Electrochemical Study of Stainless Steel Anchor Bolt Corrosion Initiation in Corrosive Underground Water
Abstract
:1. Introduction
2. Materials and Experiments
2.1. Materials
2.2. Experiments
3. Results and Discussion
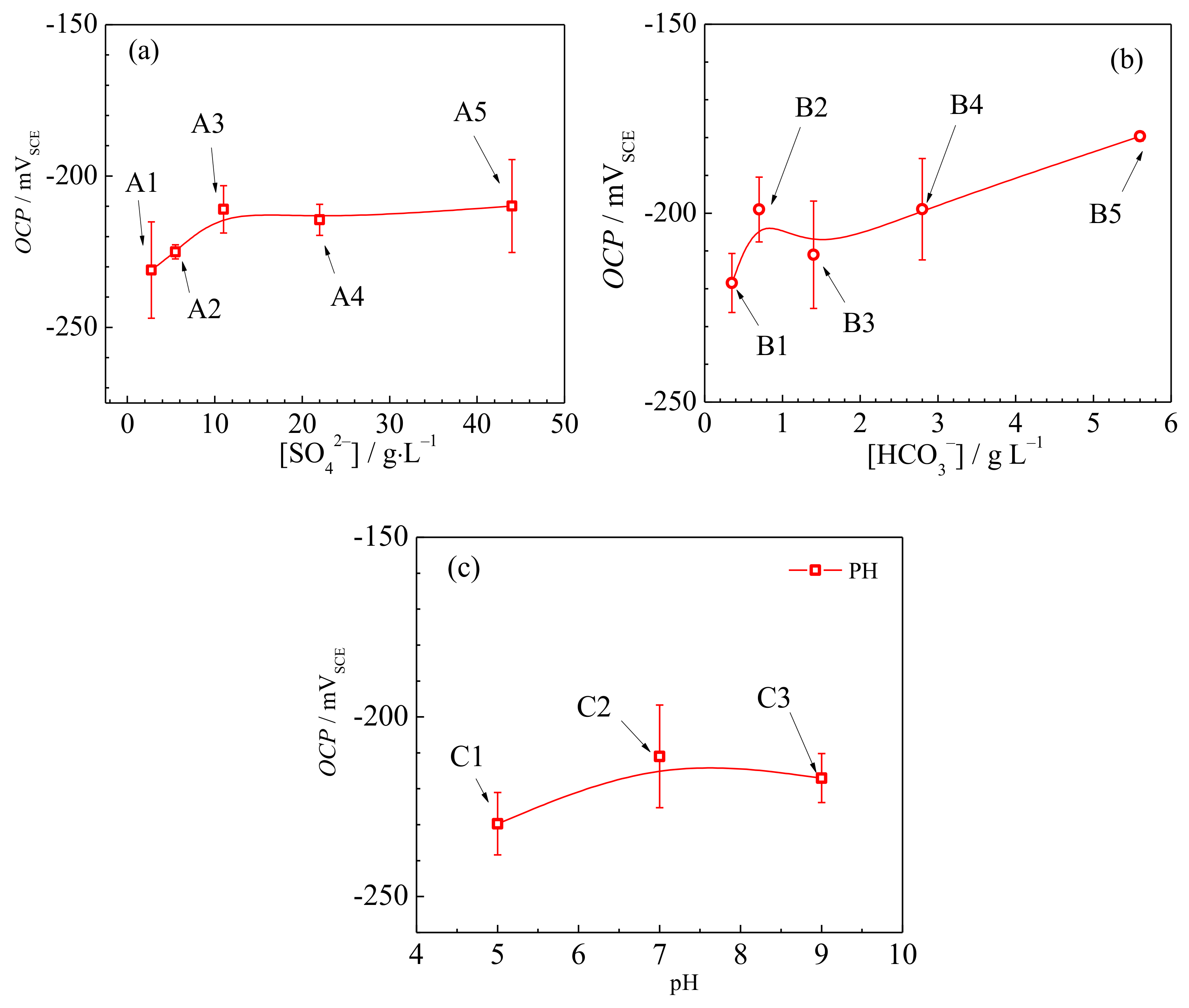
3.1. Open-Circuit Potential
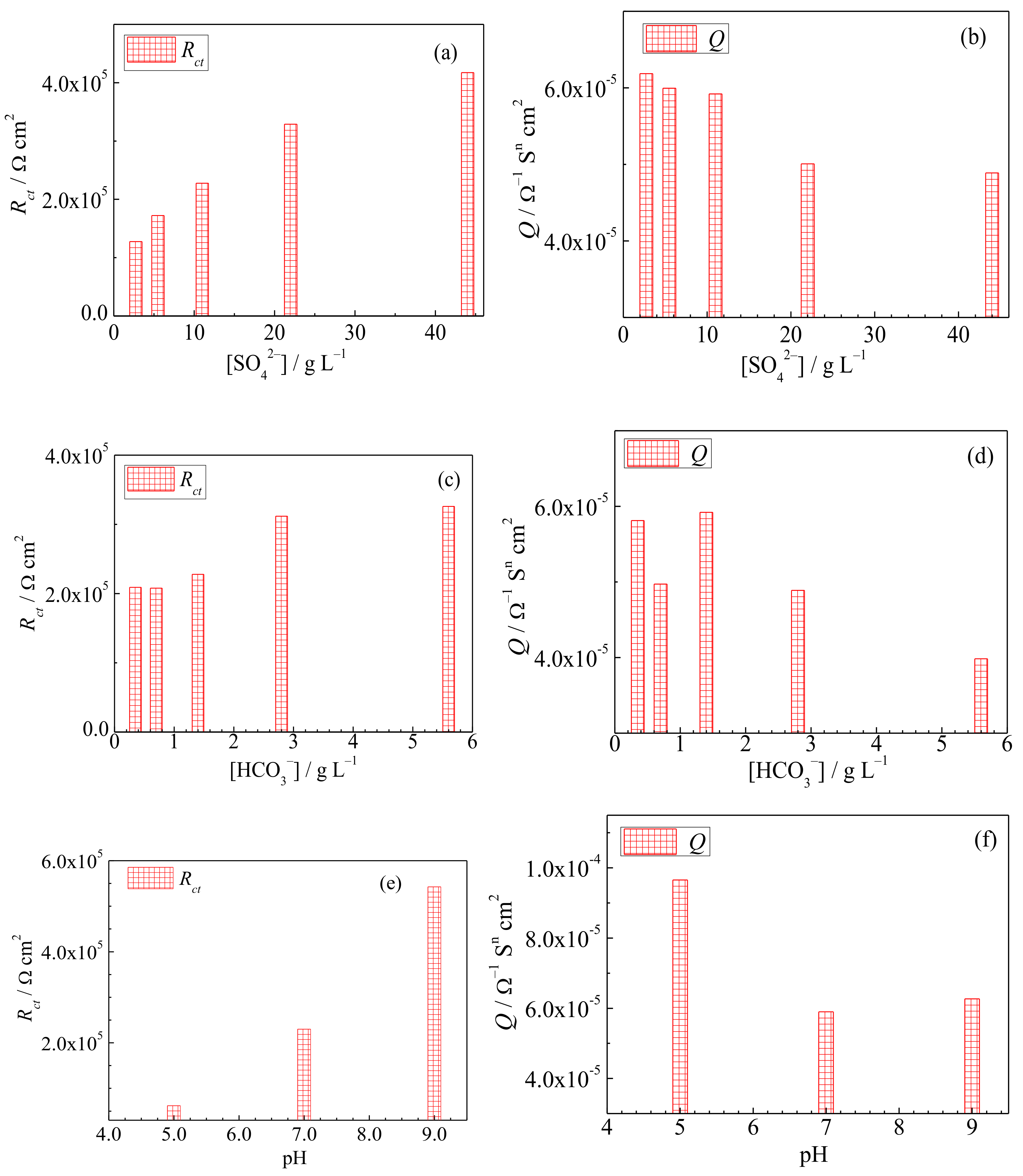
3.2. Electrochemical Impedance Spectroscopy
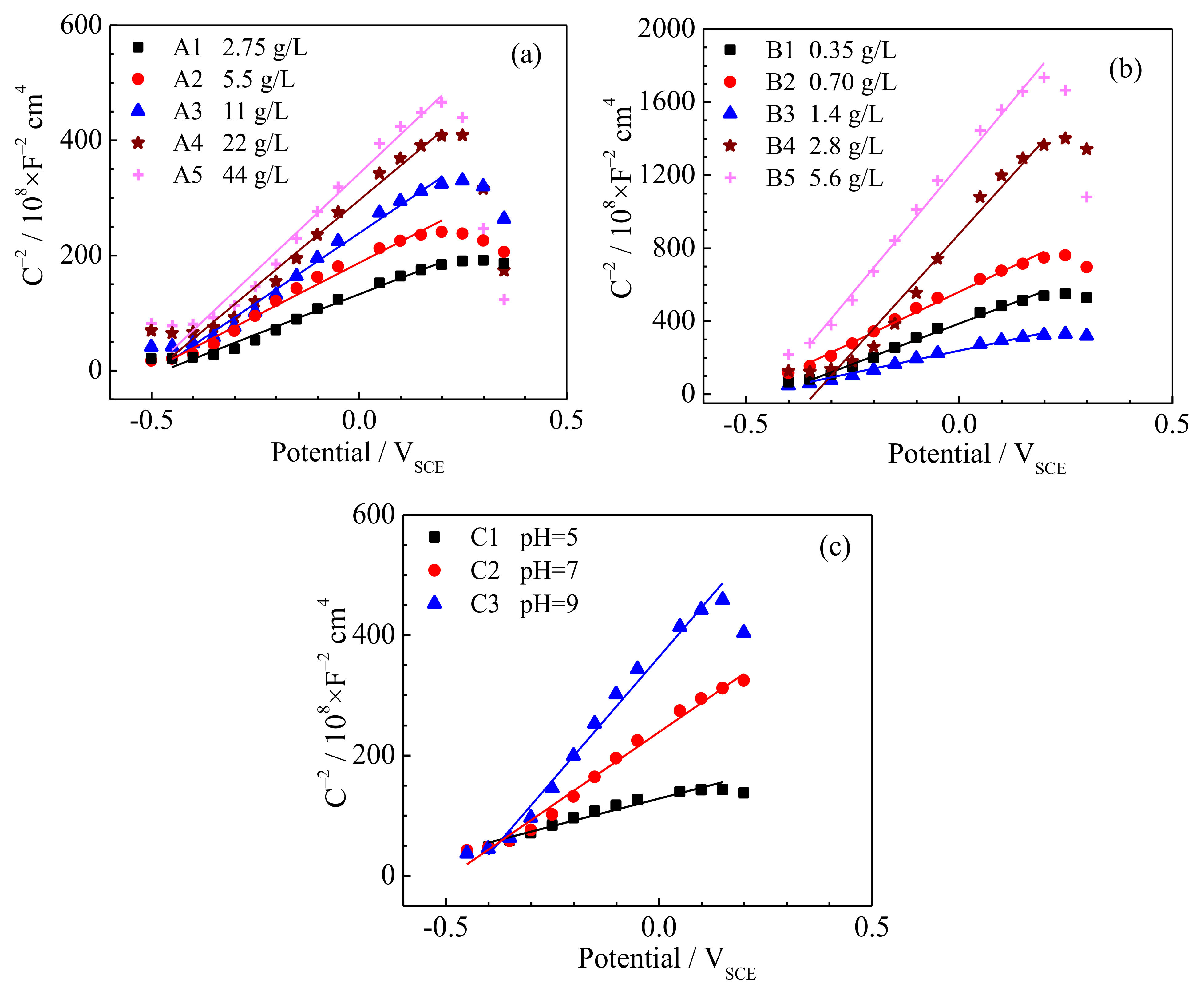
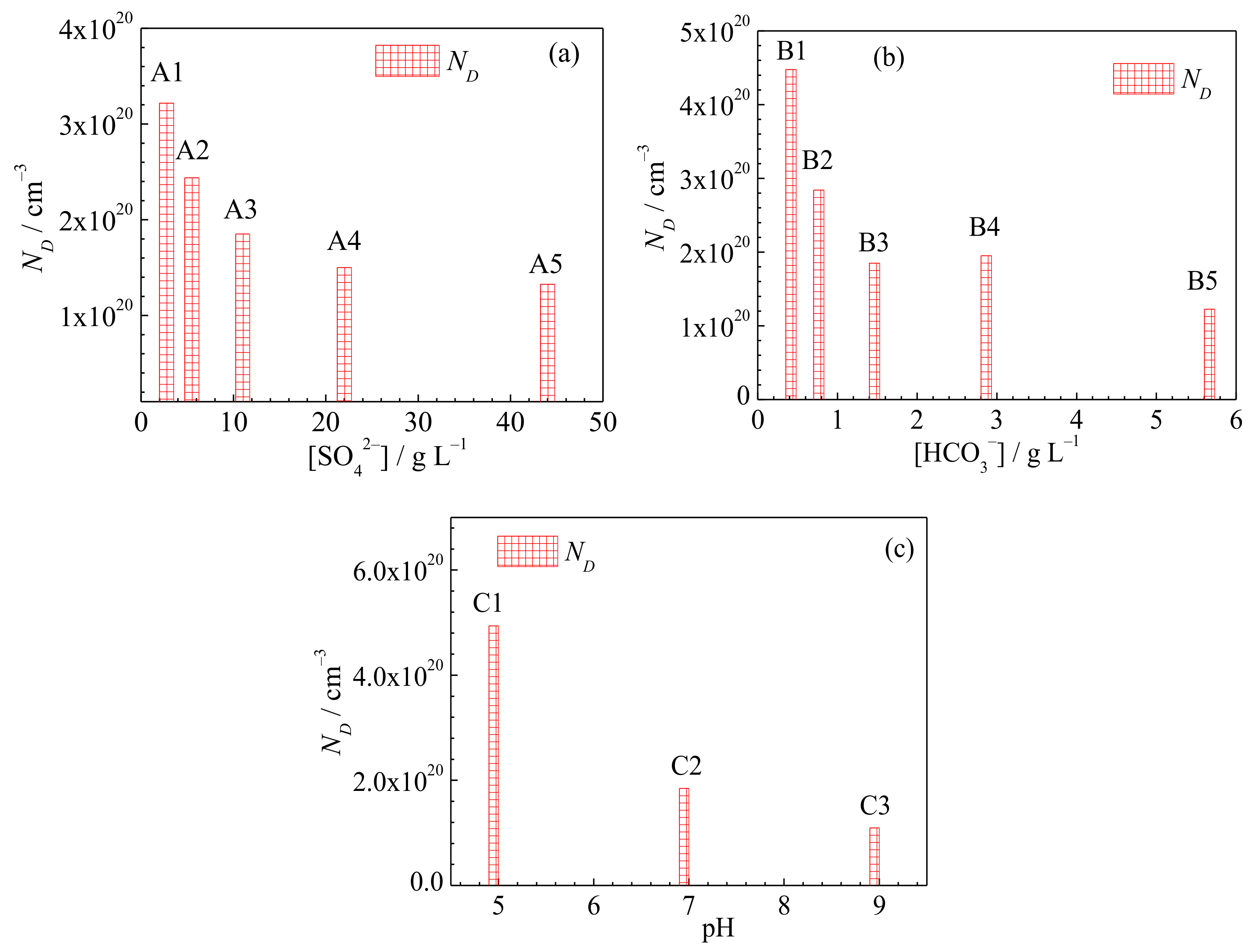
3.3. Semiconducting Properties of the Passive Film
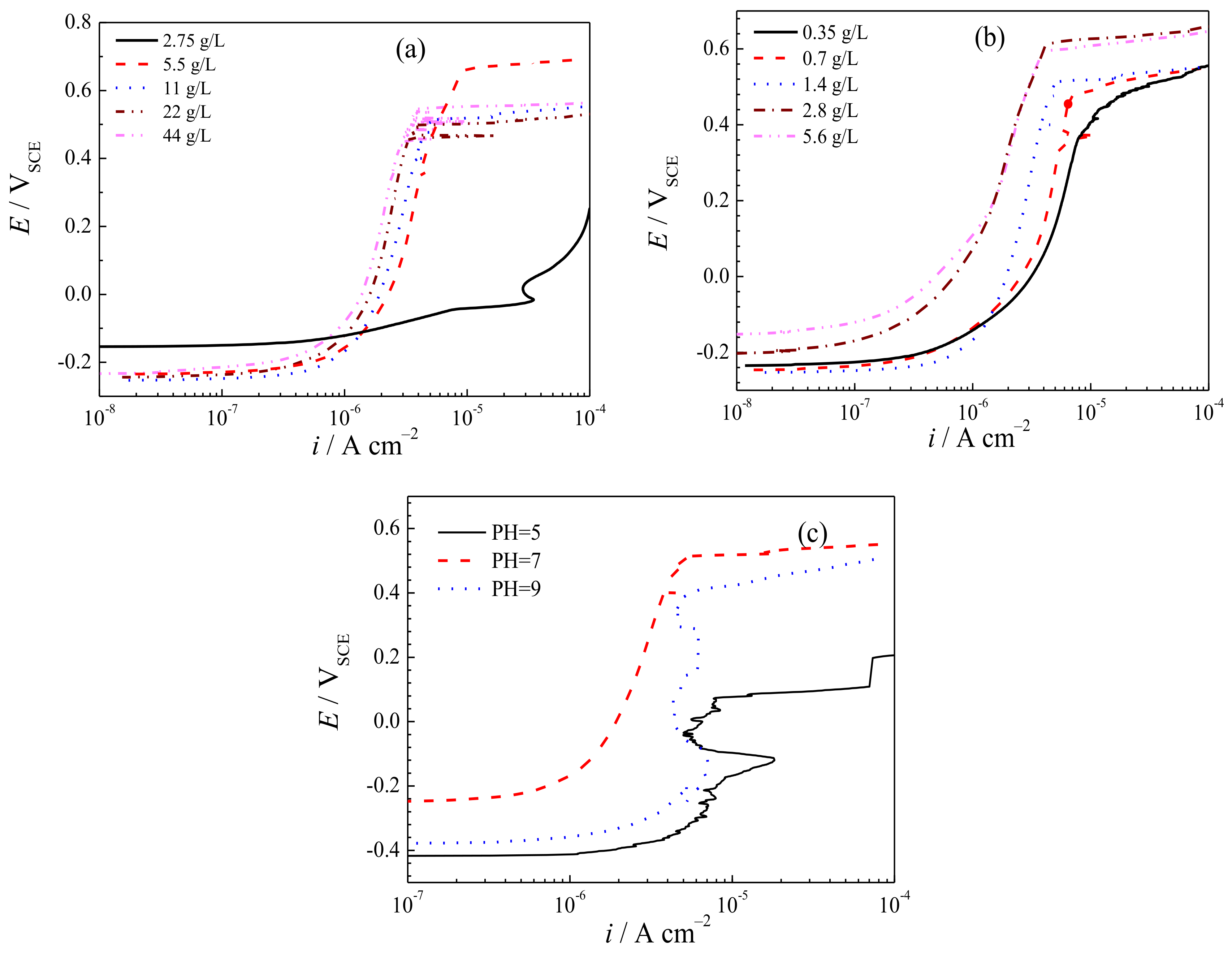
3.4. Potentiodynamic Polarization
3.5. The Corrosion Initiation of Stainless Steel in Underground Water
4. Conclusions
- The open-circuit potential and charge transfer resistance increase, while the double-layer capacitance, donor density and passive current density decrease as the SO42− concentration, HCO3− concentration or pH value of the underground water increase.
- The corrosion of 201 low-nickel stainless steel is inhibited by SO42−, HCO3− and OH− in underground water.
- The inhibitive effect of SO42− and HCO3− is significant at low concentration (for SO42−, it is less than 22 g/L, and for HCO3−, it is less than 2.8 g/L), while the excessive addition of SO42− and HCO3− only showed a little improvement on the corrosion resistance of 201 low-nickel stainless steel.
- The passivity and corrosion resistance of low-nickel stainless steel is substantially improved in alkaline underground waters.
- Anion concentrations was magnified in simulated underground waters in the present study. Corrosion behavior of 201 low-nickel stainless steel in underground waters with lower concentration of anions, and the relationship between corrosion rates in the simulated solution and that in actual groundwater environment should be investigated in the future study.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vandermaat, D.; Saydam, S.; Hagan, P.C.; Crosky, A.G. Back-calculation of failure stress of rockbolts affected by Stress Corrosion Cracking in underground coal mines. Int. J. Rock Mech. Min. 2017, 100, 310–317. [Google Scholar] [CrossRef]
- Atrens, A.; Wang, Z. Stress corrosion cracking. Mater. Forum 1995, 19, 9–34. [Google Scholar]
- Gamboa, E.; Atrens, A. Material influence on the stress corrosion cracking of rock bolts. Eng. Fail. Anal. 2004, 12, 201–235. [Google Scholar] [CrossRef]
- Mukhopadhyay, N.K.; Sridhar, G.; Parida, N.; Tarafder, S.; Ranganath, V.R. Hydrogen embrittlement failure of hot dip galvanised high tensile wires. Eng. Fail. Anal. 1998, 6, 253–265. [Google Scholar] [CrossRef]
- Toribio, J. Hydrogen-Plasticity interactions in pearlitic steel: A fractrographic and numerical study. Mater. Sci. Eng. 1996, 219, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Xia, N.; Liang, R.Y.; Payer, J.; Patnaik, A. Probabilistic modelling of the bond deterioration of fully-grouted rock bolts subject to spatiotemporally stochastic corrosion. Struct. Infrastruct. Eng. 2013, 9, 1161–1176. [Google Scholar] [CrossRef]
- Jiang, S.H.; Li, D.Q.; Zhang, L.M.; Zhou, C.B. Time-dependent system reliability of anchored rock slopes considering rock bolt corrosion effect. Eng. Geol. 2014, 175, 1–8. [Google Scholar] [CrossRef]
- Divi, S.; Chandra, D.; Daemen, J. Corrosion susceptibility of potential rock bolts in aerated multi-ionic simulated concentrated water. Tunn. Undergr. Space Technol. 2011, 26, 124–129. [Google Scholar] [CrossRef]
- Wang, B.; Guo, X.; Jin, H.; Li, F.H.; Song, Y. Experimental study on degradation behaviors of rock bolt under the coupled effect of stress and corrosion. Constr. Build. Mater. 2019, 214, 37–48. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.N.; Ren, A.W.; Peng, R.; Li, J. Corrosion Behavior and Failure Mechanism of Prestressed Rock Bolts (Cables) in the Underground Coal Mine. Adv. Civ. Eng. 2021, 2021, 6686865. [Google Scholar]
- Rahman, M.S.; Divi, S.; Chandra, D.; Daemen, J. Effect of different salts on the corrosion properties of friction type A607 steel rock bolt in simulated concentrated water. Tunn. Undergr. Space Technol. 2007, 23, 665–673. [Google Scholar] [CrossRef]
- Li, S.D.; Zhang, X.Y.; Zheng, S.K.; Duan, S.P.; Cui, J.; Zhang, H.Y. NaHCO3/Na2CO3 as an inhibitor of chloride-induced mild steel corrosion in cooling water: Electrochemical evaluation. J. Ind. Eng. Chem. 2021, 95, 235–243. [Google Scholar] [CrossRef]
- Tan, Y.T.; Wijesinghe, S.L.; Blackwood, D.J. The inhibitive effect of bicarbonate and carbonate ions on carbon steel in simulated concrete pore solution. Corros. Sci. 2014, 88, 152–160. [Google Scholar] [CrossRef]
- Qiu, J.; Leng, B.; Liu, H.J.; Macdonald, D.D.; Wu, A.J.; Jia, Y.Y.; Xue, W.D.; Yu, G.J.; Zhou, X.T. Effect of SO42- on the corrosion of 316L stainless steel in molten FLiNaK salt. Corros. Sci. 2018, 144, 224–229. [Google Scholar] [CrossRef]
- Zhu, Y.; Qiu, J.; Hou, J.; Liu, W.; Chen, H.; Ai, H.; Yu, G.; Wang, J.; Zhou, X. Effects of SO42− ions on the corrosion of GH3535 weld joint in FLiNaK molten salt. J. Nucl. Mater. 2017, 492, 122–127. [Google Scholar] [CrossRef]
- Hong, T.; Nagumo, M. The effect of SO42− concentration in NaCl solution on the early stages of pitting corrosion of type 430 stainless steel. Corros. Sci. 1997, 39, 961–967. [Google Scholar] [CrossRef]
- Ortíz, M.R.; Rodríguez, M.A.; Carranza, R.M.; Rebak, R.B. Oxyanions as inhibitors of chloride-induced crevice corrosion of Alloy 22. Corros. Sci. 2013, 68, 72–83. [Google Scholar] [CrossRef]
- Feng, X.; Shi, R.; Zhang, L.; Xu, Y.; Zhang, X.; Zhang, J.; Ding, Y.; Chen, D.; Ju, N.; Zhang, X. Degradation of passive film on low-nickel stainless steel in groundwater with different concentration of chloride ions. Int. J. Electrochem. Sci. 2018, 13, 2745–2757. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, X.; Xu, Y.; Shi, R.; Lu, X.; Zhang, L.; Zhang, J.; Chen, D. Corrosion behavior of deformed low-nickel stainless steel in groundwater solution. Eng. Fail. Anal. 2019, 98, 49–57. [Google Scholar] [CrossRef]
- Bairi, L.R.; Ningshen, S.; Mudali, U.K.; Raj, B. Corrosion investigations on metal waste form alloys of titanium-modified Type 316 stainless steel-zirconium in simulated groundwater media. Corrosion 2012, 68, 784–792. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, H.; Zhao, J.; Xiong, J. The effects of some anions on metastable pitting of 316L stainless steel. Corros. Sci. 2002, 44, 13–24. [Google Scholar] [CrossRef]
- Han, X.C.; Jun, L.I.; Zhao, K.Y.; Zhang, W.; Jie, S.U. Effect of chloride on semiconducting properties of passive films formed on supermartensitic stainless steel in NaHCO3 solution. J. Iron. Steel Res. Int. 2013, 20, 74–79. [Google Scholar] [CrossRef]
- Amri, J.; Souier, T.; Malki, B.; Baroux, B. Effect of the final annealing of cold rolled stainless steels sheets on the electronic properties and pit nucleation resistance of passive films. Corros. Sci. 2008, 50, 431–435. [Google Scholar] [CrossRef]
- Di Paola, A.; Shukla, D.; Stimming, U. Photoelectrochemical study of passive films on stainless steel in neutral solutions. Electrochim. Acta 1991, 36, 345–352. [Google Scholar] [CrossRef]
- Paola, A.D. Semiconducting properties of passive films on stainless steels. Electrochim. Acta 1989, 34, 203–210. [Google Scholar] [CrossRef]
- Renton, N.C.; Elhoud, A.M.; Deans, W.F. Effect of plastic deformation on the corrosion behavior of a super-duplex stainless steel. J. Mater. Eng. Perform. 2011, 20, 436–444. [Google Scholar] [CrossRef]
- Luo, H.; Su, H.; Dong, C.; Li, X. Passivation and electrochemical behavior of 316L stainless steel in chlorinated simulated concrete pore solution. Appl. Surf. Sci. 2017, 400, 38–48. [Google Scholar] [CrossRef]
- Luo, H.; Gao, S.; Dong, C.; Li, X. Characterization of electrochemical and passive behaviour of Alloy 59 in acid solution. Electrochim. Acta 2014, 135, 412–419. [Google Scholar] [CrossRef]
- Liu, C.; Bia, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part I. Establishment of equivalent circuits for EIS data modelling. Corros. Sci. 2003, 45, 1243–1256. [Google Scholar] [CrossRef]
- Luo, H.; Wang, X.; Dong, C.; Xiao, K.; Li, X. Effect of cold deformation on the corrosion behaviour of UNS S31803 duplex stainless steel in simulated concrete pore solution. Corros. Sci. 2017, 124, 178–192. [Google Scholar] [CrossRef]
- Sahoo, G.; Balasubramaniam, R. On the corrosion behaviour of phosphoric irons in simulated concrete pore solution. Corros. Sci. 2008, 50, 131–143. [Google Scholar] [CrossRef]
- Feng, X.; Tang, Y.; Zuo, Y. Influence of stress on passive behaviour of steel bars in concrete pore solution. Corros. Sci. 2011, 53, 1304–1311. [Google Scholar] [CrossRef]
- Feng, Z.; Cheng, X.; Dong, C.; Xu, L.; Li, X. Passivity of 316L stainless steel in borate buffer solution studied by Mott–Schottky analysis, atomic absorption spectrometry and X-ray photoelectron spectroscopy. Corros. Sci. 2010, 52, 3646–3653. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Luo, J.L. Electronic structure and pitting susceptibility of passive film on carbon steel. Electrochim. Acta 1999, 44, 2947–2957. [Google Scholar] [CrossRef]
- Dong, Z.H.; Shi, W.; Zhang, G.A.; Guo, X.P. The role of inhibitors on the repassivation of pitting corrosion of carbon steel in synthetic carbonated concrete pore solution. Electrochim. Acta 2011, 56, 5890–5897. [Google Scholar] [CrossRef]
- Carmezim, M.J.; Simoes, A.M.; Montemor, M.F.; Belo, M.D.C. Capacitance behavior of passive films on ferritic and austenitic stainless steel. Corros. Sci. 2005, 47, 581–591. [Google Scholar] [CrossRef]
- Lv, J.; Luo, H. The effects of grain refinement and deformation on corrosion resistance of passive film formed on the surface of 304 stainless steels. Mater. Res. Bull. 2015, 70, 896–907. [Google Scholar]
- Lv, J.; Guo, W.; Liang, T. The effect of pre-deformation on corrosion resistance of the passive film formed on 2205 duplex stainless steel. J. Alloys Compd. 2016, 686, 176–183. [Google Scholar] [CrossRef]
- Gomes, W.P.; Vanmackelbergh, D. Impedance spectroscopy at semiconductor electrodes: Review and recent developments. Electrochim. Acta 1996, 41, 967–973. [Google Scholar] [CrossRef]
- Hamadou, L.; Kadri, A.; Benbrahim, N. Characterization of passive films formed on low carbon steel in borate buffer solution (pH 9.2) by electrochemical impedance spectroscopy. Appl. Surf. Sci. 2005, 252, 1510–1519. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Biaggio, S.; Song, H. Steady-state passive films. J. Electrochem. Soc. 1992, 139, 170–177. [Google Scholar] [CrossRef]
- Macdonald, D.D. The point defect model for the passive state. J. Electrochem. Soc. 1992, 139, 3434–3449. [Google Scholar] [CrossRef]
- Macdonald, D.D. Passivity–the key to our metals-based civilization. Pure Appl. Chem. 1999, 71, 951–978. [Google Scholar] [CrossRef]
- Yashiro, H.; Oyama, A.; Tanno, K. Effects of temperature and potential on the inhibitive action of oxoacid salts for pitting in high-temperature chloride solutions. Corrosion 1997, 53, 290–297. [Google Scholar] [CrossRef]
- Brossia, C.S.; Kelly, R.G. Influence of alloy sulfur content and bulk electrolyte composition on crevice corrosion initiation of austenitic stainless steel. Corrosion 1998, 54, 145–154. [Google Scholar] [CrossRef]
- Ürgen, M.; Çakir, A.F. The effect of molybdate ions on the temperature dependent pitting potential of austenitic stainless steels in neutral chloride solutions. Corros. Sci. 1991, 32, 835–852. [Google Scholar] [CrossRef]
- Abd El Meguid, E.A.; Abd El Latif, A.A. Electrochemical and SEM study on Type 254 SMO stainless steel in chloride solutions. Corros. Sci. 2004, 46, 2431–2444. [Google Scholar] [CrossRef]
- Alvarez, M.G.; Galvele, J.R. The mechanism of pitting of high purity iron in NaCl solutions. Corros. Sci. 1984, 24, 27–48. [Google Scholar] [CrossRef]
- Valcarce, M.B.; Vázquez, M. Carbon steel passivity examined in alkaline solutions: The effect of chloride and nitrite ions. Electrochim. Acta 2008, 53, 5007–5015. [Google Scholar] [CrossRef]
- Valcarce, M.B.; Vázquez, M. Carbon steel passivity examined in solutions with a low degree of carbonation: The effect of chloride and nitrite ions. Mater. Chem. Phys. 2008, 115, 313–321. [Google Scholar] [CrossRef]



| Solution | A1 | A2 | A3 | A4 | A5 | B1 | B2 | B3 | B4 | B5 | C1 | C2 | C3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cl− (g/L) | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| SO42− (g/L) | 2.75 | 5.50 | 11.00 | 22.00 | 44.00 | 11.00 | 11.00 | 11.00 | 11.00 | 11.00 | 11.00 | 11.00 | 11.00 |
| HCO3− (g/L) | 1.40 | 1.40 | 1.40 | 1.40 | 1.40 | 0.35 | 0.70 | 1.40 | 2.80 | 5.60 | 1.40 | 1.40 | 1.40 |
| pH | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 5.0 | 7.0 | 9.0 |
| Solution | A1 | A2 | A3 | A4 | A5 | B1 | B2 | B3 | B4 | B5 | C1 | C2 | C3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rs (Ω cm2) | 22.4 | 16.4 | 18.2 | 18.0 | 9.52 | 15.9 | 17.3 | 18.2 | 16.3 | 14.7 | 16.9 | 18.2 | 11.2 |
| Rct (×105 Ω cm2) | 1.3 | 1.7 | 2.3 | 3.3 | 4.2 | 2.1 | 2.1 | 2.3 | 3.1 | 3.3 | 0.65 | 2.3 | 5.4 |
| Q (×10−5 Ω−1 Sn cm2) | 6.2 | 6.0 | 5.9 | 5.0 | 4.9 | 5.8 | 5.0 | 5.9 | 4.9 | 4.0 | 9.6 | 5.9 | 6.3 |
| Solution | A1 | A2 | A3 | A4 | A5 | B1 | B2 | B3 | B4 | B5 | C1 | C2 | C3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ND (×1020 cm−3) | 3.22 | 2.44 | 1.85 | 1.50 | 1.33 | 4.48 | 2.84 | 1.85 | 1.95 | 1.23 | 4.94 | 1.85 | 1.10 |
| Solution | A1 | A2 | A3 | A4 | A5 | B1 | B2 | B3 | B4 | B5 | C1 | C2 | C3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ipass (×10−6 A cm−2) | 58.8 | 2.92 | 2.38 | 1.97 | 1.69 | 4.58 | 3.69 | 2.38 | 1.12 | 0.950 | 46.4 | 2.38 | 4.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; Zeng, Q.; Lu, X.; Wu, T.; Lu, X.; Zhang, T.; Feng, X. Electrochemical Study of Stainless Steel Anchor Bolt Corrosion Initiation in Corrosive Underground Water. Processes 2021, 9, 1553. https://doi.org/10.3390/pr9091553
Ma F, Zeng Q, Lu X, Wu T, Lu X, Zhang T, Feng X. Electrochemical Study of Stainless Steel Anchor Bolt Corrosion Initiation in Corrosive Underground Water. Processes. 2021; 9(9):1553. https://doi.org/10.3390/pr9091553
Chicago/Turabian StyleMa, Fangping, Qing Zeng, Xiangyu Lu, Tong Wu, Xiao Lu, Tianyi Zhang, and Xingguo Feng. 2021. "Electrochemical Study of Stainless Steel Anchor Bolt Corrosion Initiation in Corrosive Underground Water" Processes 9, no. 9: 1553. https://doi.org/10.3390/pr9091553





