Clinical Characteristics and In-Hospital Outcomes in Patients with Iliopsoas Abscess: A Multicenter Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Patient Selection and Data Collection
2.3. Statistical Analysis
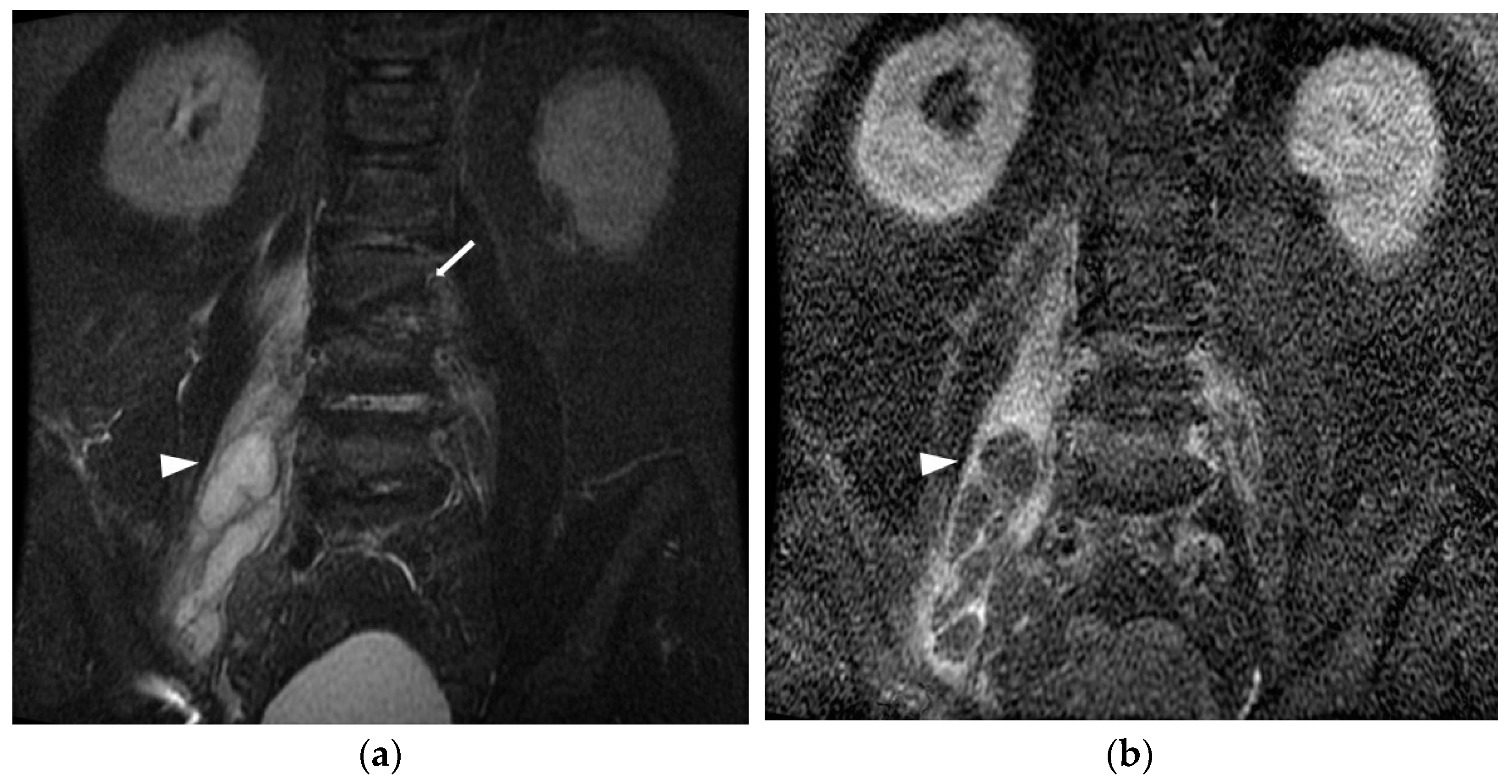
3. Results
3.1. Patient Characteristics between Survivors and Non-Survivors
3.2. Distribution of IPA According to Origin
3.3. Microbiology Results
3.4. Treatment and Outcomes
3.5. Univariate and Multivariate Analyses of Predictors of In-Hospital Mortality, Longer LOS, and Recurrence
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mynter, H. Acute psoitis. Buffalo. Med. Surg. J. 1881, 2, 202–210. [Google Scholar]
- López, V.N.; Ramos, J.M.; Meseguer, V.; Arellano, J.L.P.; Serrano, R.; Ordóñez, M.A.G.; Peralta, G.; Boix, V.; Pardo, J.; Conde, A.; et al. Microbiology and outcome of iliopsoas abscess in 124 patients. Medicine 2009, 88, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.S.; Huang, S.C.; Loh, E.W.; Tsai, C.A.; Hung, Y.Y.; Tsan, Y.T.; Huang, J.A.; Wang, L.M.; Hu, S.Y. Features and treatment modality of iliopsoas abscess and its outcome: A 6-year hospital-based study. BMC Infect. Dis. 2013, 9, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chern, C.H.; Hu, S.C.; Kao, W.F.; Tsai, J.; Yen, D.; Lee, C.H. Psoas abscess: Making an early diagnosis in the, E.D. Am. J. Emerg. Med. 1997, 15, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, L.; Hamati, M.; Flannigan, M.; Singh, M.; Bush, C.; Jones, J. Epidemiology of and risk factors for iliopsoas abscess in a large community-based study. Am. J. Emerg. Med. 2019, 37, 158–159. [Google Scholar] [CrossRef] [Green Version]
- Ricci, M.A.; Rose, F.B.; Meyer, K.K. Pyogenic psoas abscess: Worldwide variations in etiology. World J. Surg. 1986, 10, 834–843. [Google Scholar] [CrossRef]
- Shields, D.; Robinson, P.; Crowley, T.P. Iliopsoas abscess—A review and update on the literature. Int. J. Surg. 2012, 10, 466–469. [Google Scholar] [CrossRef] [Green Version]
- Mallick, I.H.; Thoufeeq, M.H.; Rajendran, T.P. Iliopsoas abscesses. Postgrad. Med. J. 2004, 80, 459–462. [Google Scholar] [CrossRef] [Green Version]
- Santaella, R.O.; Fishman, E.K.; Lipsett, P.A. Primary vs secondary iliopsoas abscess. Presentation, microbiology, and treatment. Arch. Surg. 1995, 130, 1309–1313. [Google Scholar] [CrossRef]
- Laguna, P.; Moya, M. Abscess of the psoas muscle: Analysis of 11 cases and review of the literature. Enferm. Infecc. Microbiol. Clin. 1998, 16, 19–24. [Google Scholar]
- De, U.; Pal, D.K. Seventy cases of non-tubercular psoas abscess at a rural referral centre in South Bengal. Trop. Doct. 2006, 36, 53–54. [Google Scholar] [CrossRef] [PubMed]
- Thakral, A.; Prasad, D.; Katyal, S.; Kumar, A. Characteristics and Outcomes of Psoas Abscess: Experience from a Tertiary Care Center in North India. Cureus 2022, 14, e21358. [Google Scholar] [CrossRef] [PubMed]
- Hamano, S.; Kiyoshima, K.; Nakatsu, H.; Murakami, S.; Igarashi, T.; Ito, H. Pyogenic psoas abscess: Difficulty in early diagnosis. Urol. Int. 2003, 71, 178–183. [Google Scholar] [CrossRef]
- Dietrich, A.; Vaccarezza, H.; Vaccaro, C.A. Iliopsoas abscess: Presentation, management, and outcomes. Surg. Laparosc. Endosc. Percutan. Tech. 2013, 23, 45–48. [Google Scholar] [CrossRef]
- Hu, S.Y.; Hsieh, M.S.; Chang, Y.T.; Huang, C.C.; Tsai, C.A.; Tsai, C.L.; Hsu, C.Y.; Shen, C.H.; Chang, Y.Z. Clinical features, management, and outcome of iliopsoas abscess associated with cardiovascular disorders: A hospital-based observational case series study. BMC Musculoskelet. Disord. 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Yoon, J.H.; Kim, S.I.; Wie, S.H.; Kim, Y.R. Etiology and outcome of iliopsoas muscle abscess in Korea; changes over a decade. Int. J. Surg. 2013, 11, 1056–1059. [Google Scholar] [CrossRef] [Green Version]
- Gruenwald, I.; Abrahamson, J.; Cohen, O. Psoas abscess: Case report and review of the literature. J. Urol. 1992, 147, 1624–1626. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, F.-A.; Dupon, M.; Dutronc, H.; de Barbeyrac, B.; Lawson-Ayayi, S.; Dubuisson, V.; Souillac, V. Association between psoas abscess and prosthetic hip infection: A case-control study. Acta Orthop. 2009, 80, 198–200. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-J.; Ruaan, M.-K.; Lan, R.-R.; Wang, M.-C. Acute pyogenic iliopsoas abscess in Taiwan: Clinical features, diagnosis, treatments and outcome. J. Infect. 2000, 40, 248–255. [Google Scholar] [CrossRef]
- Alonso, C.D.; Barclay, S.; Tao, X.; Auwaerter, P.G. Increasing incidence of iliopsoas abscesses with MRSA as a predominant pathogen. J. Infect. 2011, 63, 1–7. [Google Scholar] [CrossRef]
- Navarro López, V.; López García, F.; González Escoda, E.; Gregori Colomé, J.; Muñoz Pérez, A. Psoas abscess in patients infected with the human immunodeficiency virus. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Heyd, J.; Meallem, R.; Schlesinger, Y.; Rudensky, B.; Hadas-Halpern, I.; Yinnon, A.M.; Raveh, D. Clinical characteristics of patients with psoas abscess due to non-typhi Salmonella. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, W.N.; Sohn, H.J.; Chan, S.; Petrosyan, M.; Vermaire, H.M.; Kelso, R.L.; Towfigh, S.; Mason, R.J. Psoas abscess rarely requires surgical intervention. Am. J. Surg. 2008, 196, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Cantasdemir, M.; Kara, B.; Cebi, D.; Selcuk, N.D.; Numan, F. Computed tomography-guided percutaneous catheter drainage of primary and secondary iliopsoas abscesses. Clin. Radiol. 2003, 58, 811–815. [Google Scholar] [CrossRef]
- Afaq, A.; Jain, B.K.; Dargan, P.; Bhattacharya, S.K.; Rauniyar, R.K.; Kukreti, R. Surgical drainage of primary iliopsoas abscess—Safe and cost-effective treatment. Trop. Doct. 2002, 32, 133–135. [Google Scholar] [CrossRef]
- Tabrizian, P.; Nguyen, S.Q.; Greenstein, A.; Rajhbeharrysingh, U.; Divino, C.M. Management and treatment of iliopsoas abscess. Arch. Surg. 2009, 144, 946–949. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Morimoto, T.; Katsube, K.; Yamamori, Y.; Mashino, J.; Kikuchi, K. Clinical characteristics of pyogenic spondylitis and psoas abscess at a tertiary care hospital: A retrospective cohort study. J. Orthop. Surg. Res. 2018, 28, 302. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Lin, P.-C.; Wang, W.-S.; Lai, J.-I. An Update on Psoas Muscle Abscess: An 8-Year Experience and Review of Literature. Int. J. Gerontol. 2011, 5, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Yeh, H.-T.; Liau, S.-K.; Niu, K.-Y.; Hsiao, C.-H.; Yeh, C.-C.; Lu, J.-X.; Ng, C.-J.; Yen, C.-C. Clinical Characteristics and In-Hospital Outcomes in Dialysis Patients with Septic Arthritis. Medicina 2022, 58, 401. [Google Scholar] [CrossRef]
- Sato, M.; Iwasa, Y.; Otsubo, S.; Kimata, N.; Takei, T.; Miwa, N.; Akiba, T.; Nitta, K. Psoas abscess in hemodialysis patients. Int. Urol. Nephrol. 2010, 42, 1113–1116. [Google Scholar] [CrossRef]
- Syed-Ahmed, M.; Narayanan, M. Immune dysfunction and risk of infection in chronic kidney disease. Adv. Chronic Kidney Dis. 2019, 26, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Chuang, F.-R.; Lee, C.-H.; Chen, J.-B.; Cheng, Y.-F.; Yang, B.-Y.; Hsu, K.-T.; Wu, M.-S. Extra-renal abscess in chronic hemodialysis patients. Ren. Fail. 2002, 24, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Banshodani, M.; Moriishi, M.; Sato, T.; Shintaku, S.; Masaki, T.; Kawanishi, H. Iliopsoas Abscess in Hemodialysis Patients with End-Stage Kidney Disease. Ther. Apher. Dial. 2019, 23, 534–541. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total (n = 176) | Survivor (n = 163) | Non-Survivor (n = 13) | p Value |
|---|---|---|---|---|
| Age (year) | 63.0 ± 16.4 | 61.9 ± 13.6 | 76.2 ± 13.3 | 0.002 * |
| Male | 114 (64.8) | 107 (65.6) | 7 (53.8) | 0.385 |
| Systolic blood pressure (mmHg) | 130.3 ± 31.1 | 129.8 ± 30.5 | 136.9 ± 38.8 | 0.425 |
| Diastolic blood pressure (mmHg) | 73.1 ± 17.1 | 72.9 ± 16.9 | 76.0 ± 20.2 | 0.527 |
| Heart rate (beats/min) | 101.7 ± 19.5 | 101.8 ± 19.3 | 100.5 ± 23.0 | 0.823 |
| Body temperature (°C) | 37.1 ± 1.0 | 37.1 ± 1.1 | 36.5 ± 0.9 | 0.029 * |
| Respiratory rate (breaths/min) | 19.4 ± 2.9 | 19.2 ± 2.8 | 21.2 ± 3.5 | 0.020 * |
| Bedridden status | 12 (6.8) | 9 (5.5) | 3 (23.1) | 0.047 * |
| Mechanical ventilation | 32 (18.2) | 22 (13.5) | 10 (76.9) | <0.001 * |
| Intensive care unit admission | 38 (21.6) | 28 (17.2) | 10 (76.9) | <0.001 * |
| Renal replacement therapy | 21 (11.9) | 15 (9.2) | 6 (46.2) | 0.001 * |
| Length of hospital stay (day) | 32.4 ± 26.9 | 33.0 ± 26.6 | 26.1 ± 30.3 | 0.371 |
| Recurrence | 34 (19.3) | 34 (20.9) | 0 (0) | |
| Sepsis | 86 (48.9) | 73 (44.8) | 13 (100) | <0.001 * |
| Septic shock | 30 (17.0) | 18 (11) | 12 (92.3) | <0.001 * |
| Abscess features | ||||
| Gas-forming | 71 (40.3) | 63 (38.7) | 8 (61.5) | 0.105 |
| Multiple lobulated | 89 (50.6) | 83 (50.9) | 6 (46.2) | 0.781 |
| Size (cm) | 7.4 ± 4.1 | 7.4 ± 4.0 | 8.1 ± 3.3 | 0.554 |
| Involvement | 0.996 | |||
| Right | 67 (38.1) | 62 (38.0) | 5 (38.5) | |
| Left | 56 (31.8) | 52 (31.9) | 4 (30.8) | |
| Bilateral | 53 (30.1) | 49 (30.1) | 4 (30.8) | |
| Initial presentations | ||||
| Fever | 96 (54.5) | 92 (56.4) | 4 (30.8) | 0.074 |
| Abdominal pain | 40 (22.7) | 35 (21.5) | 5 (38.5) | 0.175 |
| Flank/back pain | 130 (73.9) | 125 (76.7) | 5 (38.5) | 0.006 * |
| Limp | 90 (51.1) | 86 (52.8) | 4 (30.8) | 0.127 |
| Mass | 3 (1.7) | 3 (1.8) | 0 (0) | 1.000 |
| Malaise | 70 (39.8) | 62 (38.0) | 8 (61.5) | 0.096 |
| Comorbidities | ||||
| Hypertension | 83 (47.2) | 78 (47.9) | 5 (38.5) | 0.514 |
| Diabetes mellitus | 66 (37.5) | 62 (38.0) | 4 (30.8) | 0.769 |
| CAD | 15 (8.5) | 14 (8.6) | 1 (7.7) | 1.000 |
| CKD | 32 (18.2) | 28 (17.2) | 4 (30.8) | 0.258 |
| CHF | 9 (5.1) | 5 (3.1) | 4 (30.8) | 0.002 * |
| Malignancy | 21 (11.9) | 19 (11.7) | 2 (15.4) | 0.656 |
| Prior stroke | 11 (6.3) | 10 (6.1) | 1 (7.7) | 0.581 |
| Intravenous drug abuse | 21 (11.9) | 21 (12.9) | 0 (0) | 0.370 |
| HIV infection | 4 (2.3) | 4 (2.5) | 0 (0) | 1.000 |
| Liver cirrhosis | 12 (6.8) | 11 (6.7) | 1 (7.7) | 1.000 |
| Prior TB history | 1 (0.6) | 3 (1.8) | 0 (0) | 1.000 |
| Imaging modalities | ||||
| Computed tomography | 172 (97.7) | 159 (97.5) | 13 (100.0) | 1.000 |
| Magnetic resonance imaging | 71 (40.3) | 65 (39.9) | 6 (46.2) | 0.657 |
| Blood test | ||||
| White blood count (103/μL) n = 17 | 16.6 ± 9.6 | 16.9 ± 9.8 | 13.1 ± 5.9 | 0.178 |
| Hemoglobin (g/dL) n = 175 | 11.3 ± 5.6 | 11.4 ± 5.7 | 10.8 ± 3.4 | 0.552 |
| Platelet (103/μL) n = 46 | 264.8 ± 144.0 | 273.1 ± 142.1 | 162.5 ± 133.0 | 0.007 * |
| INR n = 18 | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.3 ± 0.3 | 0.354 |
| Creatinine (mg/dL) n = 176 | 2.1 ± 5.3 | 2.1 ± 5.5 | 2.7 ± 2.2 | 0.730 |
| C-reactive protein (mg/L) n = 41 | 187.8 ± 121.9 | 189.4 ± 120.0 | 166.6 ± 151.9 | 0.534 |
| Positive culture n = 176 | 93 (52.8) | 85 (52.1) | 8 (61.5) | 0.514 |
| Treatment | ||||
| Antibiotics alone | 56 (31.8) | 50 (30.7) | 6 (46.2) | 0.352 |
| Percutaneous drainage | 88 (50.0) | 83 (50.9) | 5 (38.5) | 0.387 |
| Surgery | 32 (18.2) | 30 (18.4) | 2 (15.4) | 1.000 |
| Variable | Total (n = 176) | Survivor (n = 163) | Non-Survivor (n = 13) | p Value |
|---|---|---|---|---|
| Primary IPA | 50 (28.4) | 45 (27.6) | 5 (38.5) | 0.523 |
| Secondary IPA | ||||
| Skeletal origin | 92 (52.3) | 88 (54.0) | 4 (30.8) | 0.107 |
| Spondylitis | 79 (44.9) | 75 (46.0) | 4 (30.8) | |
| Septic arthritis | 14 (8.0) | 14 (8.6) | 0 (0) | |
| Intra-abdominal origin | 12 (6.8) | 11 (6.7) | 1 (7.7) | 1.000 |
| Intestinal perforation | 4 (2.3) | 3 (1.8) | 1 (7.7) | |
| Intestinal ischemia | 1 (0.6) | 1 (0.6) | 0 (0) | |
| Enteric fistula | 2 (1.1) | 2 (1.2) | 0 (0) | |
| Diverticulitis | 1 (0.6) | 1 (0.6) | 0 (0) | |
| Appendicitis | 4 (2.3) | 4 (2.5) | 0 (0) | |
| Colon cancer | 1 (0.6) | 1 (0.6) | 0 (0) | |
| Endometrial cancer | 1 (0.6) | 1 (0.6) | 0 (0) | |
| Urinary tract origin | 9 (5.1) | 7 (4.3) | 2 (15.4) | 0.135 |
| Pyelonephritis | 5 (2.8) | 3 (1.9) | 2 (15.4) | |
| Renal abscess | 4 (2.3) | 4 (2.5) | 0 (0) | |
| Soft tissue origin | 6 (3.4) | 5 (3.1) | 1 (7.7) | 0.373 |
| Necrotizing fasciitis | 2 (1.1) | 2 (1.2) | 0 (0) | |
| Wound infection | 1 (0.6) | 1 (0.6) | 0 (0) | |
| Pressure sore | 3 (1.7) | 2 (1.2) | 1 (7.7) | |
| Cardiovascular origin | 7 (4.0) | 7 (4.3) | 0 (0) | 1.000 |
| Abdominal aortic aneurysm post stent insertion | 1 (0.6) | 1 (0.6) | 0 (0) | |
| Infected aortic aneurysm | 3 (1.7) | 3 (1.8) | 0 (0) | |
| Infective endocarditis | 3 (1.7) | 3 (1.8) | 0 (0) |
| Total | Primary | Secondary | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skeletal | GI | GU | Soft Tissue | CV | ||||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Blood culture | 93 | 25 | 56 | 1 | 4 | 4 | 3 | |||||||
| Gram-positive | ||||||||||||||
| MSSA | 41 | (44.1) | 13 | (52.0) | 26 | (46.4) | 1 | (25.0) | 1 | (33.3) | ||||
| MRSA | 15 | (16.1) | 3 | (12.0) | 9 | (16.1) | 1 | (25.0) | 1 | (25.0) | 1 | (33.3) | ||
| CoNS | 6 | (6.5) | 1 | (4.0) | 2 | (3.6) | 1 | (25.0) | 2 | (50.0) | ||||
| GBS | 3 | (3.2) | 1 | (4.0) | 2 | (3.6) | ||||||||
| Streptococcus intermedius | 2 | (2.2) | 2 | (3.6) | ||||||||||
| Group D Streptococcus | 1 | (1.1) | 1 | (4.0) | ||||||||||
| Streptococcus gordonii | 1 | (1.1) | 1 | (1.8) | ||||||||||
| Streptococcus mitis | 1 | (1.1) | 1 | (4.0) | ||||||||||
| Anaerococcus sp. | 1 | (1.1) | 1 | (1.8) | ||||||||||
| Peptostreptococcus sp. | 1 | (1.1) | 1 | (4.0) | ||||||||||
| Gram-negative | ||||||||||||||
| Klebsiella pneumoniae | 6 | (6.5) | 2 | (8.0) | 4 | (7.1) | ||||||||
| Escherichia coli | 6 | (6.5) | 5 | (8.9) | 1 | (25.0) | ||||||||
| Salmonella enterica serogroup B | 2 | (2.2) | 1 | (1.8) | 1 | (33.3) | ||||||||
| Salmonella enterica serogroup D | 1 | (1.1) | 1 | (100.0) | ||||||||||
| Serratia marcescens | 1 | (1.1) | 1 | (25.0) | ||||||||||
| Actinobacillus actinomycetemcomitans | 1 | (1.1) | 1 | (4.0) | ||||||||||
| Aeromonas spp. | 1 | (1.1) | 1 | (1.8) | ||||||||||
| Burkholderia pseudomallei | 1 | (1.1) | 1 | (1.8) | ||||||||||
| Fungus | ||||||||||||||
| Candida albicans | 1 | (1.1) | 1 | (4.0) | ||||||||||
| Polymicrobial | 1 | (1.1) | 1 | (1.8) | ||||||||||
| Tissue culture | 94 | 25 | 50 | 7 | 6 | 4 | 2 | |||||||
| Gram-positive | ||||||||||||||
| MSSA | 26 | (27.7) | 7 | (28.0) | 19 | (38.0) | ||||||||
| MRSA | 14 | (14.9) | 6 | (24.0) | 7 | (14.0) | 1 | (16.7) | ||||||
| CoNS | 3 | (3.2) | 3 | (6.0) | ||||||||||
| GBS | 2 | (2.1) | 1 | (4.0) | 1 | (2.0) | ||||||||
| Enterococcus faecalis | 1 | (1.1) | 1 | (2.0) | ||||||||||
| Streptococcus intermedius | 1 | (1.1) | 1 | (2.0) | ||||||||||
| Viridans streptococcus | 1 | (1.1) | 1 | (4.0) | ||||||||||
| Gram-negative | ||||||||||||||
| Escherichia coli | 10 | (10.6) | 5 | (10.0) | 2 | (28.6) | 2 | (33.3) | 1 | (25.0) | ||||
| Klebsiella pneumoniae | 7 | (7.4) | 4 | (16.0) | 3 | (6.0) | ||||||||
| Salmonella enterica serogroup B | 2 | (2.1) | 2 | (4.0) | ||||||||||
| Salmonella enterica serogroup D | 1 | (1.1) | 1 | (2.0) | ||||||||||
| Bacteroides fragilis | 1 | (1.1) | 1 | (2.0) | ||||||||||
| Pseudomonas aeruginosa CR strain | 1 | (1.1) | 1 | (2.0) | ||||||||||
| Serratia marcescens | 1 | (1.1) | 1 | (2.0) | ||||||||||
| Polymicrobial | 22 | (23.4) | 6 | (24.0) | 3 | (6.0) | 5 | (71.4) | 3 | (50.0) | 3 | (75.0) | 2 | (100.0) |
| Tuberculosis | 1 | (1.1) | 1 | (2.0) | ||||||||||
| No. | Age | Sex | Underlying Diseases | Clinical Presentation | Blood Culture | Pus Culture | Location | Percutaneous Drainage | Surgical Debridement | Antibiotics Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | M | DM, HTN, ESRD, gout, HIVD | abdominal pain | N/A | Mycobacterium tuberculosis complex | L | Y | Y | Teicoplanin + Ceftriaxone (10), Piperacillin/Tazobactam + Daptomycin (8), Teicoplanin + Piperacillin/Tazobactam (7), Rifinah + Pyrazinamide (26), Teicoplanin + Cefepime (5) |
| 2 | 50 | F | cirrhosis | fever, abdominal pain, seizure | N/A | N/A | R | N | N | Piperacillin/Tazobactam (5) |
| 3 | 87 | F | HTN, asthma | flank/back pain, limp, cold sweating, chillness, SOB, conscious unclear, urine/stool retention | MSSA | N/A | B | N | N | Teicoplanin + Cefoperazone/Sulbactam + Clindamycin (2), Cefepime + Vancomycin + Metronidazole (3) |
| 4 | 81 | F | dementia, bedridden, s/p pacemaker | fever, malaise | MSSA | MSSA | L | Y | N | Piperacillin/Tazobactam (1), Cefazolin + Ciprofloxacin (6), Vancomycin + Piperacillin/Tazobactam (4), Oxacillin (9) |
| 5 | 87 | F | CHF, CKD | fever, conscious drowsy | N/A | N/A | R | Y | N | Piperacillin/Tazobactam (21), Levofloxacin (3), Cefepime (7), Tigecycline |
| 6 | 74 | M | DM | fever, abdominal pain, malaise, anorexia, body weight loss, disoriented | N/A | Klebsiella pneumoniae | L | N | N | Ceftriaxone + Metronidazole (6), Teicoplanin + Imipenem/Cilastatin (1) |
| 7 | 82 | M | HTN, CVA, bedridden, CO intoxication-related HIE, SDH/SAH/EDH Hx | malaise, limbs edema | MSSA | MSSA, Proteus mirabilis, Escherichia coli | B | N | N | Piperacillin/Tazobactam (6), Teicoplanin (3) |
| 8 | 66 | M | DM, HTN, hyperparathyroidism | flank/back pain, conscious disturbance, SOB | Escherichia coli | N/A | R | N | N | Piperacillin/Tazobactam + Vancomycin(1), Ertapenem (1), Doripenem + Doxycycline (9) |
| 9 | 83 | F | CHF, DM, ESRD, breast cancer, Af | abdominal pain, left calf erythema, conscious drowsy | MSSA | MSSA | L | Y | N | Imipenem/Cilastatin + Vancomycin (5), Oxacillin (8), Ceftriaxone(5), Ertapenem (2) |
| 10 | 83 | M | CHF, HTN, CKD, gout | flank/back pain, limp | MRSA | N/A | B | N | N | Ertapenem(2), Daptomycin(2), Teicoplanin + Cefepime(10), Teicoplanin (4), Teicoplanin + Cefepime (3), Teicoplanin + Levofloxacin (8), Vancomycin (23), Vancomycin + Meropenem (3), Ertapenem (5), Daptomycin + Meropenem (15), Levofloxacin + Micafungin + Daptomycin (8), Piperacillin/Tazobactam+ Amikacin + Amphotericin B (4) |
| 11 | 89 | F | CHF, VHD, Af | flank/back pain, limp | N/A | Escherichia coli, Bacteroides fragilis, Streptococcus anginosus, Bacteroides thetaiotaomicron, Yeast-like | R | Y | Y | Piperacillin/Tazobactam (11), Ampicillin + Sulbactam (10), Piperacillin/Tazobactam (3) |
| 12 | 49 | M | oropharyngeal cancer | flank/back pain, limp, malaise | MSSA | Escherichia coli, Enterococcus faecium, Bacteroides fragilis, Bacteroides thetaiotaomicron | B | Y | Y | Cefoperazone/Sulbactam + Vancomycin (2), Meropenem + Teicoplanin (2), Doxycycline + Ceftriaxone + Vancomycin (3), Oxacillin (12), Flomoxef (3), Ceftriaxone (6), Ertapenem (11), Ciprofloxacin (6), Teicoplanin (4), Ceftazidime (5), Teicoplanin + Ampicillin + Sulbactam + Doripenem (4) Oxacillin + Doripenem (5), Oxacillin + Metronidazole (10), Oxacillin + Piperacillin/Tazobactam (14) |
| 13 | 81 | M | asthma | abdominal pain | Peptostreptococcus sp | Bacteroides sp, Pseudomonas aeruginosa | R | Y | N | Ceftriaxone, Metronidazole (1) |
| Univariate | Multivariate (Model A) | Multivariate (Model B) | ||||
|---|---|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Age > 65 (year) | 6.68 (1.48, 30.16) | 0.013 | 5.12 (1.03,25.53) | 0.046 * | 6.06 (1.22,30.23) | 0.028 * |
| Male | 0.62 (0.21, 1.84) | 0.389 | ||||
| BT > 38 or <36 °C | 0.48 (0.11, 2.18) | 0.343 | ||||
| Heart rate > 100 (beats/min) | 1.08 (0.36, 3.22) | 0.887 | ||||
| SBP < 100 (mmHg) | 1.18 (0.26, 5.29) | 0.836 | ||||
| RR > 22 (breaths/min) | 4.98 (1.63, 15.23) | 0.005 | 1.41 (0.34, 5.95) | 0.637 | ||
| Septic shock | 71.49 (9.28, 550.63) | <0.001 | 61.90 (7.37, 519.46) | <0.001 * | ||
| Renal replacement therapy | 6.87 (2.31, 20.44) | 0.001 | 3.81 (0.94, 15.49) | 0.062 | 1.21 (0.39, 3.73) | 0.745 |
| Bedridden status | 4.42 (1.22, 16.07) | 0.024 | 2.37 (0.56, 10.08) | 0.243 | 1.37 (0.33, 5.70) | 0.669 |
| Gas-forming abscess | 2.44 (0.80, 7.44) | 0.119 | ||||
| Hypertension | 0.69 (0.23, 2.11) | 0.517 | ||||
| Diabetes mellitus | 0.73 (0.23, 2.38) | 0.604 | ||||
| Congestive heart failure | 9.20 (2.82, 29.97) | <0.001 | 5.13 (1.29, 20.45) | 0.021 * | 2.04 (0.51, 8.10) | 0.313 |
| Coronary artery disease | 0.87 (0.11, 6.70) | 0.895 | ||||
| Chronic kidney disease | 1.99 (0.61, 6.46) | 0.253 | ||||
| HIV | 0.31 (0.04, 2.24) | 0.244 | ||||
| WBC > 11 (103/μL) | 0.52 (0.17, 1.54) | 0.234 | ||||
| Hb < 8 (g/dL) | 2.33 (0.64, 8.48) | 0.198 | ||||
| Plt < 150 (103/μL) | 4.94 (1.66, 14.71) | 0.004 | 9.26 (2.59, 33.09) | 0.001 * | ||
| IPA origin | ||||||
| Primary origin | Reference | |||||
| Skeletal origin | 0.42 (0.11, 1.55) | 0.190 | ||||
| Intra-abdominal origin | 0.81 (0.09, 6.92) | 0.846 | ||||
| Urinary tract origin | 2.42 (0.47, 12.46) | 0.292 | ||||
| Soft tissue origin | 1.76 (0.21, 15.05) | 0.607 | ||||
| Cardiovascular origin | 0.55 (0.03, 11.04) | 0.985 | ||||
| Treatment | ||||||
| Antibiotics alone | Reference | |||||
| Percutaneous drainage | 0.55 (0.11, 2.74) | 0.467 | ||||
| Surgery | 0.51 (0.16, 1.67) | 0.266 | ||||
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95%CI) | p Value | OR (95%CI) | p Value | |
| Age > 65 (year) | 1.63 (0.89, 2.96) | 0.112 | ||
| Male | 1.06 (0.57, 1.96) | 0.866 | ||
| BT > 38 or <36 °C | 0.99 (0.51, 1.93) | 0.975 | ||
| Heart rate > 100 (beats/min) | 1.21 (0.67, 2.20) | 0.521 | ||
| SBP < 100 (mmHg) | 0.82 (0.34, 1.95) | 0.645 | ||
| RR > 22 (breaths/min) | 2.86 (1.10, 7.40) | 0.031 | 3.33 (1.09, 10.20) | 0.035 * |
| Renal replacement therapy | 2.08 (0.82, 5.30) | 0.125 | ||
| Bedridden status | 0.83 (0.25, 2.71) | 0.754 | ||
| Gas-forming abscess | 2.45 (1.32, 4.54) | 0.004 | 2.15 (1.03, 4.46) | 0.041 * |
| Hypertension | 0.90 (0.49, 1.62) | 0.716 | ||
| Diabetes mellitus | 1.42 (0.77, 2.63) | 0.258 | ||
| Congestive heart failure | 1.50 (0.39, 5.77) | 0.558 | ||
| Coronary artery disease | 1.85 (0.63, 5.45) | 0.262 | ||
| Chronic kidney disease | 3.79 (1.64, 8.77) | 0.002 | 5.23 (1.91, 14.31) | 0.001 * |
| HIV | 1.18 (0.16, 8.55) | 0.872 | ||
| WBC > 11 (103/μL) | 1.50 (0.78, 2.85) | 0.222 | ||
| Hb < 8 (g/dL) | 1.34 (0.54, 3.33) | 0.534 | ||
| Plt < 150 (103/μL) | 1.87 (0.89, 3.92) | 0.099 | ||
| IPA origin | ||||
| Primary origin | Reference | Reference | ||
| Skeletal origin | 3.82 (1.82, 8.05) | <0.001 | 4.92 (1.98, 12.22) | 0.001 * |
| Intra-abdominal origin | 0.23 (0.03, 1.98) | 0.183 | 0.24 (0.03, 2.18) | 0.202 |
| Urinary tract origin | 2.06 (0.48, 8.79) | 0.330 | 2.05 (0.38, 11.01) | 0.402 |
| Soft tissue origin | 1.29 (0.21, 7.83) | 0.785 | 2.52 (0.37, 17.08) | 0.343 |
| Cardiovascular origin | 6.43 (1.12, 37.07) | 0.037 | 7.18 (0.90, 57.51) | 0.063 |
| Treatment | ||||
| Antibiotics alone | Reference | Reference | ||
| Percutaneous drainage | 2.95 (1.20, 7.26) | 0.019 | 2.95 (1.01, 8.62) | 0.048 * |
| Surgery | 2.51 (1.24, 5.09) | 0.011 | 3.77 (1.58, 8.99) | 0.003 * |
| Univariate | ||
|---|---|---|
| HR (95%CI) | p Value | |
| Age > 65 (year) | 1.68 (0.34, 1.37) | 0.279 |
| Male | 0.89 (0.44, 1.79) | 0.733 |
| BT > 38 or <36 °C | 0.56 (0.23, 1.36) | 0.200 |
| Heart rate > 100 (beats/min) | 1.07 (0.55, 2.11) | 0.840 |
| SBP < 100 (mmHg) | 1.48 (0.61, 3.58) | 0.384 |
| RR > 22 (breaths/min) | 1.07 (0.38, 3.04) | 0.903 |
| Renal replacement therapy | 1.03 (0.36, 2.92) | 0.958 |
| Bedridden status | 2.40 (0.73, 7.88) | 0.148 |
| Gas-forming abscess | 1.15 (0.58, 2.26) | 0.690 |
| Hypertension | 1.10 (0.56, 2.15) | 0.786 |
| Diabetes mellitus | 1.14 (0.58, 2.25) | 0.698 |
| Congestive heart failure | 0.69 (0.09, 5.05) | 0.715 |
| Coronary artery disease | 2.14 (0.88, 5.16) | 0.092 |
| Chronic kidney disease | 0.56 (196, 1.58) | 0.271 |
| HIV | 4.69 (1.41, 15.57) | 0.012 * |
| WBC > 11 (103/μL) | 0.84 (0.41, 1.72) | 0.626 |
| Hb < 8 (g/dL) | 0.61 (0.15, 2.56) | 0.501 |
| Plt < 150 (103/μL) | 1.71 (0.80, 3.67) | 0.168 |
| IPA origin | ||
| Primary origin | Reference | |
| Skeletal origin | 0.73 (0.32, 1.70) | 0.466 |
| Intra-abdominal origin | 2.21 (0.72, 6.75) | 0.166 |
| Urinary tract origin | 1.12 (0.24, 5.31) | 0.884 |
| Soft tissue origin | 0.03 (0.002, 0.53) | 0.978 |
| Cardiovascular origin | 1.44 (0.30, 6.80) | 0.647 |
| Treatment | ||
| Antibiotics alone | Reference | |
| Percutaneous drainage | 0.66 (0.23, 1.94) | 0.454 |
| Surgery | 1.02 (0.48, 2.20) | 0.952 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-C.; Li, J.-J.; Hsiao, C.-H.; Yen, C.-C. Clinical Characteristics and In-Hospital Outcomes in Patients with Iliopsoas Abscess: A Multicenter Study. J. Clin. Med. 2023, 12, 2760. https://doi.org/10.3390/jcm12082760
Lee Y-C, Li J-J, Hsiao C-H, Yen C-C. Clinical Characteristics and In-Hospital Outcomes in Patients with Iliopsoas Abscess: A Multicenter Study. Journal of Clinical Medicine. 2023; 12(8):2760. https://doi.org/10.3390/jcm12082760
Chicago/Turabian StyleLee, Yi-Chih, Jhih-Jin Li, Chien-Han Hsiao, and Chieh-Ching Yen. 2023. "Clinical Characteristics and In-Hospital Outcomes in Patients with Iliopsoas Abscess: A Multicenter Study" Journal of Clinical Medicine 12, no. 8: 2760. https://doi.org/10.3390/jcm12082760





