Standard Bismuth Quadruple Therapy versus Concomitant Therapy for the First-Line Treatment of Helicobacter pylori Infection: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
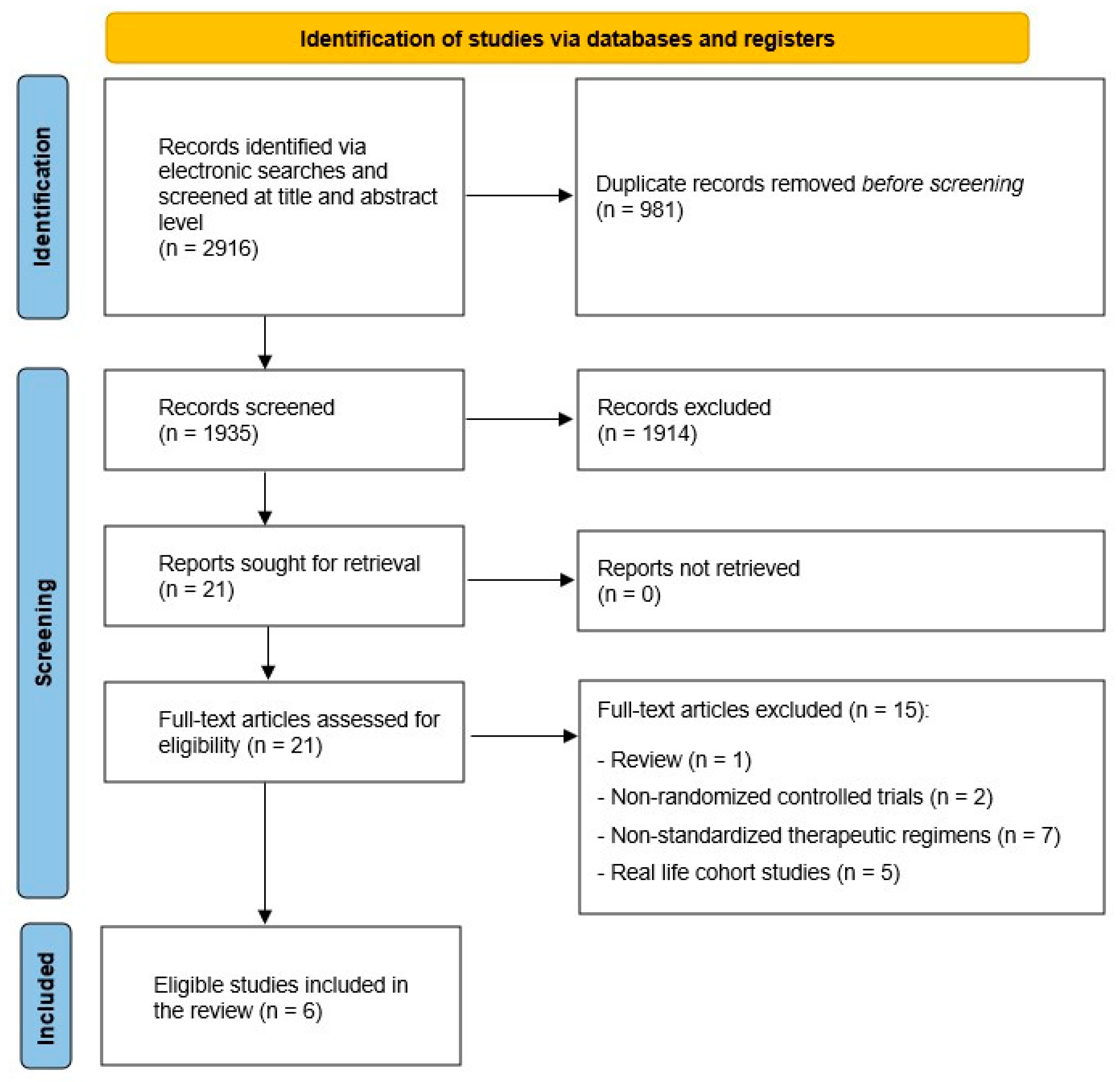
2.1. Search Strategy and Study Selection
2.2. Data Extraction
2.3. Assessing the Risk of Bias
2.4. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Risk of Bias
3.3. Eradication of H. pylori Infection
3.4. Adverse Events and Compliance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Crowe, S. Helicobacter pylori Infection. Solomon CG. N. Engl. J. Med. 2019, 380, 1158–1165. [Google Scholar] [CrossRef]
- Gravina, A.; Zagari, R.; De Musis, C.; Romano, L.; Loquercio, C.; Romao, M. Helicobacter pylori and extragastric diseases: A review. World J. Gastroenterol. 2018, 24, 3204–3221. [Google Scholar] [CrossRef]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.Y.; Crowe, S.E.; Valasek, M.A. Review article: The global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef]
- Fallone, C.A.; Moss, S.F.; Malfertheiner, P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology 2019, 157, 44–53. [Google Scholar] [CrossRef] [PubMed]
- van der Hulst, R.; Keller, J.; Rauws, E.; Tytgat, G.N. Treatment of Helicobacter pylori infection: A review of the world literature. Helicobacter 1996, 1, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Bazzoli, F.; Delchier, J.-C.; Celiñski, K.; Giguère, M.; Rivière, M.; Mégraud, F. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: A randomised, open-label, non-inferiority, phase 3 trial. Lancet 2011, 377, 905–913. [Google Scholar] [CrossRef]
- Liou, J.-M.; Fang, Y.-J.; Chen, C.-C.; Bair, M.-J.; Chang, C.-Y.; Lee, Y.-C.; Chen, M.-J.; Chen, C.-C.; Tseng, C.-H.; Hsu, Y.-C.; et al. Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: A multicentre, open-label, randomised trial. Lancet 2016, 388, 2355–2365. [Google Scholar] [CrossRef]
- Nyssen, O.; Bordin, D.; Tepes, B.; Pérez-Aisa, Á.; Vaira, D.; Caldas, M.; Bujanda, L.; Castro-Fernandez, M.; Lerang, F.; Leja, M.; et al. European Registry on Helicobacter pylori management (Hp-EuReg): Patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21,533 patients. Gut 2021, 70, 40–54. [Google Scholar] [CrossRef]
- Zagari, R.; Romiti, A.; Ierardi, E.; Gravina, A.G.; Panarese, A.; Grande, G.; Savarino, E.; Maconi, G.; Stasi, E.; Eusebi, L.H.; et al. The “three-in-one” formulation of bismuth quadruple therapy for Helicobacter pylori eradication with or without probiotics supplementation: Efficacy and safety in daily clinical practice. Helicobacter 2018, 23, e12502. [Google Scholar] [CrossRef]
- Romano, M.; Gravina, A.G.; Nardone, G.; Federico, A.; Dallio, M.; Martorano, M.; Mucherino, C.; Romiti, A.; Avallone, L.; Granata, L.; et al. Non-bismuth and bismuth quadruple therapies based on previous clarithromycin exposure are as effective and safe in an area of high clarithromycin resistance: A real-life study. Helicobacter 2020, 25, e12694. [Google Scholar] [CrossRef]
- Macías-García, F.; Bastón-Rey, I.; de la Iglesia-García, D.; Calviño-Suárez, C.; Nieto-García, L.; Domínguez-Muñoz, J.E. Bismuth-containing quadruple therapy versus concomitant quadruple therapy as first-line treatment for Helicobacter pylori infection in an area of high resistance to clarithromycin: A prospective, cross-sectional, comparative, open trial. Helicobacter 2019, 24, e12546. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology 2016, 151, 51–69.e14. [Google Scholar] [CrossRef]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol 2017, 112, 212–238. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V.; Flemyng, E. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Cochrane: London, UK, 2022; Available online: https://training.cochrane.org/handbook (accessed on 1 October 2022).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, 14898. [Google Scholar] [CrossRef]
- Deeks Jonathan, J.; Higgins Julian, P.T.; Altman Douglas, G.; The Cochrane Statistical Methods Group. Chapter 10: Analysing Data and Undertaking Meta-Analyses. Cochrane Handbook of Systeatic Reviews of Interventions Version 6.3 (Updated February 2022). Cochrane. 2022. Available online: www.training.cochrane.org/handbook (accessed on 1 October 2022).
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Page, M.J.; Higgins, J.P.; Sterne, J.A. Chapter 13: Assessing Risk of Bias Due to Missing Results in a Synthesis. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022). Available online: https://training.cochrane.org/handbook/current/chapter-13 (accessed on 1 August 2022).
- Kefeli, A.; Basyigit, S.; Yeniova, A.O.; Kefeli, T.T.; Aslan, M.; Tanas, O. Comparison of three different regimens against Helicobacter pylori as a first-line treatment: A randomized clinical trial. Bosn. J. Basic Med. Sci. 2016, 16, 52–57. [Google Scholar] [CrossRef]
- Sezikli, M.; Sirin, G.; Cetinkaya, Z.A.; Tanoglu, A.; Guzelbulut, F.; Bunul, F.; Dindar, G. Comparison of the efficacy of six different Helicobacter pylori eradication regimens: Greater than or equal to another. Biomed. Res. 2018, 29, 1143–1148. [Google Scholar] [CrossRef]
- De Francesco, V.; Pontone, S.; Bellesia, A.; Serviddio, G.; Panetta, C.; Palma, R.; Zullo, A. Quadruple, sequential, and concomitant first-line therapies for H. pylori eradication: A prospective, randomized study. Dig Liver Dis. 2018, 50, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chung, J.-W.; Woo, H.S.; Kim, S.Y.; Kim, J.H.; Kim, Y.J.; Kim, K.O.; Kwon, K.A.; Park, D.K. Two-week bismuth-containing quadruple therapy and concomitant therapy are effective first-line treatments for Helicobacter pylori eradication: A prospective open-label randomized trial. World J. Gastroenterol. 2019, 25, 6790–6798. [Google Scholar] [CrossRef] [PubMed]
- Veliev, A.M.; Maev, I.V.; Andreev, D.N.; Dicheva, D.T.; Zaborovskii, A.V.; Lobanova, E.G.; Bektemirova, L.G. The efficacy and safety of quadruple therapy without bismuth (concomitant therapy) in the treatment of patients with Helicobacter pylori-associated gastric and duodenal peptic ulcer disease. Ter. Arkhiv 2019, 91, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Cao, N.-W.; Zhou, H.-Y.; Chu, X.-J.; Li, B.-Z. Efficacy and safety of bismuth-containing quadruple treatment and concomitant treatment for first-line Helicobacter pylori eradication: A systematic review and meta-analysis. Microb. Pathog. 2021, 152, 104661. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Hernaez, R.; Rokkas, T. Cross-roads for meta-analysis and network meta-analysis of H. pylori therapy. Gut 2022, 71, 643–650. [Google Scholar] [CrossRef]
- Liu, W.Z.; Xie, Y.; Lu, H.; Cheng, H.; Zeng, Z.R.; Zhou, L.Y.; Chen, Y.; Wang, J.B.; Du, Y.Q.; Lu, N.H.; et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter 2018, 23, e12475. [Google Scholar] [CrossRef]
- Bujanda, L.; Nyssen, O.P.; Vaira, D.; Saracino, I.M.; Fiorini, G.; Lereng, F.; Georgopoulos, S.; Tepes, B.; Heluwaert, F.; Gasbarrini, A.; et al. Antibiotic Resistance Prevalence and Trends in Patients Infected with Helicobacter pylori in the Period 2013–2020: Results of the European Registry on H. pylori Management (Hp-EuReg). Antibiotics 2021, 10, 1058. [Google Scholar] [CrossRef]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, A.J. Chapter 8: Assessing Risk of Bias in a Randomized Trial. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022). Available online: www.training.cochrane.org/handbook (accessed on 1 August 2022).




| Study, Year | Country | Total Patients, n. | Age Mean, Year | Males n, (%) | Medical Condition | Tests for H. pylori Diagnosis | Test for H. pylori Eradication | Eradication Rate (ITT) | Side Effects | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BQT n. (%) | CT n. (%) | BQT n. (%) | CT n. (%) | ||||||||
| Kefeli, 2016 [21] | Turkey | 260 | 37.3 | 138 (53.1) | PU, NUD | Histology | UBT after ≥ 6 weeks | 114/130 (87.7) | 113/130 (86.9) | 54/130 (41.5) | 52/130 (40) |
| Liou, 2016 [8] | Taiwan | 1080 | 53.3 | 534 (49.4) | PU, NUD, asymptomatic individuals | 2 out of 4: histology, RUT, culture, serology | UBT after > 6 weeks | 488/540 (90.4) | 464/540 (85.9) | 358/533 (67.2) | 309/535 (57.8) |
| Sezikli, 2018 [22] | Turkey | 70 | 41.8 | 30 (42.9) | NUD | Histology | UBT after 6–8 weeks | 20/35 (57.1) | 22/35 (62.8) | 5/35 (14.3) | 7/35 (20) |
| De Francesco, 2018 [23] | Italy | 124 | 53.6 | 55 (44.3) | PU, NUD | Histology and RUT | UBT after 6–8 weeks | 52/61 (85.2) | 60/63 (95.2) | 15/61 (24.6) | 15/63 (23.8) |
| Kim, 2019 [24] | Korea | 136 | 58.7 | 72 (52.9) | PU, NUD | Histology or RUT | UBT after ≥ 4 weeks | 60/68 (88.2) | 54/68 (79.4) | 23/68 (33.8) | 35/68 (51.5) |
| Veliev 2019 [25] | Russia | 140 | N/A | N/A | PU | RUT | UBT after ≥ 4 weeks | 56/70 (80) | 59/70 (84.2) | 14/70 (20) | 17/70 (24.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagari, R.M.; Dajti, E.; Cominardi, A.; Frazzoni, L.; Fuccio, L.; Eusebi, L.H.; Vestito, A.; Lisotti, A.; Galloro, G.; Romano, M.; et al. Standard Bismuth Quadruple Therapy versus Concomitant Therapy for the First-Line Treatment of Helicobacter pylori Infection: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 3258. https://doi.org/10.3390/jcm12093258
Zagari RM, Dajti E, Cominardi A, Frazzoni L, Fuccio L, Eusebi LH, Vestito A, Lisotti A, Galloro G, Romano M, et al. Standard Bismuth Quadruple Therapy versus Concomitant Therapy for the First-Line Treatment of Helicobacter pylori Infection: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2023; 12(9):3258. https://doi.org/10.3390/jcm12093258
Chicago/Turabian StyleZagari, Rocco Maurizio, Elton Dajti, Anna Cominardi, Leonardo Frazzoni, Lorenzo Fuccio, Leonardo Henry Eusebi, Amanda Vestito, Andrea Lisotti, Giuseppe Galloro, Marco Romano, and et al. 2023. "Standard Bismuth Quadruple Therapy versus Concomitant Therapy for the First-Line Treatment of Helicobacter pylori Infection: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 12, no. 9: 3258. https://doi.org/10.3390/jcm12093258
APA StyleZagari, R. M., Dajti, E., Cominardi, A., Frazzoni, L., Fuccio, L., Eusebi, L. H., Vestito, A., Lisotti, A., Galloro, G., Romano, M., & Bazzoli, F. (2023). Standard Bismuth Quadruple Therapy versus Concomitant Therapy for the First-Line Treatment of Helicobacter pylori Infection: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 12(9), 3258. https://doi.org/10.3390/jcm12093258







