Influence of Leachate and Nitrifying Bacteria on Photosynthetic Biogas Upgrading in a Two-Stage System
Abstract
:1. Introduction
2. Materials and Methods
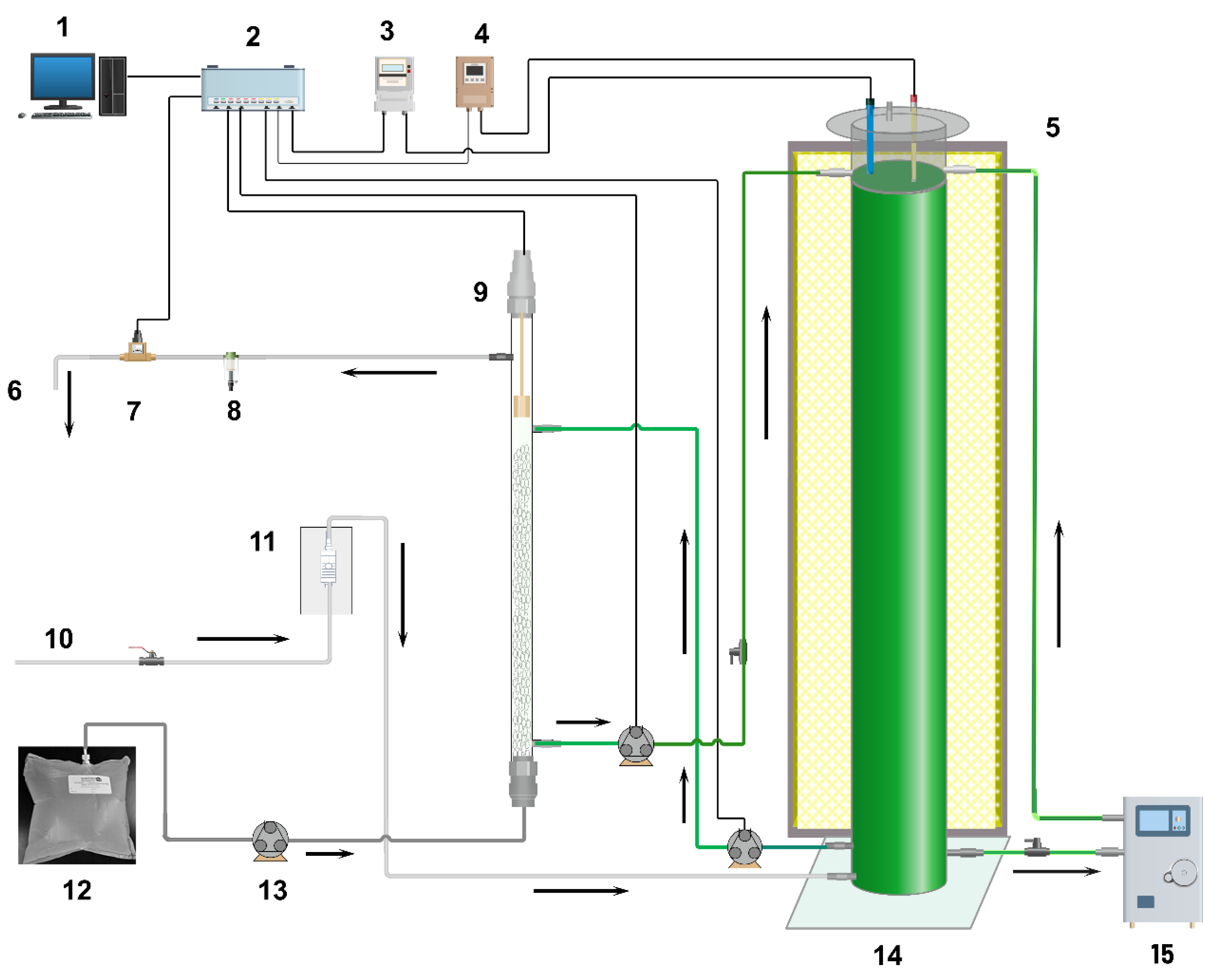
2.1. Experimental Set-Up
2.2. Experimental Conditions
- Inoculation of the photobioreactor: 2 L of nitrifying bacterial culture were added directly to the photobioreactor. To avoid light inhibition of the nitrifying bacteria, the lower third of the bubble column was covered.
- Inoculation of the absorption column: the absorption column recirculated the nitrifying culture for 15 days, thus allowing biofilm formation on the Rasching rings.
2.3. Analytical Methods
2.4. Fitting to Empirical Model
3. Results and Discussion
3.1. Optimization of the Two-Stage System
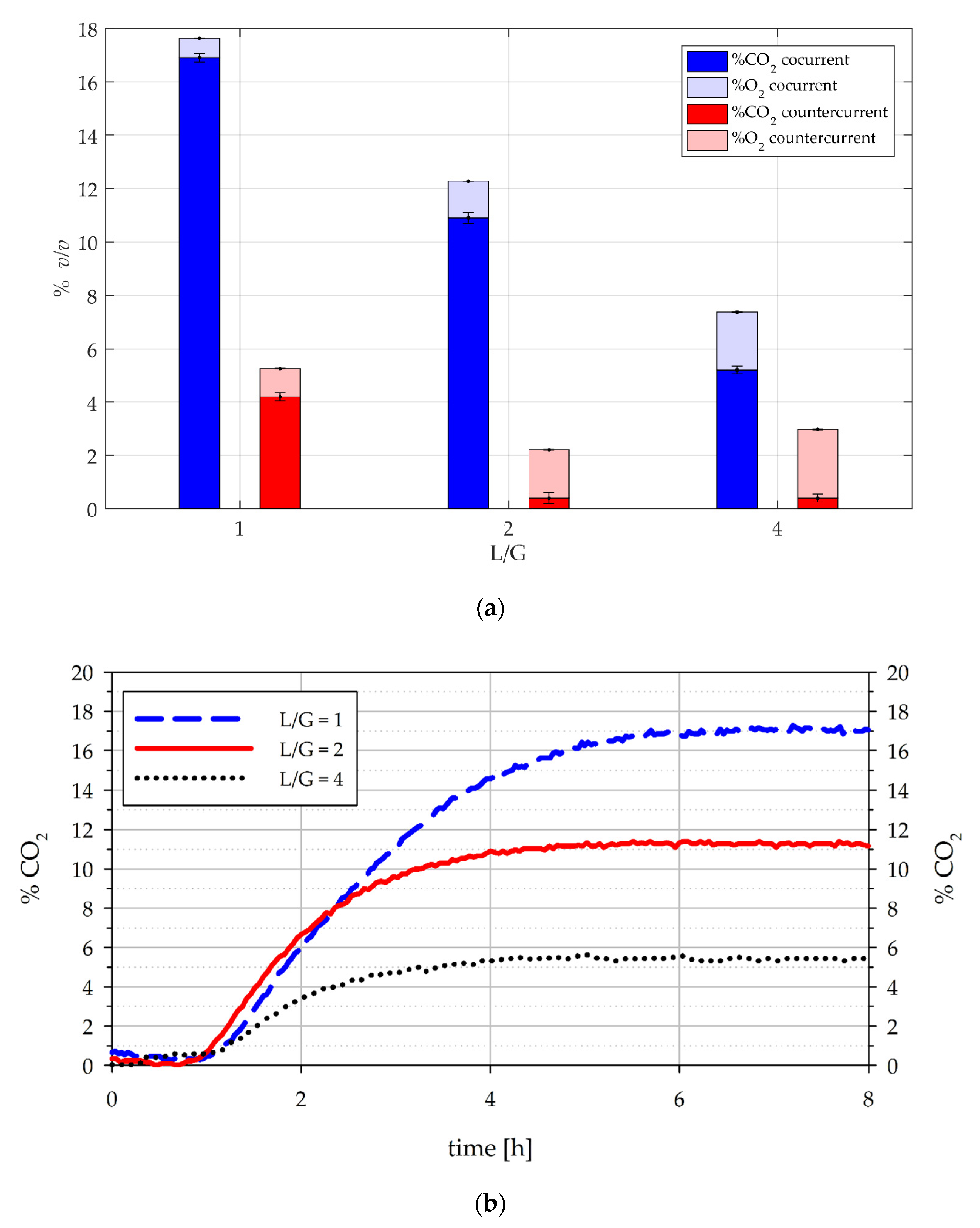
3.1.1. Effect of Flow Mode in the Absorption Column
3.1.2. Influence of L/G and Inlet Concentration
3.1.3. Empirical Model
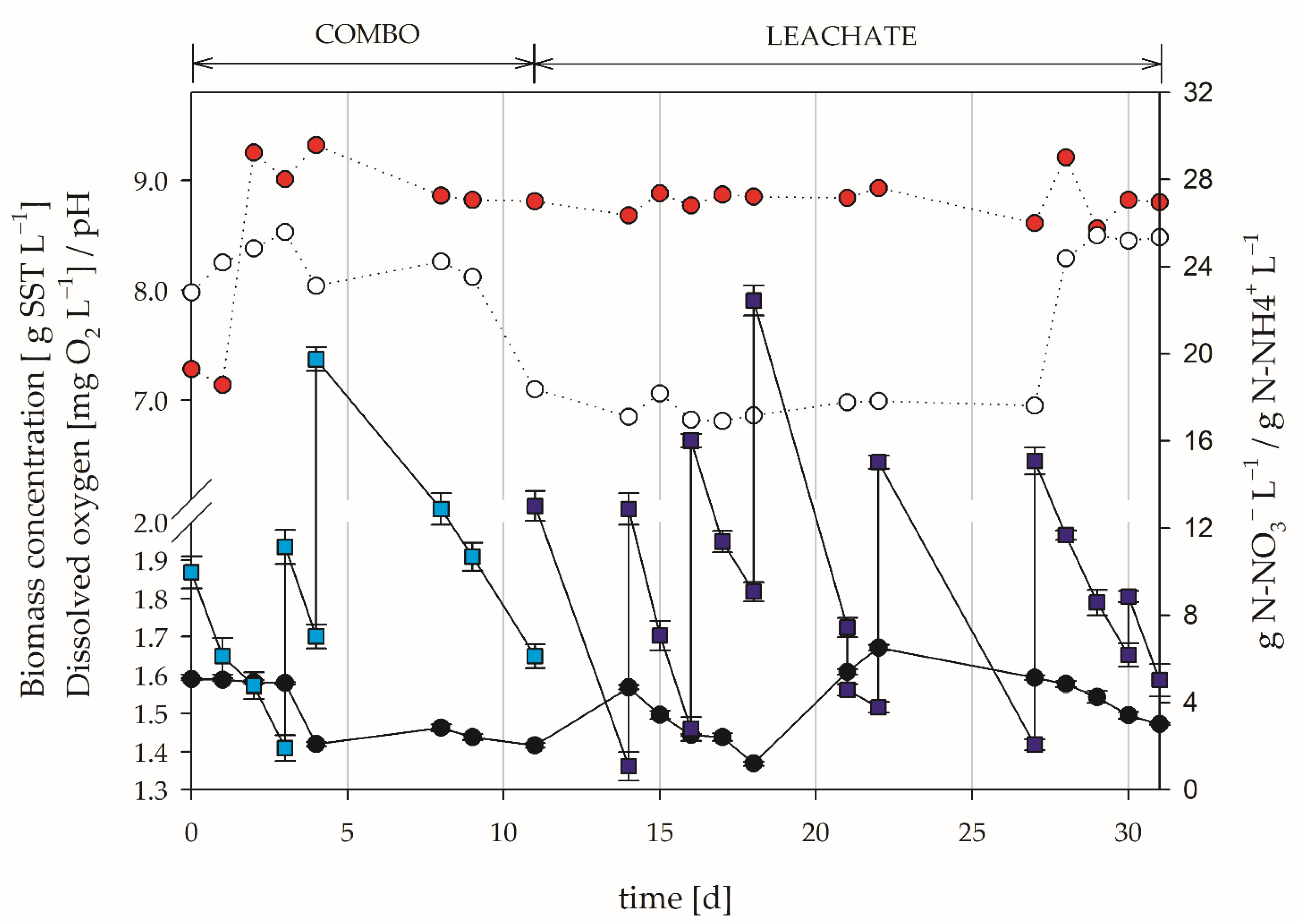
3.2. Use of Leachate as Culture Medium
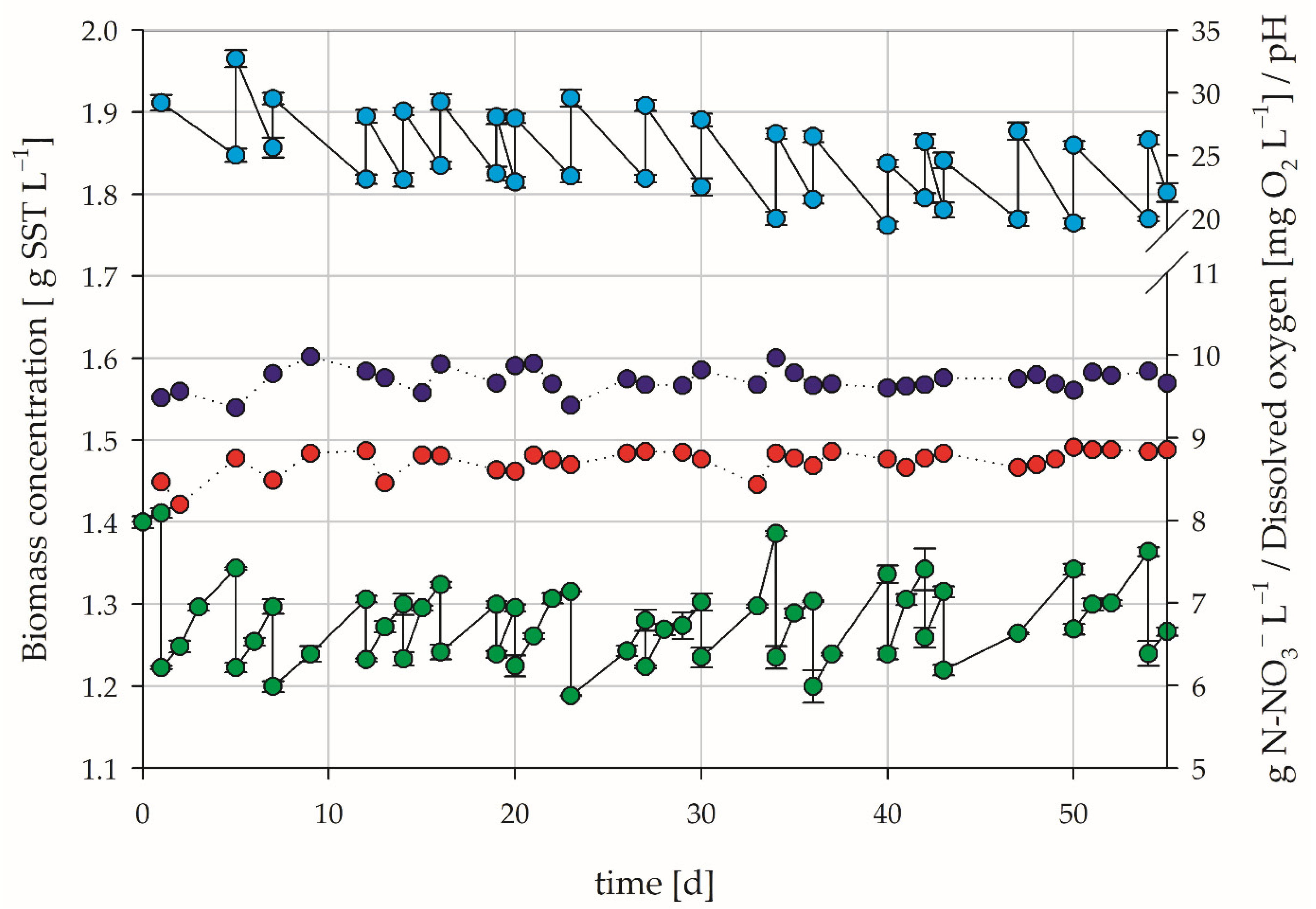
3.3. Inoculation with Nitrifying Bacteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee, the Committee of the Regions and the European Investment Bank a Clean Planet for All a European Strategic Long-Term. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52018DC0773 (accessed on 16 November 2020).
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions a Roadmap for Moving to a Competitive Low Carbon Economy in 2050. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:52011DC0112 (accessed on 21 June 2021).
- Sun, Q.; Li, H.; Yan, J.; Liu, L.; Yu, Z.; Yu, X. Selection of appropriate biogas upgrading technology-a review of biogas cleaning, upgrading and utilisation. Renew. Sustain. Energy Rev. 2015, 51, 521–532. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Vilches, L.F.; Navarrete, B. Review: Recent advances in biogas purifying technologies. Int. J. Green Energy 2019, 16, 401–412. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhao, Y.; Sun, S.; Hu, C.; Ping, L. Effect of light intensity on the capability of different microalgae species for simultaneous biogas upgrading and biogas slurry nutrient reduction. Int. Biodeterior. Biodegrad. 2015, 104, 157–163. [Google Scholar] [CrossRef]
- Srinuanpan, S.; Cheirsilp, B.; Prasertsan, P. Effective biogas upgrading and production of biodiesel feedstocks by strategic cultivation of oleaginous microalgae. Energy 2018, 148, 766–774. [Google Scholar] [CrossRef]
- Bahr, M.; Díaz, I.; Dominguez, A.; González Sánchez, A.; Muñoz, R. Microalgal-Biotechnology as a platform for an integral biogas upgrading and nutrient removal from anaerobic effluents. Environ. Sci. Technol. 2014, 48, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Cervantes, A.; Madrid-Chirinos, C.; Cantera, S.; Lebrero, R.; Muñoz, R. Influence of the gas-liquid flow configuration in the absorption column on photosynthetic biogas upgrading in algal-bacterial photobioreactors. Bioresour. Technol. 2017, 225, 336–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo-Cervantes, A.; Morales, T.; González, Á.; Muñoz, R.; Lebrero, R. Long-term photosynthetic CO2 removal from biogas and flue-gas: Exploring the potential of closed photobioreactors for high-value biomass production. Sci. Total Environ. 2018, 640–641, 1272–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, L.; Pérez, R.; Azócar, L.; Rivas, M.; Jeison, D. Photosynthetic CO2 uptake by microalgae: An attractive tool for biogas upgrading. Biomass Bioenergy 2015, 73, 102–109. [Google Scholar] [CrossRef]
- Prandini, J.M.; da Silva, M.L.B.; Mezzari, M.P.; Pirolli, M.; Michelon, W.; Soares, H.M. Enhancement of nutrient removal from swine wastewater digestate coupled to biogas purification by microalgae Scenedesmus spp. Bioresour. Technol. 2016, 202, 67–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilanovic, D.; Holland, M.; Starosvetsky, J.; Armon, R. Co-cultivation of microalgae and nitrifiers for higher biomass production and better carbon capture. Bioresour. Technol. 2016, 220, 282–288. [Google Scholar] [CrossRef]
- Mairet, F.; Ramírez, C.H.; Rojas-Palma, A. Modeling and stability analysis of a microalgal pond with nitrification. Appl. Math. Model. 2017, 51, 448–468. [Google Scholar] [CrossRef]
- Rada-Ariza, A.M.; Lopez-Vazquez, C.M.; van der Steen, N.P.; Lens, P.N.L. Nitrification by microalgal-bacterial consortia for ammonium removal in flat panel sequencing batch photo-bioreactors. Bioresour. Technol. 2017, 245, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Das, A.; Yu, C.-P.; Hu, Z. Nitrifying bacterial growth inhibition in the presence of algae and cyanobacteria. Biotechnol. Bioeng. 2010, 107, 1004–1011. [Google Scholar] [CrossRef]
- Noorain, R.; Kindaichi, T.; Ozaki, N.; Aoi, Y.; Ohashi, A. Biogas purification performance of new water scrubber packed with sponge carriers. J. Clean. Prod. 2019, 214, 103–111. [Google Scholar] [CrossRef]
- Läntelä, J.; Rasi, S.; Lehtinen, J.; Rintala, J. Landfill gas upgrading with pilot-scale water scrubber: Performance assessment with absorption water recycling. Appl. Energy 2012, 92, 307–314. [Google Scholar] [CrossRef]
- Tan, L.S.; Shariff, A.M.; Lau, K.K.; Bustam, M.A. Factors affecting CO2 absorption efficiency in packed column: A review. J. Ind. Eng. Chem. 2012, 18, 1874–1883. [Google Scholar] [CrossRef]
- Sunda, W.G.; Price, N.M.; Morel, F.M.M. Trace metal ion buffers and their use in culture studies. In Algal Culturing Techniques; Elsevier: Amsterdam, The Netherlands, 2005; pp. 35–63. [Google Scholar]
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aleman-Nava, G.S.; Chandra, R.; Garcia-Perez, J.S.; Contreras-Angulo, J.R.; Markou, G.; Muylaert, K.; Rittmann, B.E.; Parra-Saldivar, R. Nutrients utilization and contaminants removal. A review of two approaches of algae and cyanobacteria in wastewater. Algal Res. 2017, 24, 438–449. [Google Scholar] [CrossRef]
- Park, K.C.; Whitney, C.G.E.; Kozera, C.; O’Leary, S.J.B.; McGinn, P.J. Seasonal isolation of microalgae from municipal wastewater for remediation and biofuel applications. J. Appl. Microbiol. 2015, 119, 76–87. [Google Scholar] [CrossRef]
- Khanzada, Z.T.; Övez, S. Microalgae as a sustainable biological system for improving leachate quality. Energy 2017, 140, 757–765. [Google Scholar] [CrossRef]
- Nordin, N.; Yusof, N.; Samsudin, S. Biomass Production of Chlorella sp., Scenedesmus sp., and Oscillatoria sp. in nitrified landfill leachate. Waste Biomass Valorization 2017, 8, 2301–2311. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Huang, S.; Qiu, D.; Schideman, L.; Chai, X.; Zhao, Y. Characterization of microalgae-bacteria consortium cultured in landfill leachate for carbon fixation and lipid production. Bioresour. Technol. 2014, 156, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Kilham, S.S.; Kreeger, D.A.; Lynn, S.G.; Goulden, C.E.; Herrera, L. COMBO: A defined freshwater culture medium for algae and zooplankton. Hydrobiologia 1998, 377, 147–159. [Google Scholar] [CrossRef]
- Saldarriaga, L.F.; Almenglo, F.; Ramírez, M.; Cantero, D. Kinetic characterization and modeling of a microalgae consortium isolated from landfill leachate under a high CO2 concentration in a bubble column photobioreactor. Electron. J. Biotechnol. 2020, 44, 47–57. [Google Scholar] [CrossRef]
- González-Cortés, J.J.; Almenglo, F.; Ramírez, M.; Cantero, D. Simultaneous removal of ammonium from landfill leachate and hydrogen sulfide from biogas using a novel two-stage oxic-anoxic system. Sci. Total Environ. 2021, 750, 141664. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Chiu, S.-Y.; Kao, C.-Y.; Tsai, M.-T.; Ong, S.-C.; Chen, C.-H.; Lin, C.-S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Arbib, Z.; Álvarez-Díaz, P.D.; Garrido-Pérez, C.; Barragán, J.; Perales, J.A. Photobiotreatment model (PhBT): A kinetic model for microalgae biomass growth and nutrient removal in wastewater. Environ. Technol. 2013, 34, 979–991. [Google Scholar] [CrossRef]
- Serejo, M.L.; Posadas, E.; Boncz, M.A.; Blanco, S.; García-Encina, P.; Muñoz, R. Influence of biogas flow rate on biomass composition during the optimization of biogas upgrading in microalgal-bacterial processes. Environ. Sci. Technol. 2015, 49, 3228–3236. [Google Scholar] [CrossRef]
- Toledo-Cervantes, A.; Serejo, M.L.; Blanco, S.; Pérez, R.; Lebrero, R.; Muñoz, R. Photosynthetic biogas upgrading to bio-methane: Boosting nutrient recovery via biomass productivity control. Algal Res. 2016, 17, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Franco-Morgado, M.; Alcántara, C.; Noyola, A.; Muñoz, R.; González-Sánchez, A. A study of photosynthetic biogas upgrading based on a high rate algal pond under alkaline conditions: Influence of the illumination regime. Sci. Total Environ. 2017, 592, 419–425. [Google Scholar] [CrossRef] [Green Version]
- del Rosario Rodero, M.; Lebrero, R.; Serrano, E.; Lara, E.; Arbib, Z.; García-Encina, P.A.; Muñoz, R. Technology validation of photosynthetic biogas upgrading in a semi-industrial scale algal-bacterial photobioreactor. Bioresour. Technol. 2019, 279, 43–49. [Google Scholar] [CrossRef]
- Marín, D.; Carmona-Martínez, A.A.; Blanco, S.; Lebrero, R.; Muñoz, R. Innovative operational strategies in photosynthetic biogas upgrading in an outdoors pilot scale algal-bacterial photobioreactor. Chemosphere 2021, 264, 128470. [Google Scholar] [CrossRef] [PubMed]
- Marín, D.; Ortíz, A.; Díez-Montero, R.; Uggetti, E.; García, J.; Lebrero, R.; Muñoz, R. Influence of liquid-to-biogas ratio and alkalinity on the biogas upgrading performance in a demo scale algal-bacterial photobioreactor. Bioresour. Technol. 2019, 280, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.D.; Dlugogorski, B.Z.; Kennedy, E.M. Elementary reaction step model of the N-nitrosation of ammonia. Int. J. Chem. Kinet. 2007, 39, 645–656. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; De-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Value | Unit |
|---|---|---|
| pH | 7.86 ± 0.01 | - |
| Conductivity | 41.4 ± 0.46 | mS cm−1 |
| Chemical oxygen demand (COD) | 8991 ± 227 | mg O2 L−1 |
| Alkalinity | 17,977 ± 244 | mg CaCO3 L−1 |
| Total suspended solids (TSS) | 17,418 ± 137 | mg L−1 |
| Total volatile solids (TVS) | 6297 ± 61 | mg L−1 |
| Total phosphorous | 82.77 ± 0.77 | mg L−1 |
| P-PO43− | 43.99 ± 1.20 | mg L−1 |
| Total nitrogen | 4613 ± 93 | mg L−1 |
| N-NH4+ | 3785 ± 174 | mg L−1 |
| N-NO3− | n.d. | mg L−1 |
| N-NO2− | n.d. | mg L−1 |
| S-SO42− | 92.72 ± 0.57 | mg L−1 |
| Cl- | 5939 ± 172 | mg L−1 |
| Br- | 24.01 ± 2.83 | mg L−1 |
| Na | 3920 ± 12 | mg L−1 |
| K | 1957 ± 22 | mg L−1 |
| Ca | 42.1 ± 0.6 | mg L−1 |
| Mg | 49.0 ± 1.3 | mg L−1 |
| Si | <40 * | mg L−1 |
| Sr | 3.34 ± 0.10 | mg L−1 |
| V | <0.200 * | mg L−1 |
| Mn | 0.160 ± 0.010 | mg L−1 |
| Fe | 8.10 ± 0.10 | mg L−1 |
| Co | 0.075 ± 0.002 | mg L−1 |
| Cu | 0.102 ± 0.001 | mg L−1 |
| Zn | 0.970 ± 0.170 | mg L−1 |
| Se | <0.240 * | mg L−1 |
| Hg | <0.030 * | mg L−1 |
| Pb | 0.020 ± 0.002 | mg L−1 |
| Experimental Conditions | Nutrient Solution | Flow Mode | L/G | Inlet CO2 Concentration |
|---|---|---|---|---|
| 1 | COMBO | co-current or counter-current | 1, 2, 4 | 40% |
| 2 | COMBO | counter-current | 1, 1.5, 2, 4 | 20%, 40% |
| 3 | Leachate | counter-current | 1.5 | 20%, 40% |
| 4 | Leachate | counter-current | 1.5 | 20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saldarriaga, L.F.; Almenglo, F.; Cantero, D.; Ramírez, M. Influence of Leachate and Nitrifying Bacteria on Photosynthetic Biogas Upgrading in a Two-Stage System. Processes 2021, 9, 1503. https://doi.org/10.3390/pr9091503
Saldarriaga LF, Almenglo F, Cantero D, Ramírez M. Influence of Leachate and Nitrifying Bacteria on Photosynthetic Biogas Upgrading in a Two-Stage System. Processes. 2021; 9(9):1503. https://doi.org/10.3390/pr9091503
Chicago/Turabian StyleSaldarriaga, Luis Fernando, Fernando Almenglo, Domingo Cantero, and Martín Ramírez. 2021. "Influence of Leachate and Nitrifying Bacteria on Photosynthetic Biogas Upgrading in a Two-Stage System" Processes 9, no. 9: 1503. https://doi.org/10.3390/pr9091503








