
Abstract
:1. Introduction
2. Experimental Method
3. Optimization Experiment by Response Surface Methodology
3.1. Experimental Design
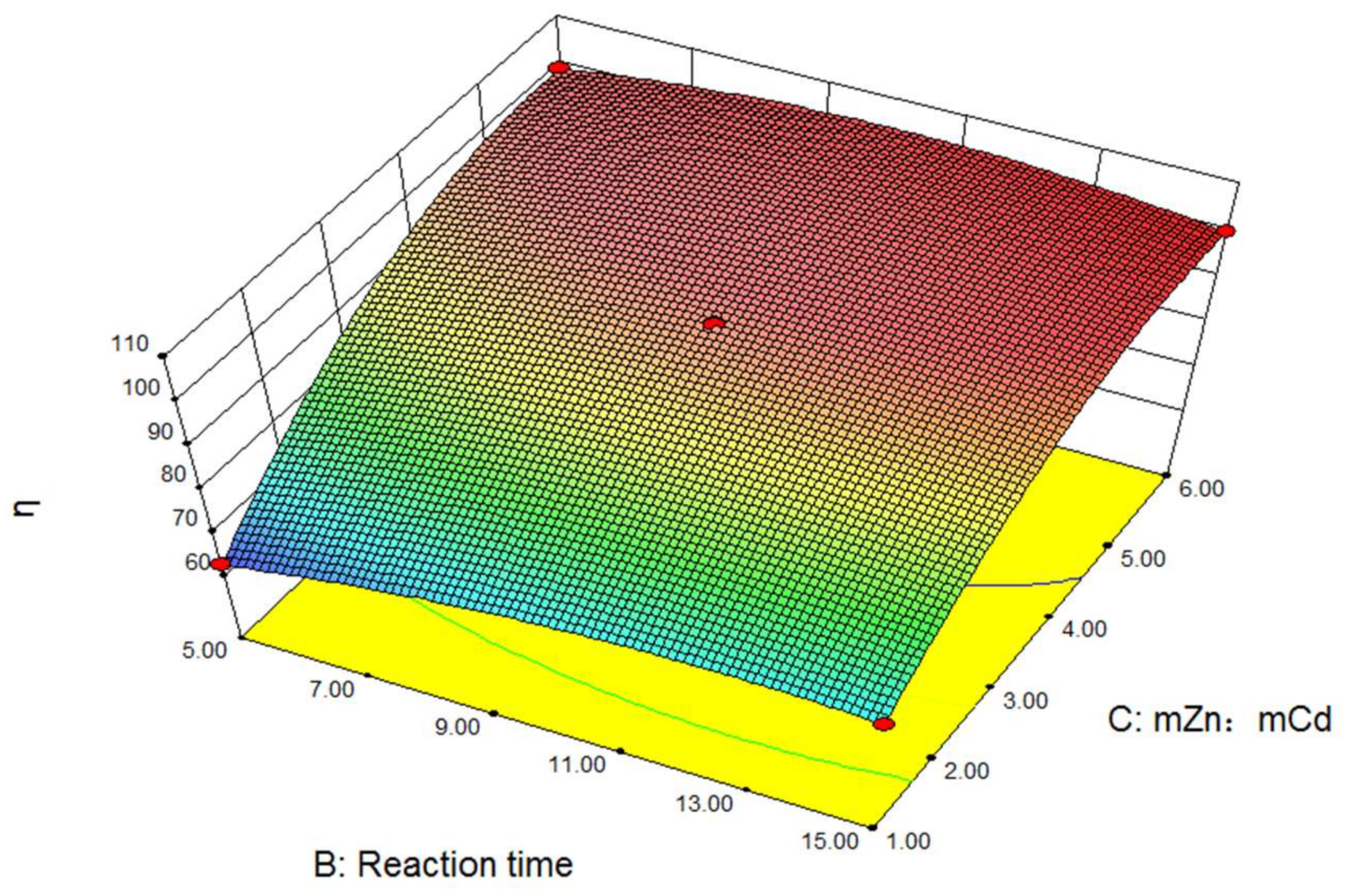
3.2. Experimental Results and Regression Analysis
4. Kinetic Analysis of Cd Removal Reaction
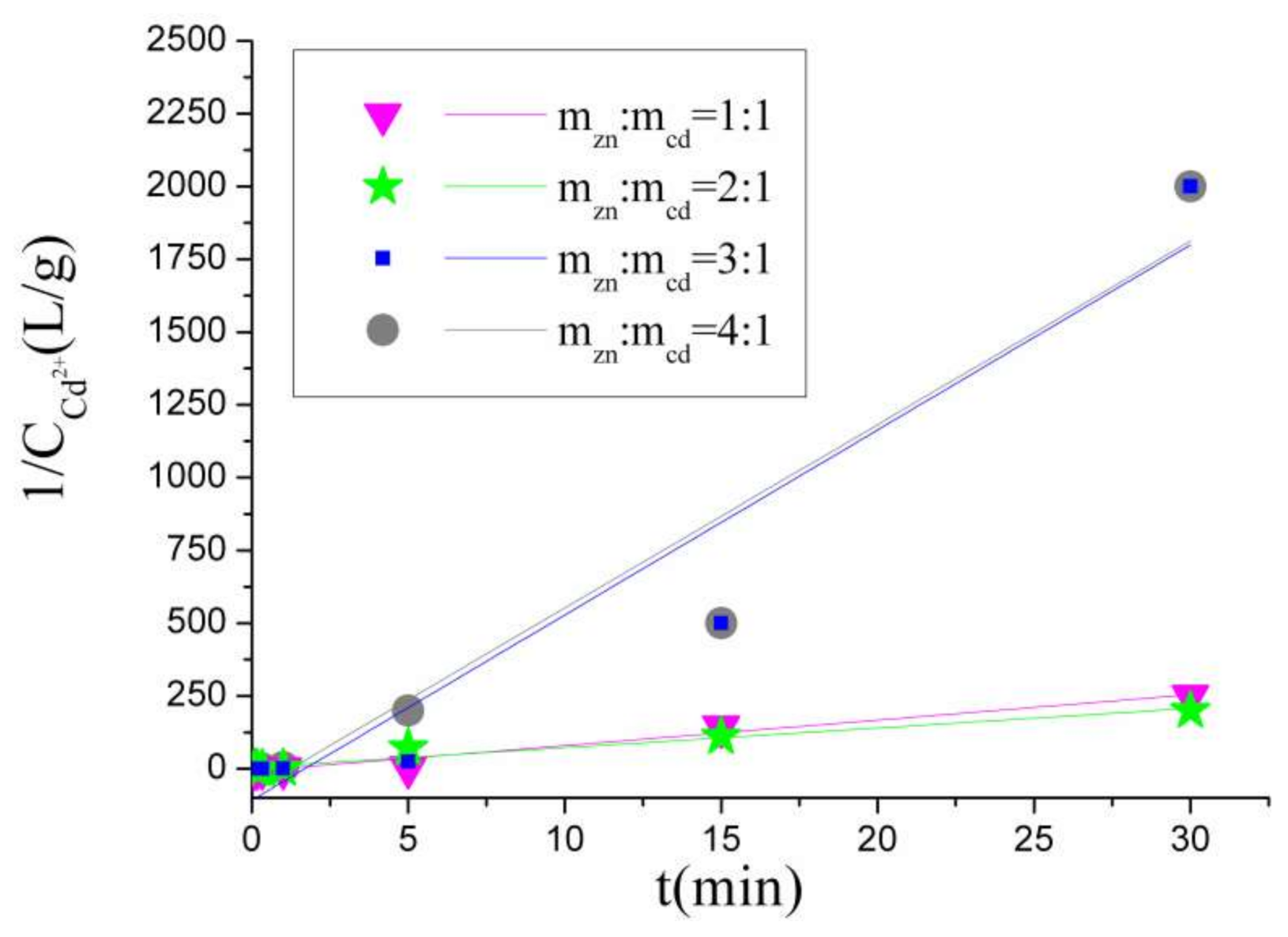
4.1. Reaction Order Judgment
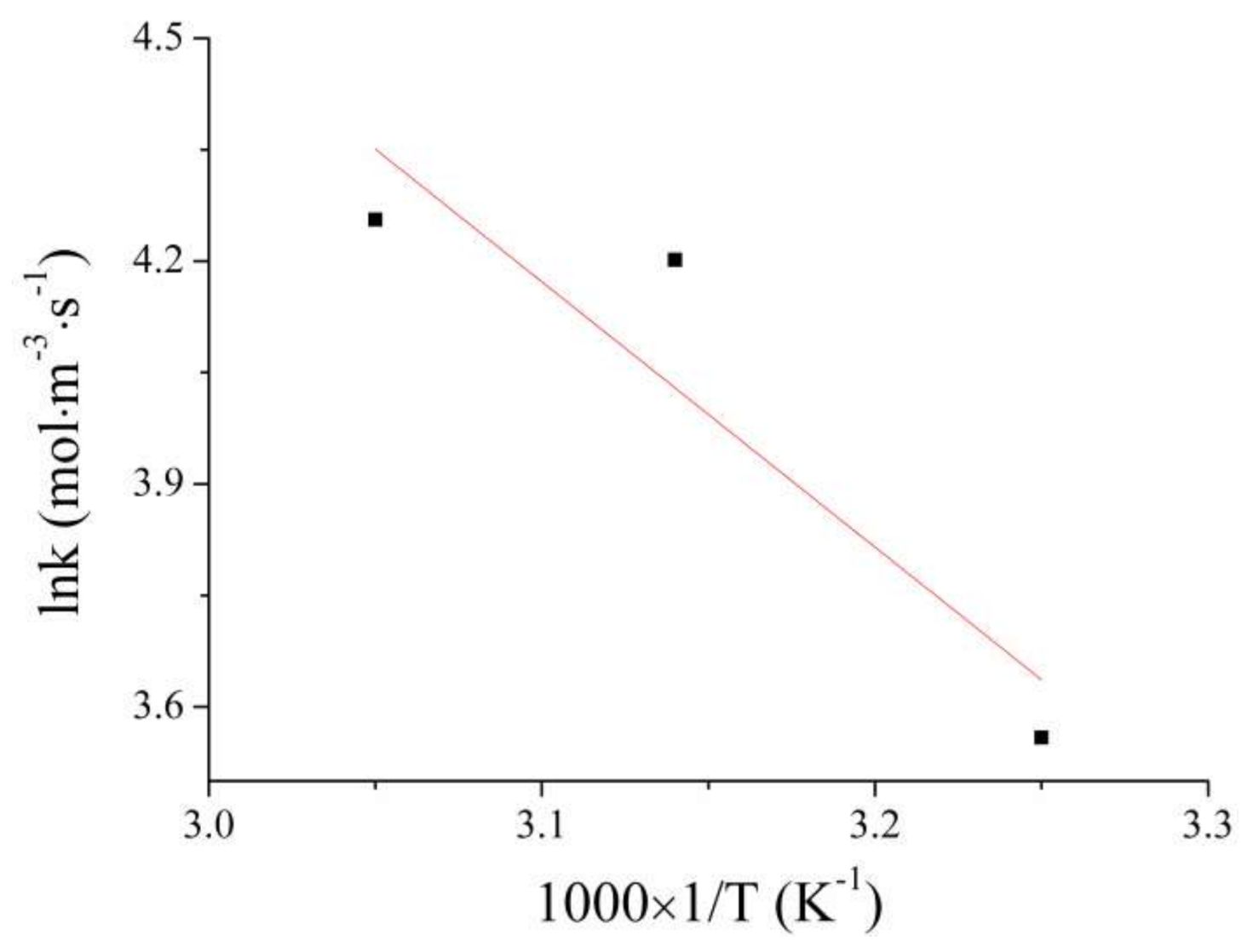
4.2. Activation Energy Calculation
5. Conclusions
- (1)
- According to the response surface analysis, it is not the case that the longer the time or the higher the temperature, the better the Cd removal effect. The Cd removal rate is higher when zinc powder is used more. However, the cost is higher when zinc powder is used more. Therefore, there is an optimal process condition for Cd removal production. The specific conditions are as follows: reaction temperature 55 °C, reaction time 13.43 min, and the zinc powder dosage should be 2.14 times that of Cd. The main effects of the three variables from large to small are the zinc powder dosage, reaction temperature and reaction time. The three variables of reaction temperature, reaction time and zinc powder dosage are regression-fitted with the Cd removal rate. The third-order regression equation is obtained. That is: η = 98.03 + 4.11T + 2.49t + 16.15N − 5.07Tt − 4.07TN − 2.22tN − 2.26T2 − 4.39t2 − 10.11N2 + 5.36T2t − 2.01T2N + 1.48TN2. The predicted value of the equation is close to the real experimental result, and so the fitting degree is high.
- (2)
- According to the kinetics analysis, the Cd removal reaction is a second-order reaction. That is, the reaction rate has a great relationship with the amount of reactants. Furthermore, the activation energy of this reaction is 29.6986 kJ/mol, so the reaction conforms to the diffusion control mechanism.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, X.; Wu, Y.F.; Gong, Y.; Zuo, T.Y. The lead-acid battery industry in China: Outlook for production and recycling. Waste Manag. Res. 2015, 33, 1–9. [Google Scholar] [CrossRef]
- Yu, H.J.; Zhang, T.Z.; Yuan, J.; Li, C.D. Trial study on EV battery recycling standardization development. Adv. Mater. Res. 2013, 610–613, 2170–2173. [Google Scholar] [CrossRef]
- Li, M.Y.; Yang, J.K.; Sha, L.G.; Hou, H.J.; Hua, J.P.; Liu, B.C.; Kumar, R.V. Review on clean recovery of discarded/spent lead-acid battery and trends of recycled products. J. Power Sources 2019, 436, 226853. [Google Scholar] [CrossRef]
- Li, L.L. Research and practice on the resources utilization of waste acid in waste lead acid battery. Sulphuric Acid Ind. China. 2019, 1, 33–36. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2019&filename=LSGY201901014&v=icvBmaR3U%25mmd2B%25mmd2FNuolbo70LHD2IdvyFWThVyWmvtXpHD9R3F%25mmd2Fl0YVWKGkyUGK9SnYP4 (accessed on 11 August 2021).
- Li, B.; Wei, Y.G.; Wang, H.; Yang, Y.D.; Yin, Y.G. Preparation of ZnSO4·7H2O and separation of zinc from blast furnace sludge by leaching-purification-crystallization method. ISIJ Int. 2019, 59, 201–207. [Google Scholar] [CrossRef]
- Palden, T.; Machiels, L.; Regadío, M.; Koen, B. Antimony recovery from lead-rich dross of lead smelter and conversion into antimony oxide chloride (Sb4O5Cl2). ACS Sustain. Chem. Eng. 2021, 9, 5074–5084. [Google Scholar] [CrossRef]
- She, X.F.; Wang, J.S.; Wang, G.; Xue, Q.G.; Zhang, X.X. Removal mechanism of Zn, Pb and alkalis from metallurgical dust in direct reduction process. J. Iron Steel Res. Int. 2014, 21, 488–495. [Google Scholar] [CrossRef]
- Feng, H.; Liu, M.; Zeng, W.; Chen, Y. Optimization of the O3/H2O2 process with response surface methodology for pretreatment of mother liquor of gas field wastewater. Front. Environ. Sci. Eng. 2021, 15, 78. [Google Scholar] [CrossRef]
- Tasca, A.L.; Mannarino, G.; Gori, R.; Vitolo, S.; Puccini, M. Phosphorus recovery from sewage sludge hydrochar: Process optimization by response surface methodology. Water Sci. Technol. 2021, 82, 2331–2343. [Google Scholar] [CrossRef] [PubMed]
- Nanda, V.; Singh, S.; Raina, C.S.; Jindal, N.; Singh, K.; Saxena, D.C. Optimization of the process variables for the preparation of processed paneer using response surface methodology. Eur. Food Res. Technol. 2004, 218, 529–534. [Google Scholar] [CrossRef]
- Mei, Z.Y.; Zhang, R.F.; Zhao, Z.M.; Zheng, G.D.; Xu, X.J.; Yang, D.P. Extraction process and method validation for bioactive compounds from citrus reticulata cv. chachiensis: Application of response surface methodology and hplc–dad. Acta Chromatogr. 2020, 33, 270–280. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Canas, S.; Taladrid, D.; Segovia, A.; Bartolome, B.; Aguilera, Y.; Martin-Cabrejas, M.A. Extraction of phenolic compounds from cocoa shell: Modeling using response surface methodology and artificial neural networks. Sep. Purif. Technol. 2021, 270, 118779. [Google Scholar] [CrossRef]
- Feki, F.; Klisurova, D.; Masmoudi, M.A.; Choura, S.; Denev, P.; Trendafilova, A.; Chamkha, M.; Sayadi, S. Optimization of microwave assisted extraction of simmondsins and polyphenols from jojoba (simmondsia chinensis) seed cake using box-behnken statistical design. Food Chem. 2021, 356, 129670. [Google Scholar] [CrossRef] [PubMed]
- Mgm, A.; Ht, B.; Jcd, B.; Jpkb, C.; Actvd, A. Understanding the chemistry of cation leaching in illite/water interfacial system using reactive molecular dynamics simulations and hydrothermal experiments. Acta Mater. 2020, 186, 564–574. [Google Scholar] [CrossRef]
- Adamou, A.; Nicolaides, A.; Varotsis, C. Bio-hydrometallurgy dynamics of copper sulfide-minerals probed by micro-ftir mapping and raman microspectroscopy. Miner. Eng. 2019, 132, 39–47. [Google Scholar] [CrossRef]
- Zekavat, M.; Yoozbashizadeh, H.; Khodaei, A. Leaching of antimony from stibnite ore in koh solution for sodium pyroantimonate production: Systematic optimization and kinetic study. Miner. Met. Mater. Soc. 2021, 73, 903–912. [Google Scholar] [CrossRef]
- Peng, H.; Shang, Q.; Chen, R.H.; Leng, Y.M.; Guo, J.; Liu, Z.H.; Tao, C.Y. Oxidative leaching kinetics of vanadium from the vanadium-chromium-reducing residue with K2Cr2O7. ACS OMEGA 2021, 5, 8777–8783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.P. Hydrometallurgy—Leaching Technology; Metallurgical Industry Press: Beijing, China, 2010; pp. 28–50. [Google Scholar]
- Wu, S.H.; Liu, C.Y.; Lan, Y.Z. Kinetic study on the precipitation of spongy bismuth by substitution of iron powder. Hydrometall. China 2007, 26, 139–141. [Google Scholar] [CrossRef]
- Jin, Y.H.; Wu, S.X. Kinetics of surface transpositional coating of copper on iron powder. Mater. Rev. China 2007, 21, 226–229. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2007&filename=CLDB2007S1069&v=vAUlZHPBoRhbFoJsrzzULpaJONrl05hJ%25mmd2FOJ1a3uCTd8Xe9XAWtUYiJ3MO03oEkDI (accessed on 11 August 2021).
- Li, S.L. Physical Chemistry; Higher Education Press: Beijing, China, 2017; pp. 551–569. [Google Scholar]




| Levels | Factors | ||
|---|---|---|---|
| T (°C) | t (min) | mZn:mCd | |
| −1 | 35 | 5 | 1:1 |
| 0 | 45 | 10 | 3:1 |
| 1 | 55 | 15 | 6:1 |
| Sequence Number | T (°C) | t (min) | N (mZn:mCd) | η/% |
|---|---|---|---|---|
| 1 | 35 | 5 | 3:1 | 72.85 |
| 2 | 55 | 5 | 3:1 | 94.19 |
| 3 | 35 | 15 | 3:1 | 98.71 |
| 4 | 55 | 15 | 3:1 | 99.76 |
| 5 | 35 | 10 | 1:1 | 63.35 |
| 6 | 55 | 10 | 1:1 | 79.71 |
| 7 | 35 | 10 | 6:1 | 99.75 |
| 8 | 55 | 10 | 6:1 | 99.85 |
| 9 | 45 | 5 | 1:1 | 62.67 |
| 10 | 45 | 15 | 1:1 | 72.10 |
| 11 | 45 | 5 | 6:1 | 99.40 |
| 12 | 45 | 15 | 6:1 | 99.95 |
| 13 | 45 | 10 | 3:1 | 97.17 |
| 14 | 45 | 10 | 3:1 | 98.08 |
| 15 | 45 | 10 | 3:1 | 98.19 |
| 16 | 45 | 10 | 3:1 | 98.42 |
| 17 | 45 | 10 | 3:1 | 98.30 |
| Sources of Variation | Quadratic Sum | Degree of Freedom | Mean Sum of Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Regression model | 3067.91 | 12 | 255.66 | 1030.18 | <0.0001 | Highly significant |
| A(T) | 67.73 | 1 | 67.73 | 272.93 | <0.0001 | Highly significant |
| B(t) | 24.90 | 1 | 24.90 | 100.33 | 0.0006 | Highly significant |
| C(N) | 1042.64 | 1 | 1042.64 | 4201.33 | <0.0001 | Highly significant |
| AB | 102.92 | 1 | 102.92 | 414.72 | <0.0001 | Highly significant |
| AC | 66.10 | 1 | 66.10 | 266.34 | <0.0001 | Highly significant |
| BC | 19.71 | 1 | 19.71 | 79.44 | 0.0009 | Highly significant |
| A2 | 24.97 | 1 | 24.97 | 100.62 | 0.0006 | Highly significant |
| B2 | 87.10 | 1 | 87.10 | 350.96 | <0.0001 | Highly significant |
| C2 | 711.48 | 1 | 711.48 | 2866.92 | <0.0001 | Highly significant |
| A2B | 47.43 | 1 | 47.43 | 191.14 | 0.0002 | Highly significant |
| A2C | 8.08 | 1 | 8.08 | 32.56 | 0.0047 | significant |
| AB2 | 0.88 | 1 | 0.88 | 3.54 | 0.1330 | Nonsignificant |
| Pure error | 0.99 | 4 | 0.25 | |||
| Total deviation | 3068.90 | 16 |
| Reaction Time (S) | mzn:mcd | |||
|---|---|---|---|---|
| 1:1 | 2:1 | 3:1 | 4:1 | |
| 5 | 9.1 | 8.6 | 8.0 | 6.8 |
| 10 | 6.95 | 5.7 | 5.8 | 2.7 |
| 20 | 6 | 3.4 | 3.1 | 1.7 |
| 60 | 2.4 | 1.5 | 1.5 | 0.19 |
| 300 | 0.3 | 0.014 | 0.04 | 0.005 |
| 900 | 0.007 | 0.009 | 0.002 | 0.002 |
| 1800 | 0.004 | 0.005 | 0.0005 | 0.0005 |
| mzn:mcd | Reaction Series | ||
|---|---|---|---|
| Zero-Order Reaction | First-Order Reaction | Second-Order Reaction | |
| 1:1 | 0.35 | 0.83 | 0.97 |
| 2:1 | 0.23 | 0.60 | 0.95 |
| 3:1 | 0.25 | 0.81 | 0.92 |
| 4:1 | 0.08 | 0.65 | 0.93 |
| Temperature | Reaction Time | ||||
|---|---|---|---|---|---|
| 20 s | 1 min | 5 min | 15 min | 30 min | |
| 35 | 4.2 | 1.6 | 0.03 | 0.002 | 0.001 |
| 45 | 3.1 | 1.5 | 0.04 | 0.002 | 0.0005 |
| 55 | 2.8 | 0.8 | 0.03 | 0.001 | 0.0005 |
| Temperature | K (mol·m−3·s−1) | ln K (mol·m−3·s−1) | T (K) | 1000 × 1/T (K−1) |
|---|---|---|---|---|
| 35 | 35.12 | 3.56 | 308.15 | 3.25 |
| 45 | 66.81 | 4.20 | 318.15 | 3.14 |
| 55 | 70.52 | 4.26 | 328.15 | 3.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Shu, X.; Li, H.; Deng, J.; Li, P.; Qin, S. Optimization of Process Parameters and Kinetics Analysis of Cd Removal in ZnSO4 Production. Processes 2021, 9, 1437. https://doi.org/10.3390/pr9081437
Ren X, Shu X, Li H, Deng J, Li P, Qin S. Optimization of Process Parameters and Kinetics Analysis of Cd Removal in ZnSO4 Production. Processes. 2021; 9(8):1437. https://doi.org/10.3390/pr9081437
Chicago/Turabian StyleRen, Xiaoling, Xinqian Shu, Haisheng Li, Jiushuai Deng, Peng Li, and Sixue Qin. 2021. "Optimization of Process Parameters and Kinetics Analysis of Cd Removal in ZnSO4 Production" Processes 9, no. 8: 1437. https://doi.org/10.3390/pr9081437







