A New Homo-Hexamer Mn-Containing Catalase from Geobacillus sp. WCH70
Abstract
:1. Introduction
2. Results
2.1. DNA Sequence and Phylogenetic Analysis of ACS24898.1
2.2. Gene Cloning Expression and Purification of Recombinant Enzyme
2.3. Manganese Catalase Identification
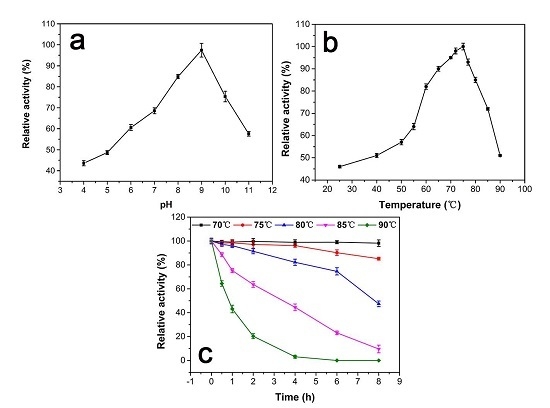
2.4. Characteristics of Purified Recombinant Catalase GWC
2.4.1. pH Effect on Catalase Activity
2.4.2. Temperature Effect and Thermostability Testing
2.4.3. Kinetic Parameter Determination
3. Discussion
4. Materials and Methods
4.1. Amino Acid Sequences Analysis
4.2. Expression Vector Construction
4.3. Expression and Purification of Recombinant Enzyme
4.4. Metal Analysis and Molecular Size Determination
4.5. Enzyme Activity Characterization
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oscar, L.; Oscar, C.B. Physiological Studies of Connecticut Leaf Tobacco; United States Department of Agriculture: Washington, DC, USA, 1900; Volume 56, pp. 5–57.
- Qin, J.; Lu, M.-X.; Zheng, Y.-T.; Du, Y.-Z. Molecular cloning, characterization, and functional analysis of catalase in Frankliniella occidentalis (Thysanoptera: Thripidae). Ann. Entomol. Soc. Am. 2017, 110, 212–220. [Google Scholar]
- Wang, H.-X.; Tokusige, Y.; Shinoyama, H.; Fujii, T.; Urakami, T. Purification and characterization of a thermostable catalase from culture broth of Thermoascus aurantiacus. J. Ferment. Bioeng. 1998, 85, 169–173. [Google Scholar] [CrossRef]
- Sharif, A.; Ashraf, M.; Javeed, A.; Anjum, A.A.; Akhtar, M.F.; Akhtar, B.; Saleem, A. Oxidative stress responses in Wistar rats on subacute exposure to pharmaceutical wastewater. Environ. Sci. Pollut. Res. 2016, 23, 24158–24165. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-W.; Cai, Y.-J.; Liao, X.-R.; Qian, S.-L.; Zhang, F.; Zhang, D.-B. Optimization of catalase production and purification and characterization of a novel cold-adapted Cat-2 from mesophilic bacterium Serratia marcescens SYBC-01. Ann. Microbiol. 2010, 60, 701–708. [Google Scholar] [CrossRef]
- Paar, A.; Costa, S.; Tzanov, T.; Gudelj, M.; Robra, K.H.; Cavaco-Paulo, A.; Gübitz, G.M. Thermo-alkali-stable catalases from newly isolated Bacillus sp. for the treatment and recycling of textile bleaching effluents. J. Biotechnol. 2001, 89, 147–153. [Google Scholar] [CrossRef]
- Tzanov, T.; Costa, S.; Guebitz, G.M.; Cavaco-Paulo, A. Dyeing in catalase-treated bleaching baths. Color. Technol. 2010, 117, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.-H.; Wang, W.; Hao, J.-H.; Zhu, X.-L.; Sun, M. Purification and characterization of catalase from marine bacterium Acinetobacter sp. YS0810. BioMed Res. Int. 2014, 2014, 409626. [Google Scholar] [CrossRef] [PubMed]
- Josef, D.; Wolfgang, S. The mechanism of hydrogen peroxide bleaching. Text. Chem. Color. 1996, 28, 24–28. [Google Scholar]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, A.G. Catalase immobilization—A review. Biochem. Eng. J. 2017, 117, 1–20. [Google Scholar] [CrossRef]
- Pradhan, A.; Herrero-de-Dios, C.; Belmonte, R.; Budge, S.; Garcia, A.L.; Kolmogorova, A.; Lee, K.K.; Martin, B.D.; Ribeiro, A.; Bebes, A.; et al. Elevated catalase expression in a fungal pathogen is a double-edged sword of iron. PLoS Pathog. 2017, 13, e1006405. [Google Scholar] [CrossRef] [PubMed]
- Switala, J.; Loewen, P.C. Diversity of properties among catalases. Arch. Biochem. Biophys. 2002, 401, 145–154. [Google Scholar] [CrossRef]
- Jia, X.-B.; Chen, J.-C.; Lin, C.-Q.; Lin, X.-J. Cloning, expression, and characterization of a novel thermophilic monofunctional catalase from Geobacillus sp. CHB1. BioMed Res. Int. 2016, 2016, 7535604. [Google Scholar] [CrossRef] [PubMed]
- Thompson, V.S.; Schaller, K.D.; Apel, W.A. Purification and characterization of a novel thermo-alkali-stable catalase from Thermus brockianus. Biotechnol. Prog. 2003, 19, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Ebara, S.; Shigemori, Y. Alkali-tolerant high-activity catalase from a thermophilic bacterium and its overexpression in Escherichia coli. Protein Expr. Purif. 2008, 57, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-X.; Zheng, H.-C.; Zhao, X.-Y.; Li, S.-F.; Xu, J.-Y.; Song, H. High level extracellular production of a recombinant alkaline catalase in E. coli BL21 under ethanol stress and its application in hydrogen peroxide removal after cotton fabrics bleaching. Bioresour. Technol. 2016, 214, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.W. Non-heme manganese catalase—The ‘other’ catalase. Arch. Biochem. Biophys. 2011, 525, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Fridovich, I. Isolation and characterization of the pseudocatalase of Lactobacillus plantarum. J. Biol. Chem. 1983, 258, 6015–6019. [Google Scholar] [PubMed]
- Barynin, V.V.; Grebenko, A.I. T-catalase is a nonheme catalase of the extremally thermophilic bacterium Thermus thermophilus HB8. Dokl. Akad. Nauk SSSR 1986, 286, 461–464. [Google Scholar]
- Kagawa, M.; Murakoshi, N.; Nishikawa, Y.; Matsumoto, G.; Kurata, Y.; Mizobata, T.; Kawata, Y.; Nagai, J. Purification and cloning of a thermostable manganese catalase from a thermophilic bacterium. Arch. Biochem. Biophys. 1999, 362, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Allgood, G.S.; Perry, J.J. Characterization of a manganese-containing catalase from the obligate thermophile Thermoleophilum album. J. Bacteriol. 1986, 168, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Amo, T.; Atomi, H.; Imanaka, T. Unique presence of a manganese catalase in a hyperthermophilic archaeon, Pyrobaculum calidifontis VA1. J. Bacteriol. 2002, 184, 3305–3312. [Google Scholar] [CrossRef] [PubMed]
- Bihani, S.C.; Chakravarty, D.; Ballal, A. KatB, a cyanobacterial Mn-catalase with unique active site configuration: Implications for enzyme function. Free Radic. Biol. Med. 2016, 93, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Baginski, R.; Sommerhalter, M. A manganese catalase from Thermomicrobium roseum with peroxidase and catecholase activity. Extremophiles 2016, 21, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-L.; Feng, M.-Q.; Zhao, Y.-J.; Guo, X.; Zhou, P. Overexpression, purification and characterization of a recombinant secretary catalase from Bacillus subtilis. Biotechnol. Lett. 2008, 30, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-L.; Feng, M.-Q.; Shi, J.; Shi, Z.-H.; Zhong, J.; Zhou, P. High-level expression and purification of recombinant human catalase in Pichia pastoris. Protein Expr. Purif. 2007, 54, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Gellissen, G.; Piontek, M.; Dahlems, U.; Jenzelewski, V.; Gavagan, J.E.; Dicosimo, R.; Anton, D.L.; Janowicz, Z.A. Recombinant Hansenula polymorpha as a biocatalyst: Coexpression of the spinach glycolate oxidase (GO) and the S. cerevisiae catalase T (CTT1) gene. Appl. Microbiol. Biotechnol. 1996, 46, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Rochat, T.; Miyoshi, A.; Gratadoux, J.J.; Duwat, P.; Sourice, S.; Azevedo, V.; Langella, P. High-level resistance to oxidative stress in Lactococcus lactis conferred by Bacillus subtilis catalase KatE. Microbiology 2005, 151, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Betancor, L.; Moreno, R.; Zafra, O.; Cava, F.; Fernández-Lafuente, R.; Guisán, J.M.; Berenguer, J. Thermus thermophilus as a cell factory for the production of a thermophilic Mn-dependent catalase which fails to be synthesized in an active form in Escherichia coli. Appl. Environ. Microbiol. 2004, 70, 3839–3844. [Google Scholar] [CrossRef] [PubMed]
- Brumm, P.J.; Land, M.L.; Mead, D.A. Complete genome sequences of Geobacillus sp. WCH70, a thermophilic strain isolated from wood compost. Stand. Genom. Sci. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.-B.; Zhang, X.-H.; Chen, J.-X.; Chi, Z.-H.; Sun, B.-G.; Li, Y.; Austin, B. Overexpression, purification, characterization, and pathogenicity of Vibrio harveyi hemolysin VHH. Infect. Immun. 2006, 74, 6001–6005. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-W.; Cai, Y.-J.; Liao, X.-R.; Zhang, F.; Zhang, D.-B. Production, characterization, cloning and sequence analysis of a monofunctional catalase from Serratia marcescens SYBC08. J. Basic Microbiol. 2011, 51, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, H.; Yoshimune, K.; Matsuyma, H.; Yumoto, I. Characterization of catalase from psychrotolerant Psychrobacter piscatorii T-3 exhibiting high catalase activity. Int. J. Mol. Sci. 2012, 13, 1733–1746. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, M.S.; Moe, E.; Jouve, H.M.; Willassen, N.P. Cold adapted features of Vibrio salmonicida catalase: Characterisation and comparison to the mesophilic counterpart from Proteus mirabilis. Extremophiles 2006, 10, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Yumoto, I.; Ichihashi, D.; Iwata, H.; Istokovics, A.; Ichise, N.; Matsuyama, H.; Okuyama, H.; Kawasaki, K. Purification and characterization of a catalase from the facultatively psychrophilic bacterium Vibrio rumoiensis S-1T exhibiting high catalase activity. J. Bacteriol. 2000, 182, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Phucharoen, K.; Hoshino, K.; Takenaka, Y.; Shinozawa, T. Purification, characterization, and gene sequencing of a catalase from an alkali- and halo-tolerant bacterium, Halomonas sp. SK1. Biosci. Biotechnol. Biochem. 2002, 66, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, M.; Liu, W.-S.; Zhang, B. Purification and characterization of a psychrophilic catalase from Antarctic Bacillus. Can. J. Microbiol. 2008, 54, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [PubMed]
- Hildebrandt, A.G.; Roots, I. Reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent formation and breakdown of hydrogen peroxide during mixed function oxidation reactions in liver microsomes. Arch. Biochem. Biophys. 1975, 171, 385–397. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]




| Method | Total Activity (U) | Total Protein (mg) | Sp Act (Specific Activity) (U/mg) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Crude extract | 116,800 | 317 | 368 | 100 | 1 |
| Heat treatment 1 | 87,200 | 128 | 681 | 75 | 2 |
| Ni-NTA (Ni2+ and tris (carboxymethyl) | 62,700 | 10 | 6270 | 54 | 17 |
| Heat treatment 2 | 60,600 | 4 | 15,150 | 52 | 41 |
| Mn-Catalase | kcat (s−1·subunit−1) | Km (mM) | kcat/Km (M−1 s−1) | Reference |
|---|---|---|---|---|
| Lactobacillus plantarum | 3.3 × 104 | 350 | 9.4 × 104 | [19] |
| Thermus thermophiles | 2.6 × 105 | 83 | 3.1 × 106 | [20] |
| Thermoleophilum album | 6.2 × 103 | 15 | 4.1 × 105 | [22] |
| Pyrobaculum caldifontis | 2.9 × 104 | 170 | 1.7 × 105 | [23] |
| Anabaena PCC7120 | 2.23 × 104 | 1.63 | 1.35 × 107 | [24] |
| Thermomicrobium roseum | 2.02 × 104 | 20 | 1.01 × 106 | [25] |
| Geobacillus sp. WCH70 | 2.9 × 104 | 67.26 | 4.22 × 105 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-C.; Yu, Q.; Wang, H.; Cao, X.-Y.; Ma, L.; Li, Z.-Q. A New Homo-Hexamer Mn-Containing Catalase from Geobacillus sp. WCH70. Catalysts 2017, 7, 277. https://doi.org/10.3390/catal7090277
Li H-C, Yu Q, Wang H, Cao X-Y, Ma L, Li Z-Q. A New Homo-Hexamer Mn-Containing Catalase from Geobacillus sp. WCH70. Catalysts. 2017; 7(9):277. https://doi.org/10.3390/catal7090277
Chicago/Turabian StyleLi, Hai-Chao, Qing Yu, Hui Wang, Xin-Yu Cao, Li Ma, and Zheng-Qiang Li. 2017. "A New Homo-Hexamer Mn-Containing Catalase from Geobacillus sp. WCH70" Catalysts 7, no. 9: 277. https://doi.org/10.3390/catal7090277
APA StyleLi, H.-C., Yu, Q., Wang, H., Cao, X.-Y., Ma, L., & Li, Z.-Q. (2017). A New Homo-Hexamer Mn-Containing Catalase from Geobacillus sp. WCH70. Catalysts, 7(9), 277. https://doi.org/10.3390/catal7090277