Universal Model for the Cogeneration of Heat, Power and Char in a Decoupled Pyrolysis and Gasification Process for Techno-Economic Assessment
Abstract
:1. Introduction
- –
- evaluation and calculation of the theoretical process model of CHPC process based on elemental (C, H, O) and species (CO, CO2, H2, H2O, N2) composition at each process stage;
- –
- building typical-standard case using wet wood chips input;
- –
- upgrading and fulfilment of published process technical data;
- –
- developing a model of mass and heat balance adapted to support TEA entirely,
- –
- clarifying of mass and energy streams details presenting the potential for process improvement.
2. Materials and Methods
2.1. Materials
2.2. Wood Biomass
2.3. Char
2.4. Brief Overview of the Gasification Processes in the EU Market
2.5. CHPC Process Description
2.6. The CHPC Process Model and Simultaneous Mass and Heat Balance
2.7. Pyrolysis and Gasification Model Equations
2.8. Case Studies Description
3. Results and Discussion
3.1. Unit Mass at Input Case
3.2. TEA Relevant Cases
Cases of Plant Capacity Definition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| AC | Ash content in solid residue (%) |
| AR | As Received definition of samples |
| BuM | Business Model |
| CAPEX | Capital Expenditures |
| Cfix | Fixed (pure) carbon in the solid residue (%) |
| CH | Char |
| CHO | Hydrocarbons with CHO content |
| CHPC | Cogeneration of Heat Power and Char |
| Cp | Specific heat at constant pressure for Biomass |
| ∆H | Enthalpy of assigned stream (MJ kg−1) |
| ER | Equivalence Ratio; actual air-fuel ratio to the stoichiometric air-fuel ratio for combustion |
| GS | Gas storage reservoir |
| HE | Heat Exchanger |
| HEfg | Heat exchanger for flue gases cooling |
| HEge | Heat exchanger for electric generator cooling |
| HEmc | Heat exchanger for motor block (cylinders) cooling |
| HEoc | Heat exchanger for engine oil cooling |
| HEs | Heat exchanger for syngas cooling after char separation |
| HEd | Heat exchanger for heating drying air |
| ICE | Internal Combustion Engine |
| LHV | Lower Heating Value of assigned stream(MJ kg−1) |
| ṁ | Mass flow (kg h−1) |
| m | Mass of stream (kg) |
| MAF | Moisture and Ash Free definition of samples |
| MF | Moisture Free |
| OPEX | Operational Expenses |
| P | Power (kW) |
| P(A) | Probability function |
| PAH | Polyaromatic hydrocarbons |
| PERM | Permanent gases |
| PG | Pyrolysis Gases |
| Q | Energy (heat) (J) |
| SC | Scrubber for water |
| TEA | Techno Economic Assessment |
| W1 | Hot (90 °C) Water stream |
| W2 | Low temperature (90 °C) Water stream |
| W3 | Waste-water stream |
| W4 | Char cooling and moisturising water |
| WC | Water content of the material based on wet (AR-as received) material (%) |
| X | Water vapor concentration in gas (g/g) |
| θ | Celsius Temperature of the assigned stream or process inside the whole process (°C) |
| η | Efficiency (%) |
Subscripts
| airSP | Air Specific Parameter |
| BM | Biomass (wood chips) |
| BM+vh | Biomass and vapor heating |
| BMwe | Biomass water evaporating |
| CH | Char material (Cfix > 50%) |
| CHarh | Energy to heat char |
| CHdry | Char dry |
| CHsep | Char separation process |
| CHwet | Char wet |
| dA | Drying Air |
| Ddem | Gasification demand |
| dr | Drying |
| el | Electric |
| fg | Flue gasses |
| G | Gasification |
| g | Gas phase |
| Gair | Heating of air needed for gasification |
| Gburn | Burning in gasification process |
| gin | Gas at input |
| Glos | Energy Losses in Gasification process |
| gout | Gas at output |
| Gproc | Gasification process |
| in | Input, assignment of characteristics at input |
| out | Parameter of the output stream |
| p | Pyrolysis |
| Pair | Heating of air need for running pyrolysis |
| Pburn | Burning in Pyrolysis process |
| Pdem | Pyrolysis demand |
| PGheat | Energy needed to heat Pyrolysis Gases |
| Ploss | Energy Losses in pyrolysis process |
| Pproc | Pyrolysis process |
| proc | Process |
| py | Pyrolysis |
| ref | Reforming |
| syg | Syngas |
| th | Thermal |
| tot | Total |
| V | Volume (m3) |
| w | Water |
| we | Water evaporation |
| wh | Water heating |
| WV | Water vapor |
| wv | Water vapors |
| wvap | Water evaporation |
References
- Bórawski, P.; Bełdycka-Bórawska, A.; Szymańska, E.; Jankowski, K.J.; Dubis, B.; Dunn, J.W. Development of renewable energy sources market and biofuels in The European Union. J. Clean. Prod. 2019, 228, 467–484. [Google Scholar] [CrossRef]
- Sherwood, J. The significance of biomass in a circular economy. Bioresour. Technol. 2020, 300, 122755. [Google Scholar] [CrossRef] [PubMed]
- Böcker-Riese, B.; Bräkow, D. Status quo from Biomass gasification CHP-plant systems in Germany. In Proceedings of the Task33 Workshop. Small Scale Gasification for CHP, Innsbruck, Austria, 2–4 May 2017; FEE—Society for the Promotion of Renewable Energies: Berlin, Germany, 2017. [Google Scholar]
- Dahmen, N.; Sauer, J. Evaluation of Techno-Economic Studies on the bioliq® Process for Synthetic Fuels Production from Biomass. Processes 2021, 9, 684. [Google Scholar] [CrossRef]
- Klinar, D. Universal model of slow pyrolysis technology producing biochar and heat from standard biomass needed for the techno-economic assessment. Bioresour. Technol. 2016, 206, 112–120. [Google Scholar] [CrossRef] [PubMed]
- EBC. European Biochar Certificate—Guidelines for a Sustainable Production of Biochar; European Biochar Foundation (EBC): Arbaz, Switzerland, 2012; Version 8.3E of 1st September 2019; Available online: http://www.europeanbiochar.org/en/download (accessed on 14 December 2020). [CrossRef]
- Ciuta, S.; Tsiamis, D.; Castaldi, M.J. Gasification of Waste Materials: Technologies for Generating Energy, Gas, and Chemicals from Municipal Solid Waste, Biomass, Nonrecycled Plastics, Sludges, and Wet Solid Wastes; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128127162. [Google Scholar]
- Patuzzi, F.; Prando, D.; Vakalis, S.; Rizzo, A.M.; Chiaramonti, D.; Tirler, W.; Mimmo, T.; Gasparella, A.; Baratieri, M. Small-scale biomass gasification CHP systems: Comparative performance assessment and monitoring experiences in South Tyrol (Italy). Energy 2016, 112, 285–293. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 3): Gasification technologies. Bioresour. Technol. 2002, 83, 55–63. [Google Scholar] [CrossRef]
- Vakalis, S.; Patuzzi, F.; Baratieri, M. Thermodynamic modeling of small scale biomass gasifiers: Development and assessment of the ‘‘Multi-Box’’ approach. Bioresour. Technol. 2016, 206, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, H.; Rauch, R.; Bosch, K.; Koch, R.; Aichernig, C. Biomass CHP Plant Güssing—A Success Story. In Proceedings of the Expert Meeting on Pyrolysis and Gasification of Biomass and Waste, Strasbourg, France, 30 September–1 October 2002; Bridgewater, A.V., Ed.; CPL Press: Newbury, UK, 2003; pp. 527–536. [Google Scholar]
- Huber, M.; Huemer, M.; Hofmann, A.; Dumfort, S. Floating-fixed-bed-gasification: From Vision to Reality. Energy Procedia 2016, 93, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Damartzis, T.; Zabaniotou, A. Thermochemical conversion of biomass to second generation biofuels through integrated process design—A review. Renew. Sustain. Energy Rev. 2011, 15, 366–378. [Google Scholar] [CrossRef]
- Hansen, V.; Müller-Stöver, D.; Ahrenfeldt, J.; Holm, J.K.; Henriksen, U.B.; Hauggaard-Nielsen, H. Gasification biochar as a valuable by-product for carbon sequestration and soil amendment. Biomass Bioenergy 2015, 72, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Huber, M. Technical Specification CraftWERK CW1800-500; SynCraft Engineering GmbH: Schwaz, Austria, 2017. [Google Scholar]
- Crombie, K.; Mašek, O. Investigating the potential for a self-sustaining slow pyrolysis system under varying operating conditions. Bioresour. Technol. 2014, 162, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Higman, C.; van der Burgt, M. Gasification, 2nd ed.; Gulf Professional Publishing: Burlington, MA, USA, 2008; ISBN 9780750685283. [Google Scholar]
- Yu, J.; Smith, J.D. Validation and application of a kinetic model for biomass gasification simulation and optimization in updraft gasifiers. Chem. Eng. Process. Process. Intensif. 2018, 125, 214–226. [Google Scholar] [CrossRef]
- Narobe, M.; Golob, J.; Klinar, D.; Francetič, V.; Likozar, B. Co-gasification of biomass and plastics: Pyrolysis kinetics studies, experiments on 100 kW dual fluidized bed pilot plant and development of thermodynamic equilibrium model and balances. Bioresour. Technol. 2014, 162, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, V.; Patuzzi, F.; Baratieri, M. Characterization of char from biomass gasification and its similarities with activated carbon in adsorption applications. Appl. Energy 2018, 227, 92–99. [Google Scholar] [CrossRef]
- Dumfort, S.; Huemer, M.; Hofmann, A.; Dumfort, S.; Huemer, M.; Hofmann, A.; Huber, M.B.; Krueger, J. Tar Decomposition at low Temperatures within staged Gasification Reactors-first Approach towards Mechanisms and Background. Artic. J. Eng. Technol. 2015, 3, 45–49. [Google Scholar]

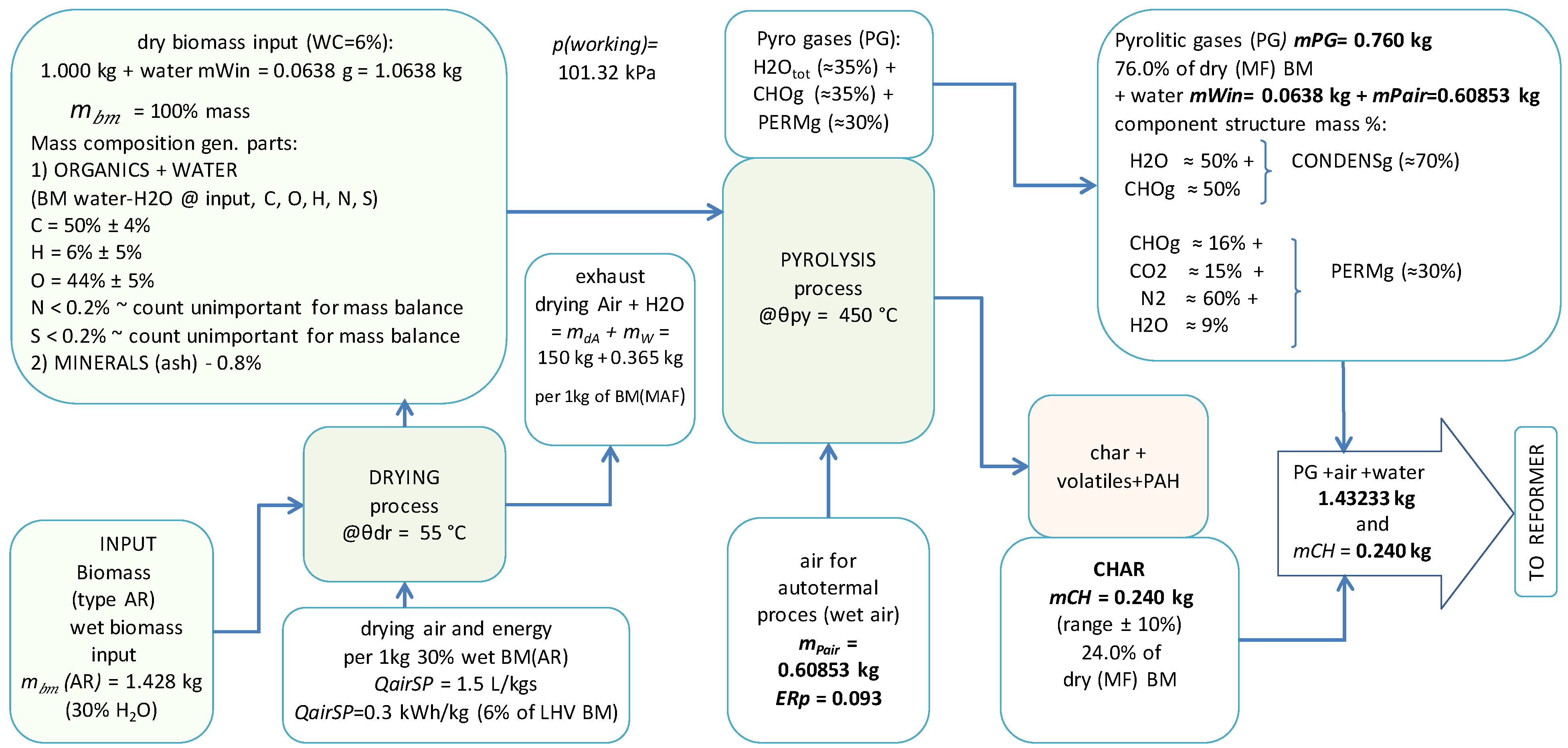
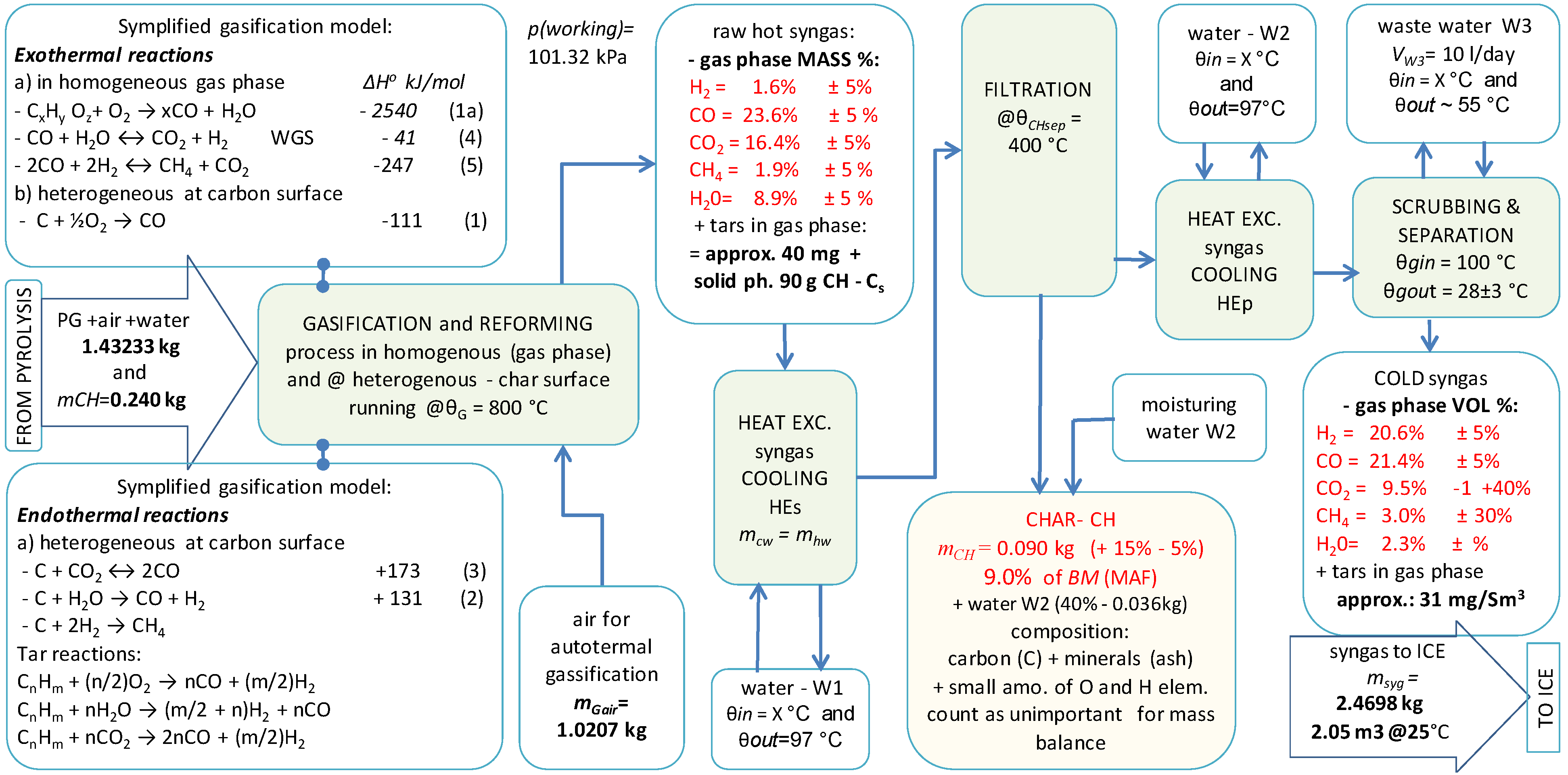
| Parameter Description | Notation | Units | Value |
|---|---|---|---|
| Biomass water content | WC | % | 6 |
| Biomass ash content | AC | % | 0.8 |
| Biomass lower heating value | LHVBM | MJ kg−1 | 18 |
| Process input temperature | θin | °C | 20 |
| Process heat up temperature difference | ∆Tpy | K | 480 |
| Pyrolysis temperature | θpy | °C | 500 |
| BM specific heat in temp. range up to θpy | CpBM | kJ kg−1 K−1 | 2.16 |
| Char specific heat in temp. range up to θpy | CpCH | kJ kg−1 K−1 | 1.67 |
| Enthalpy of water vaporisation | ∆Hwvap | MJ kg−1 | 2.260 |
| Enthalpy to heat water vapor to θpy | ∆Hwv | MJ kg−1 | 0.9696 |
| Char heating value | LHVCH | MJ kg−1 | 29 |
| Corrected—MAF BM lower heating value | MJ kg−1 | 16.2 |
| Mass Unit Results | Pyrolysis | Gasification | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Qin BM input | mWin BM water input | qBM+wh | qBMwe | qPair | qPlos | ∑ener. QPdem | qPGheat | qCHarh | qGair | qGlos | ∑ener. QGdem | qCH energy of char (CH) output | qdry energy to dry input BM | qwcfg energy of water cond. from flue gas | mCH char mass (CH) output | msyg syngasmass output |
| Units: MJ per kg input *) | 18.0 | 0.0638 (kg) | 1.0270 | 0.1443 | 0.2702 | 0.3960 | 1.837 | 1.3212 | 0.1242 | 0.7931 | 0.1800 | 2.419 | 2.520 | 11.006 | 1.2312 | 0.090 (kg) | 2.4698 (kg) |
| % | 100 | 6.0 | 5.71 | 0.80 | 1.50 | 2.20 | 10.21 | 7.34 | 0.69 | 4.41 | 1.00 | 13.44 | 14.00 | 6.11 | 6.84 | 9.0 w% | |
| given | given | calc. | calc. | calc. | sel. | calc. | calc. | calc. | calc. | sel. | calc. | calc. | calc. | calc. | calc. | calc. | |
| Mass Unit Results | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Qin BM input | qdrying | qcond-fg | qHEp | qHEs | qsc | Pin | Pth | Pel | Pth lowT | energy efficiency el. + highT | energy efficiency el.+ highT + lowT | energy losses total | waste- water W3 Vw3 | |
| Units: MJ per kg input *) | 18.0 | 1.1006 | 1.2312 | 1.038 | 1.1015 | 0.558 | Units: kW perkg input *) | 5.00 | 2.1944 | 1.425 | 0.7125 | ηe+h | ηtot | 1 − ηtot | 10 L/day |
| % | 100 | 6.11 | 6.84 | 5.8 | 6.12 | 3.10 | η (fa) | 100 | 43.9 | 28.50 | 14.3 | 72.4 | 86.6 | 13.4 | - |
| given | calc. | calc. | calc. | sel. | calc. | given | calc. | calc. | calc. | calc. | calc. | calc. | - | ||
| Cases: | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Units | (1) | (2) | (3) | (4) | Px/Pin | ṁx/ṁBM | Equation No.: |
| Ptot = Pin | kW | 1754 | 1368 | 1067 | 721 | 100% | / | 8/10 |
| Pel | kW | 500 | 400 | 300 | 200 | 28.5% | / | 12 |
| Pth | kW | 770 | 615 | 488 | 326 | 43.9% | / | 9 |
| ṁBM(MAF) | kg/h | 342 | 267 | 208 | 140 | / | 1 | 10 |
| ṁBM(MAF) | t/day | 8.21 | 6.41 | 5.00 | 3.40 | / | / | / |
| VBM(MAF) * | m3/day | 37.30 | 29.10 | 22.70 | 15.30 | / | / | / |
| Pth lowT | kW | 250 | 195 | 152 | 103 | 14.25% | / | / |
| ṁCHdry | kg/h | 30.8 | 24.3 | 18.7 | 12.6 | 12.5% | 9.0% | / |
| ṁCHdry | kg/day | 739 | 577 | 449 | 302 | / | / | |
| ṁCHwet | kg/h | 43.1 | 33.6 | 26.2 | 17.6 | / | 12.6% (40% water) | |
| VCHwet** | m3/h | 0.196 | 0.153 | 0.12 | 0.08 | / | / | / |
| VCHwet** | m3/day | 4.7 | 3.7 | 2.9 | 1.9 | / | / | / |
| Vwaste water | m3/day | 3.42 | 2.67 | 2.08 | 1.4 | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klinar, D.; Huber, M.; Belšak Šel, N.; Rižnar, K. Universal Model for the Cogeneration of Heat, Power and Char in a Decoupled Pyrolysis and Gasification Process for Techno-Economic Assessment. Processes 2021, 9, 1442. https://doi.org/10.3390/pr9081442
Klinar D, Huber M, Belšak Šel N, Rižnar K. Universal Model for the Cogeneration of Heat, Power and Char in a Decoupled Pyrolysis and Gasification Process for Techno-Economic Assessment. Processes. 2021; 9(8):1442. https://doi.org/10.3390/pr9081442
Chicago/Turabian StyleKlinar, Dušan, Marcel Huber, Nataša Belšak Šel, and Klavdija Rižnar. 2021. "Universal Model for the Cogeneration of Heat, Power and Char in a Decoupled Pyrolysis and Gasification Process for Techno-Economic Assessment" Processes 9, no. 8: 1442. https://doi.org/10.3390/pr9081442






