Effect of Combined Hydrophilic Activation on Interface Characteristics of Si/Si Wafer Direct Bonding
Abstract
:1. Introduction
2. Materials and Methods
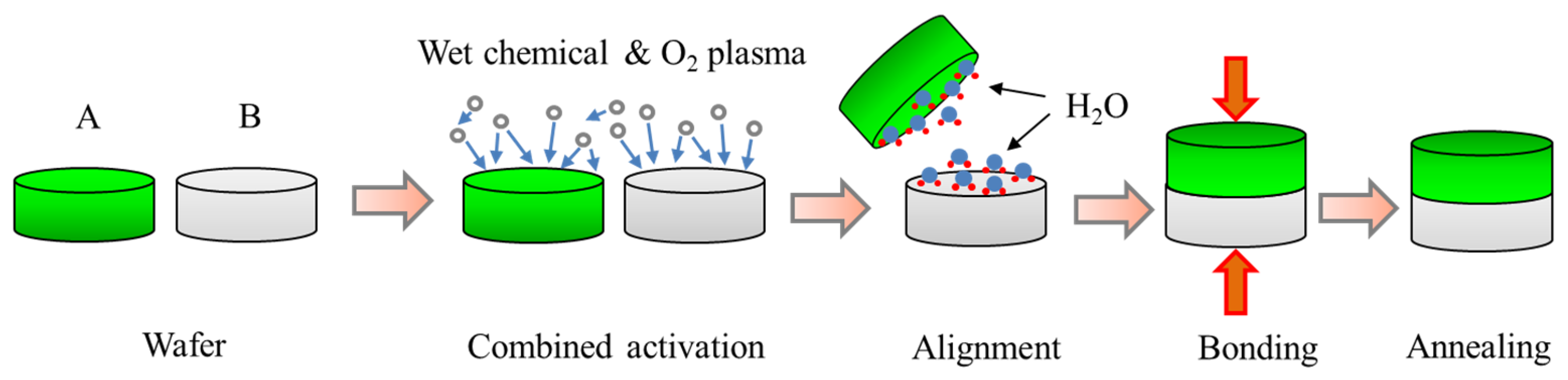
3. Mechanism for Combined Hydrophilic Bonding
4. Results and Discussion
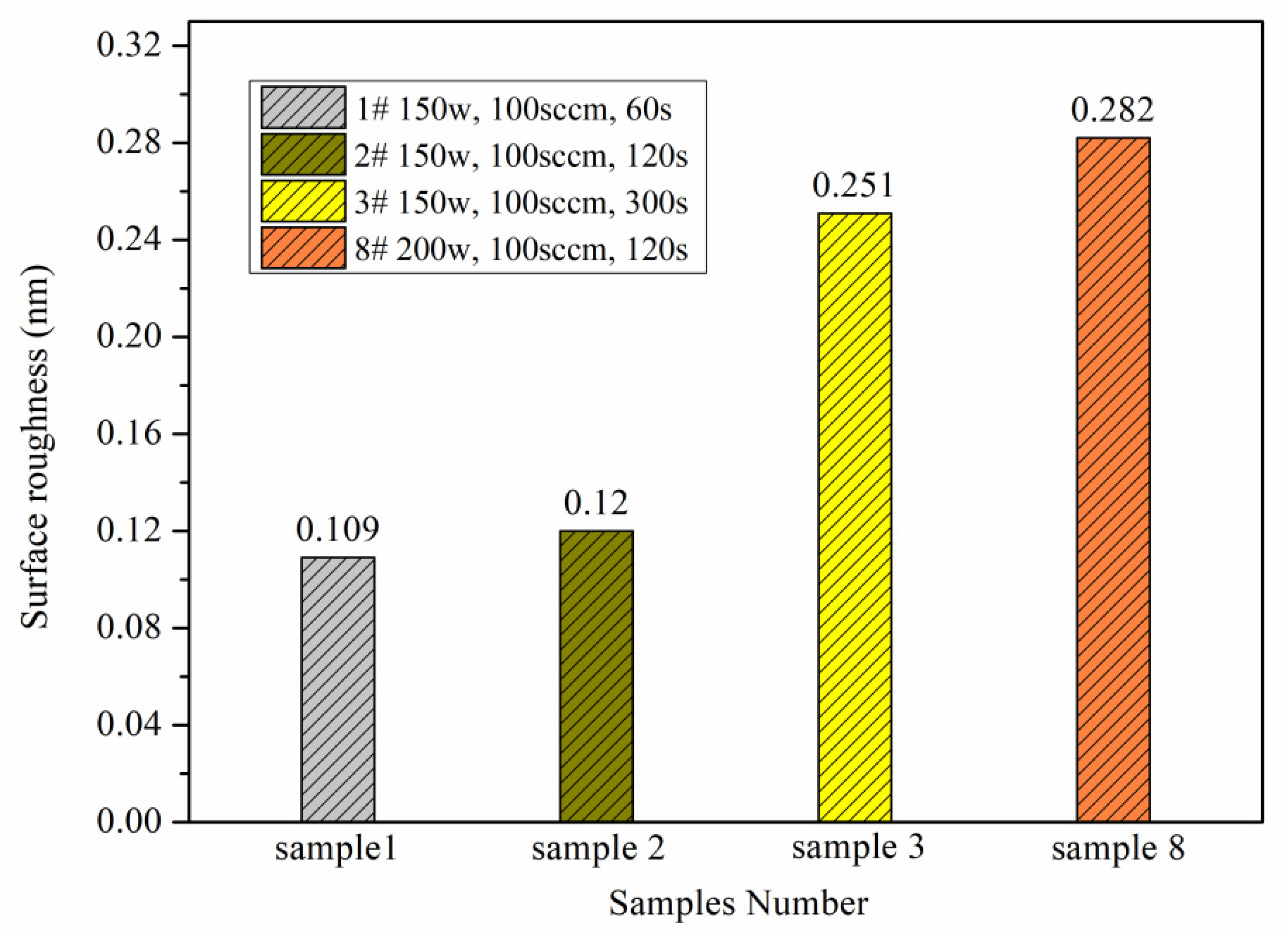
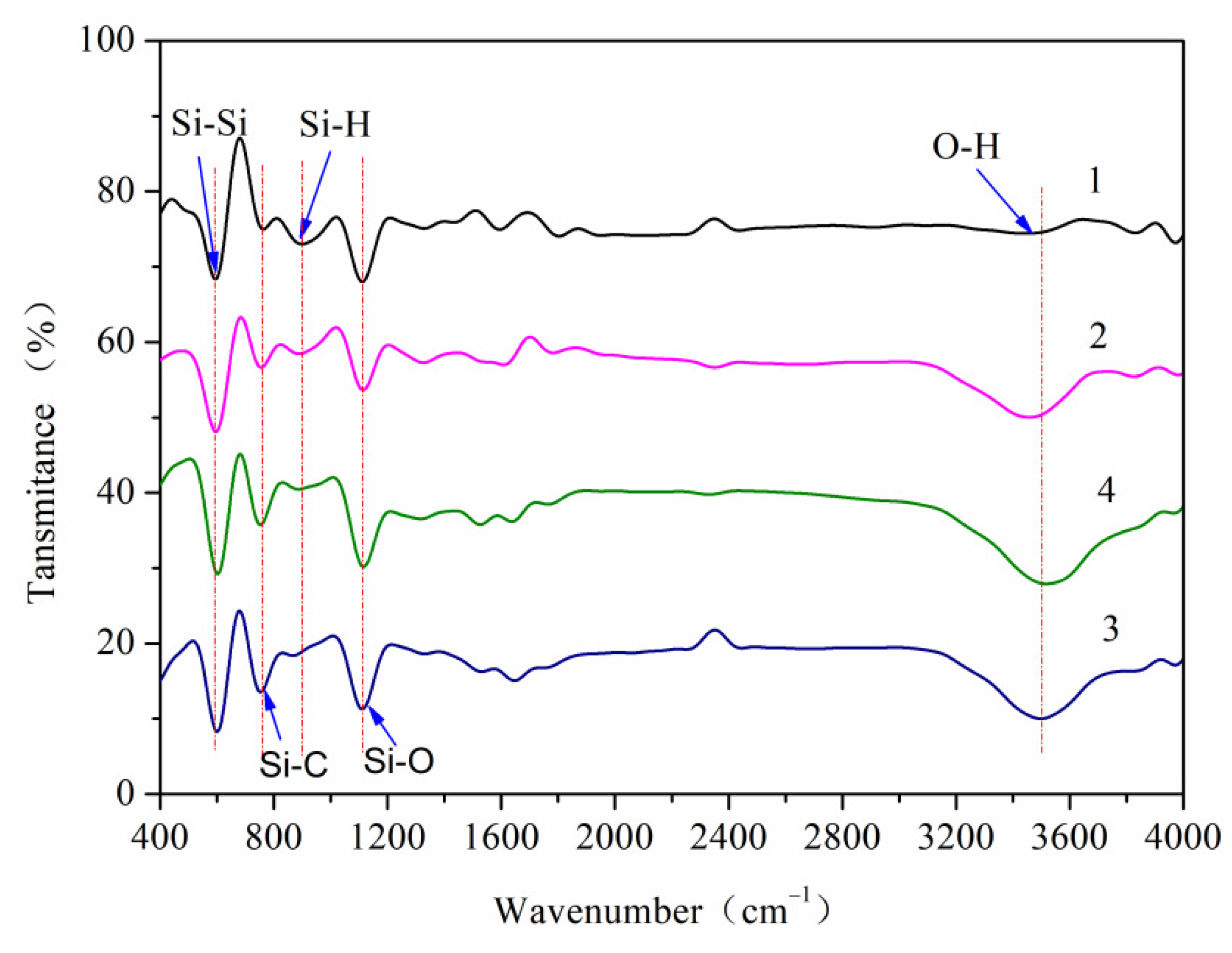
4.1. Surface Hydrophilicity
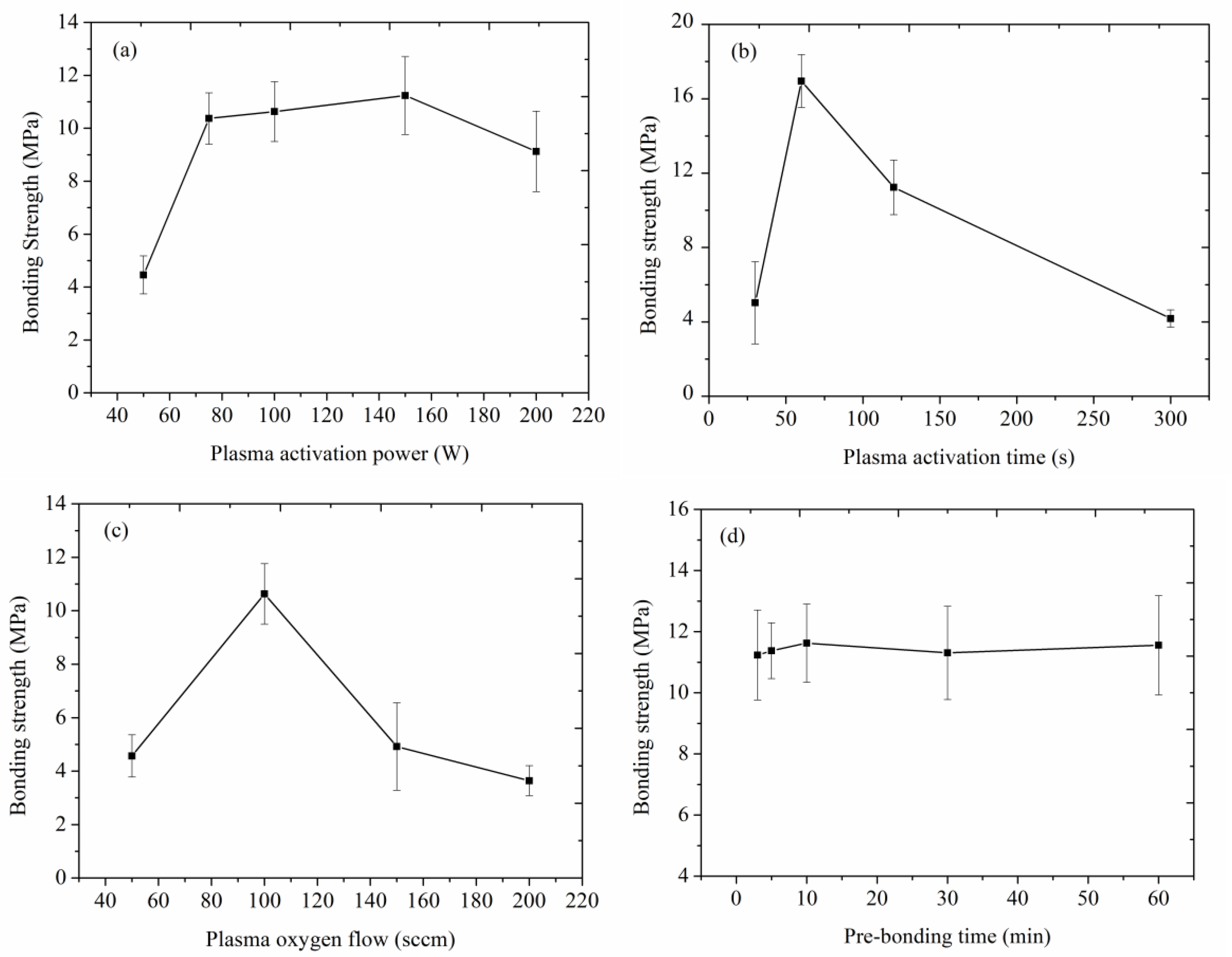
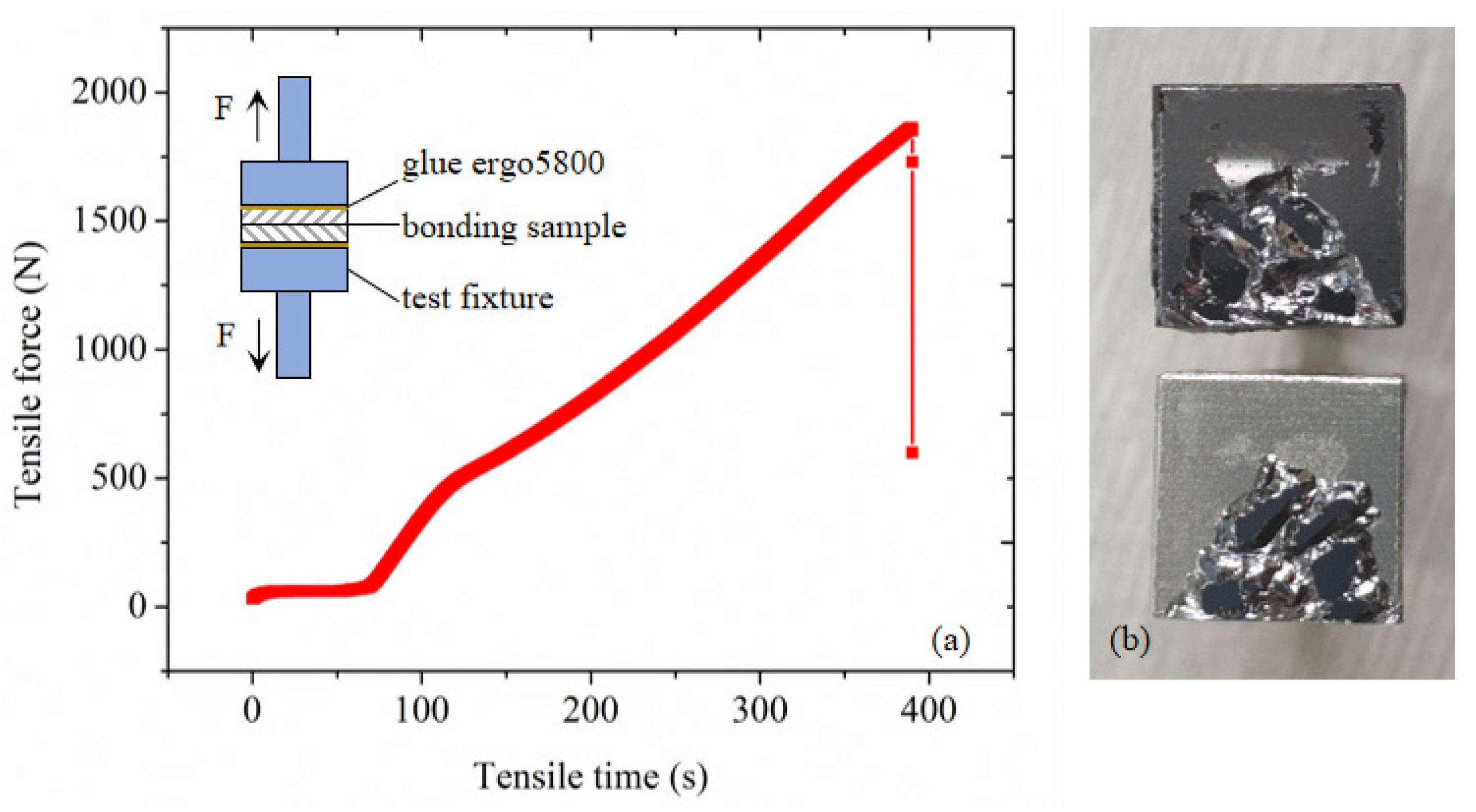
4.2. Bonding Strength
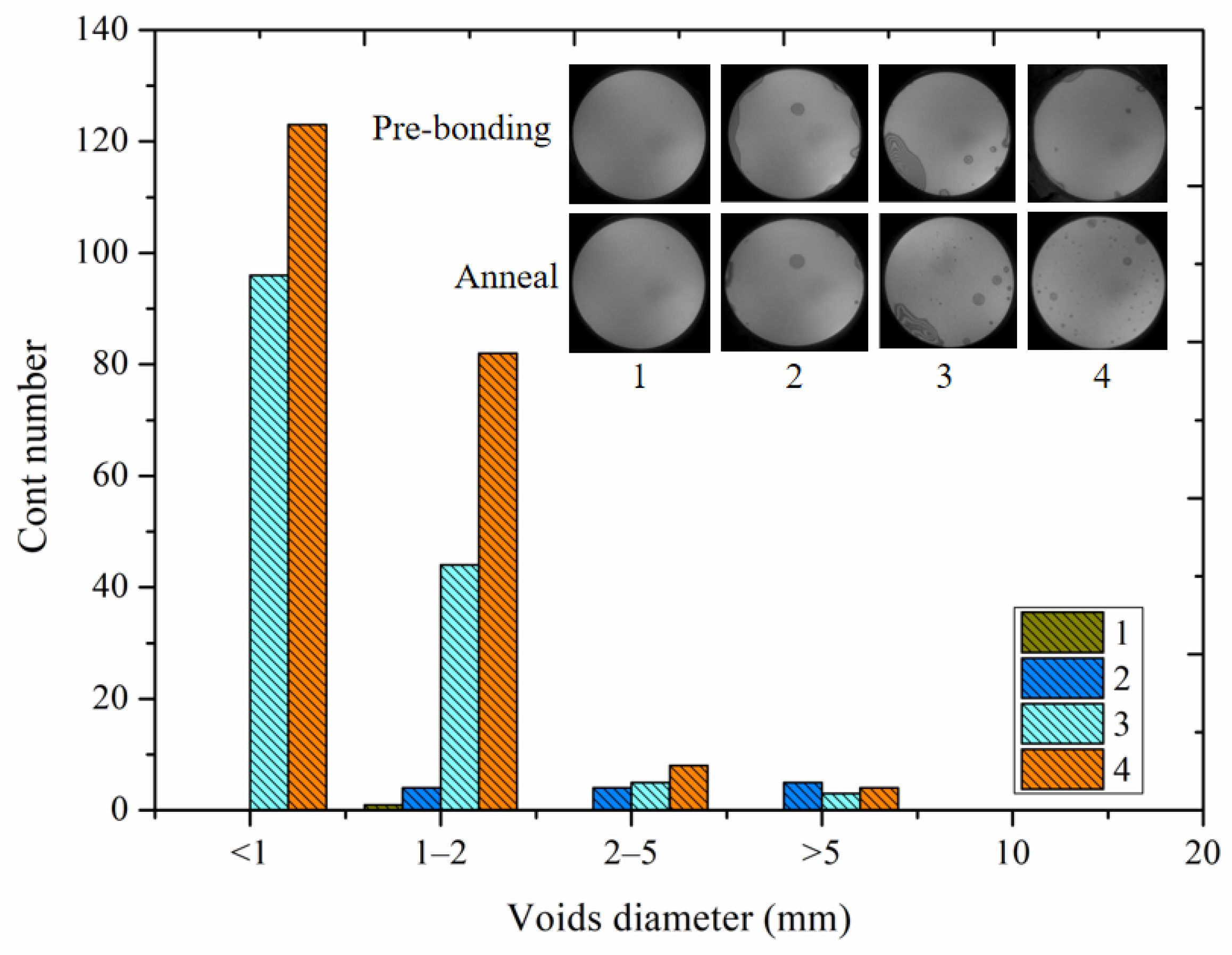
4.3. Microstructure of Bonding Interface
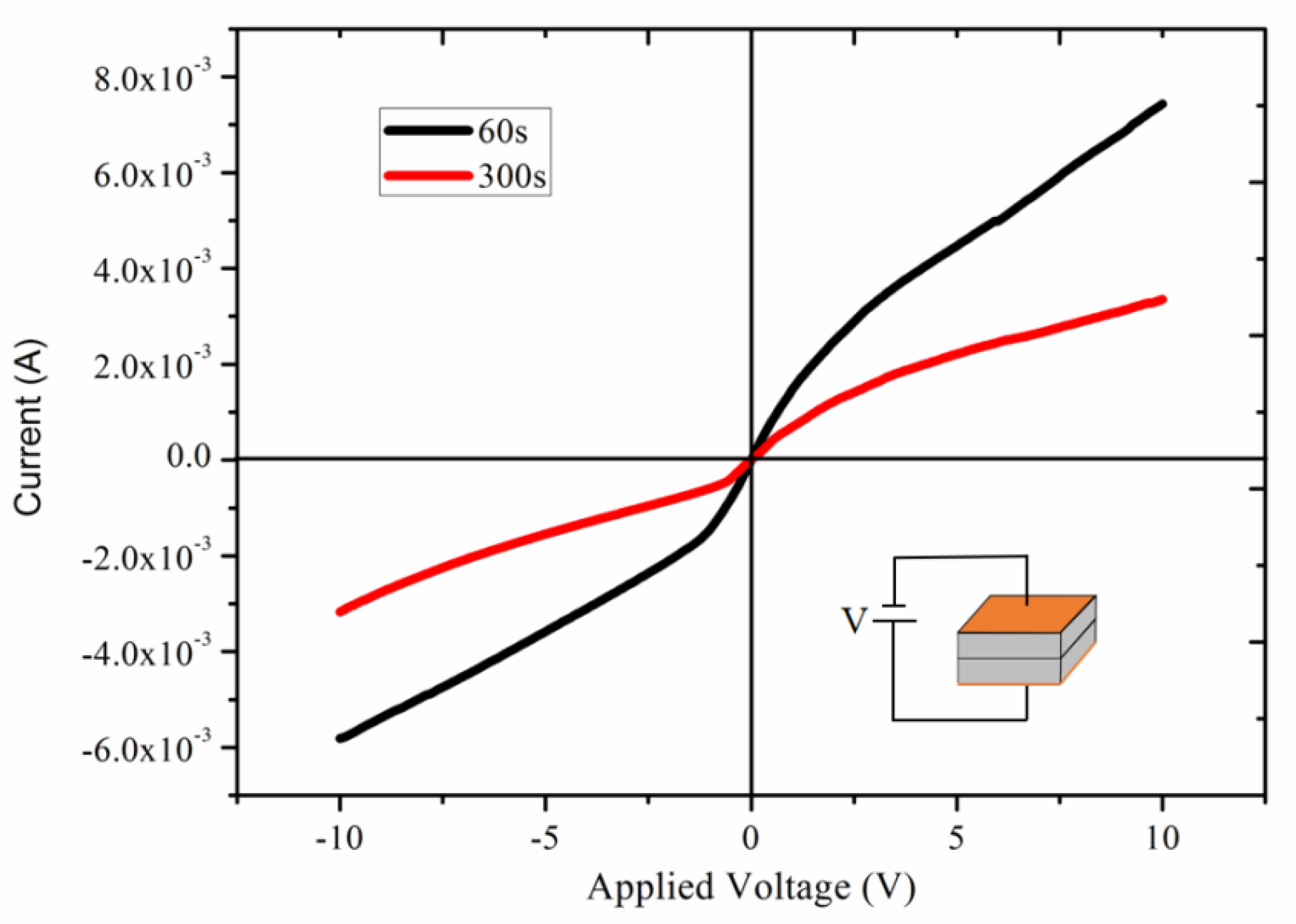
4.4. Electrical Characteristics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeon, Y.R.; Han, H.; Choi, C. Thin Si wafer substrate bonding and de-bonding below 250 °C for the monolithic 3D integration. Sens. Actuators A 2018, 281, 222–228. [Google Scholar] [CrossRef]
- Zhenga, Z.; Yao, Y.; Liu, J.A.; Sun, Y.H.; Yeow, J.T.W. Highly sensitive CMUT-based humidity sensors built with nitride-to-oxide wafer bonding technology. Sens. Actuators B Chem. 2019, 294, 123–131. [Google Scholar] [CrossRef]
- Kikuchi, T.; Bai, L.; Mitarai, T.; Yagi, H.; Furukawa, M.; Amemiya, T.; Nishiyama, N.; Arai, H. Enhanced bonding strength of InP/Si chip-on-wafer by plasma-activated bonding using stress-controlled interlayer. Jpn. J. Appl. Phys. 2020, 59, SBBD02. [Google Scholar] [CrossRef]
- Takigawa, R.; Higurashi, E.; Asano, T. Room-temperature wafer bonding of LiNbO3 and SiO2 using a modified surface activated bonding method. Jpn. J. Appl. Phys. 2018, 57, 06HJ12. [Google Scholar] [CrossRef]
- Ke, S.Y.; Ye, Y.J.; Wu, J.Y.; Ruan, Y.J.; Zhang, X.Y.; Huang, H.; Wang, J.Y.; Xu, J.F.; Li, C.; Chen, S.Y. Interface characteristics of different bonded structures fabricated by low-temperature a-Ge wafer bonding and the application of wafer-bonded Ge/Si photoelectric device. J. Mater. Sci. 2019, 54, 2406–2416. [Google Scholar] [CrossRef]
- Koga, Y.; Kurita, K. Fabrication of silicon on insulator wafer with silicon carbide insulator layer by surface-activated bonding at room temperature. Jpn. J. Appl. Phys. 2020, 59, 051002. [Google Scholar] [CrossRef]
- Veerappan, M.; Mukannan, A.; Salleh, F.; Shimura, Y.; Hayakawa, Y.; Ikeda, H. Fabrication of high quality, thin Ge-on-insulator layers by direct wafer-bonding for nanostructured thermoelectric devices. Semicond. Sci. Technol. 2017, 32, 035021. [Google Scholar] [CrossRef]
- Yokoyama, M.; Yokoyama, H.; Takenaka, M.; Takagi, S. InGaSb-on-insulator p-channel metal-oxide semiconductor field-effect transistors on Si fabricated by direct wafer bonding. J. Appl. Phys. 2019, 125, 114501. [Google Scholar] [CrossRef]
- Christiansen, S.H.; Singh, R.; Gosele, U. Wafer direct bonding: From advanced substrate engineering to future applications in micro/nano electronics. Proc. IEEE 2006, 94, 2060–2106. [Google Scholar] [CrossRef]
- Wang, C.X.; Xu, J.K.; Zeng, X.R.; Tian, Y.H.; Wang, C.Q.; Suga, T. Low-temperature wafer direct bonding of silicon and quartz glass by a two-step wet chemical surface cleaning. Jpn. J. Appl. Phys. 2018, 57, 02BD02. [Google Scholar] [CrossRef] [Green Version]
- Gong, K.W.; Sun, C.Z.; Xiong, B.; Han, Y.J.; Hao, Z.B.; Wang, J.; Wang, L.; Li, H.T. Effect of NH4OH treatment on plasma-assisted InP/Al2O3/SOI direct wafer bonding. Phys. Status Solidi A 2018, 215, 1700739. [Google Scholar] [CrossRef]
- Howlader, M.M.R.; Zhang, F.; Kim, M.J. Annealing temperature-dependent interfacial behavior of sequentially plasma-activated silicon bonded wafers. J. Microelectromech. Syst. 2011, 20, 17–20. [Google Scholar] [CrossRef]
- Fukushima, T.; Hashiguchi, H.; Yonekura, H.; Kino, H.; Murugesan, M.; Bea, J.-C.; Lee, K.-W.; Tanaka, T.; Koyanagi, M. Oxide-Oxide thermocompression direct bonding technologies with capillary self-assembly for multichip-to-wafer heterogeneous 3d system integration. Micromachines 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, R.; Yamauchi, A.; Suga, T. Sequential plasma activation methods for hydrophilic direct bonding at sub-200 degrees C. Jpn. J. Appl. Phys. 2018, 57, 6. [Google Scholar] [CrossRef]
- Wang, C.X.; Higurashi, E.J.; Suga, T. Silicon wafer bonding by modified surface activated bonding methods. In Proceedings of the 6th International IEEE Conference on Polymers and Adhesives in Microelectronics and Photonics, Polytronic 2007, Tokyo, Japan, 16–18 January 2007; pp. 36–40. [Google Scholar]
- Gong, K.W.; Sun, C.Z.; Xiong, B.; Han, Y.J.; Hao, Z.B.; Wang, J.; Wang, L.; Li, H.T. Oxides formation on hydrophilic bonding interface in plasma-assisted InP/Al2O3/SOI direct wafer bonding. AIP Adv. 2017, 7, 015039. [Google Scholar] [CrossRef] [Green Version]
- Mu, F.; Iguchi, K.; Nakazawa, H.; Takahashi, Y.; He, R.; Fujino, M.; Suga, T. Room temperature SiC-SiO2 wafer bonding enhanced by using an intermediate si nano layer. ECS J. Solid State Sci. Technol. 2017, 6, 227–230. [Google Scholar] [CrossRef]
- Takigawa, R.; Matsumae, T.; Yamamoto, M.; Higurashi, E.; Asano, T.; Kanaya, H. Demonstration of GaN/LiNbO3 hybrid wafer using room temperature surface activated bonding. ECS J. Solid State Sci. Technol. 2020, 9, 045005. [Google Scholar] [CrossRef]
- Eichler, M.; Hennecke, P.; Nagel, K.; Gabriel, M.; Klages, C.P. Plasma activation as a pretreatment tool for low temperature direct wafer bonding in microsystems technology. ECS Trans. 2012, 50, 265–276. [Google Scholar] [CrossRef]
- Wang, C.X.; Wang, Y.; Tian, Y.H.; Wang, C.Q.; Suga, T. Room-temperature direct bonding of silicon and quartz glass wafers. Appl. Phys. Lett. 2017, 110, 221602. [Google Scholar] [CrossRef]
- Wang, C.X.; Liu, Y.N.; Suga, T. A comparative study: Void formation in silicon wafer direct bonding by oxygen plasma activation with and without fluorine. ECS J. Solid State Sci. Technol. 2017, 6, 7–13. [Google Scholar] [CrossRef]
- Cocheteau, N.; Maurel-Pantel, A.; Lebon, F.; Mazerolle, F.; Rosu, I.; Ait-Zaid, S.; De Larclause, I.S. Influence of roughness on mechanical strength of direct bonded silica and Zerodur® glasses. Int. J. Adhes. Adhes. 2016, 68, 87–94. [Google Scholar] [CrossRef]
- Rieutord, F.; Tardif, S.; Nikitskiy, I.; Fournel, F.; Tedjini, M.; Larrey, V.; Bridoux, C.; Morales, C.; Landruc, D.; Kononchuk, O. Water transport within silicon direct bonding gap. ECS Trans. 2018, 86, 39–47. [Google Scholar] [CrossRef]
- Kibria, M.G.; Zhang, F.; Lee, T.H.; Kim, M.J.; Howlader, M.M.R. Comprehensive investigation of sequential plasma activated Si/Si bonded interfaces for nanointegration on the wafer scale. Nanotechnology 2010, 21, 134011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, M.; Li, D.L.; Liu, Y.F. Investigation of plasma activated Si-Si bonded interface by infrared image based on combination of spatial domain and morphology. Micromachines 2019, 10, 445. [Google Scholar] [CrossRef] [Green Version]
- Fournela, F.; Continnia, L.; Moralesa, C.; Da Fonseca, J.; Moriceau, H.; Martin-Cocher, C.; Rieutord, F.; Barthelemy, A.; Radu, I. Direct bonding energy in anhydrous atmosphere. ECS Trans. 2012, 50, 3–16. [Google Scholar] [CrossRef]
- He, R.; Fujino, M.; Yamauchi, A.; Suga, T. Combined surface-activated bonding technique for low-temperature hydrophilic direct wafer bonding. Jpn. J. Appl. Phys. 2016, 55, 04EC02. [Google Scholar] [CrossRef]
- Tedjini, M.; Fournel, F.; Moriceau, H.; Larrey, V.; Landru, D.; Kononchuk, O.; Tardif, S.; Rieutord, F. Interface water diffusion in silicon direct bonding. Appl. Phys. Lett. 2016, 109, 111603. [Google Scholar] [CrossRef]
- Egorov, S.A.; Binder, K. When does Wenzel’s extension of Young’s equation for the contact angle of droplets apply A density functional study. J. Chem. Phys. 2020, 152, 194707. [Google Scholar] [CrossRef]
- Li, D.L.; Shang, Z.G.; Wang, S.Q.; Wen, Z.Y. Low temperature Si/Si wafer direct bonding using a plasma activated method. J. Zhejiang Univ.-Sci. C 2013, 14, 244–251. [Google Scholar] [CrossRef]
- Jung, A.; Zhang, Y.; Dasilva, Y.A.R.; Isa, F.; von Känel, H. Electrical properties of Si-Si interfaces obtained by room temperature covalent wafer bonding. J. Appl. Phys. 2018, 123, 085701. [Google Scholar] [CrossRef]




| No. | Power /W | O2 Flow /Sccm | Time /S | Contact Angle/° | Bonding Strength/Mpa Mean and SD | |||
|---|---|---|---|---|---|---|---|---|
| Testing Results | Mean and SD 1 | |||||||
| 1 | 150 | 100 | 60 | 4.73 | 4.59 | 4.35 | 4.56 ± 0.19 | 16.95 ± 1.42 |
| 2 | 150 | 100 | 120 | 3.71 | 3.35 | 4.21 | 3.76 ± 0.43 | 11.23 ± 1.47 |
| 3 | 150 | 100 | 300 | 2.77 | 3.88 | 3.73 | 3.46 ± 0.60 | 4.18 ± 0.47 |
| 4 | 100 | 50 | 120 | 3.98 | 4.39 | 4.04 | 4.14 ± 0.22 | 4.46 ± 0.72 |
| 5 | 100 | 100 | 120 | 3.76 | 4.04 | 4.26 | 4.02 ± 0.25 | 10.63 ± 1.63 |
| 6 | 100 | 150 | 120 | 3.27 | 3.93 | 3.86 | 3.69 ± 0.36 | 3.64 ± 0.56 |
| 7 | 50 | 100 | 120 | 5.50 | 5.28 | 4.97 | 5.25 ± 0.27 | 6.80 ± 0.26 |
| 8 | 200 | 100 | 120 | 3.49 | 3.35 | 3.71 | 3.52 ± 0.18 | 9.12 ± 1.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Cui, X.; Du, M.; Zhou, Y.; Lan, F. Effect of Combined Hydrophilic Activation on Interface Characteristics of Si/Si Wafer Direct Bonding. Processes 2021, 9, 1599. https://doi.org/10.3390/pr9091599
Li D, Cui X, Du M, Zhou Y, Lan F. Effect of Combined Hydrophilic Activation on Interface Characteristics of Si/Si Wafer Direct Bonding. Processes. 2021; 9(9):1599. https://doi.org/10.3390/pr9091599
Chicago/Turabian StyleLi, Dongling, Xiaohan Cui, Mao Du, Ying Zhou, and Fenfen Lan. 2021. "Effect of Combined Hydrophilic Activation on Interface Characteristics of Si/Si Wafer Direct Bonding" Processes 9, no. 9: 1599. https://doi.org/10.3390/pr9091599
APA StyleLi, D., Cui, X., Du, M., Zhou, Y., & Lan, F. (2021). Effect of Combined Hydrophilic Activation on Interface Characteristics of Si/Si Wafer Direct Bonding. Processes, 9(9), 1599. https://doi.org/10.3390/pr9091599






