Structure of Dihydrochalcones and Related Derivatives and Their Scavenging and Antioxidant Activity against Oxygen and Nitrogen Radical Species
Abstract
:1. Introduction


2. Results and Discussion
| Species | PhOH | PhO. | ΔEiso |
|---|---|---|---|
| au | au | Kcal/mol | |
| I | −307.478467 | −306.835385 | 0.00 |
| II | −382.698201 | −382.056648 | (−0.96) |
| III | −457.919066 | −457.275369 | 0.39 |
| IV | −460.143138 | −459.472936 | 17.02 |
| V (6) | −610.584324 | −609.945225 | (−2.50) |
| VI (6) | −535.362116 | −534.723866 | (−3.03) |
| VII (4) | −535.366132 | −534.715737 | 4.59 |
| VIII | −574.666026 | −573.991339 | 19.83 |
| IX (6) | −956.175410 | −955.537571 | (−3.29) |
| X (4’) | −1566.909704 | −1566.267755 | (−0.71) |
| Compouds | BDE (O – H) | IPin gas | IPCPCM | PON | LPO |
|---|---|---|---|---|---|
| (kcal mol-1) | (kcal mol-1) | (kcal mol-1) | IC50 | IC50 | |
| I | 81.87 | 186.66 | 137.28 | 552 | >1,000 |
| II | 80.88 | 180.29 | 131.51 | 58 | >1,000 |
| III | 82.02 | 178.14 | 133.20 | 39 | 624 |
| IV | 97.63 | 188.71 | 139.68 | >1,000 | >1,000 |
| V-OH(2) | 101.80 | - | - | - | - |
| V-OH(4) | 85.59 | - | - | - | - |
| V-OH(6) | 78.41 | 181.95 | 134.24 | 5.5 | 106 |
| VI-OH(2) | 99.76 | - | - | - | - |
| VI-OH(6) | 78.02 | 182.63 | 133.26 | 7.8 | 95 |
| VII-OH(2) | 99.58 | - | - | - | - |
| VII-OH(4) | 85.05 | 187.04 | 139.39 | >1,000 | >1,000 |
| VIII | 99.91 | 179.65 | 132.46 | >1,000 | >1,000 |
| IX-OH(2) | 98.38 | - | - | - | - |
| IX-OH(4) | 98.63 | - | - | - | - |
| IX-OH(6) | 76.32 | 167.48 | 132.86 | 3.1 | 24 |
| IX-OH(4’) | 78.63 | - | - | - | - |
| X -OH(4) | 82.50 | - | - | - | - |
| X -OH(6) | 77.40 | - | - | - | - |
| X-OH(4’) | 77.14 | 169.12 | 133.29 | 55 | 435 |

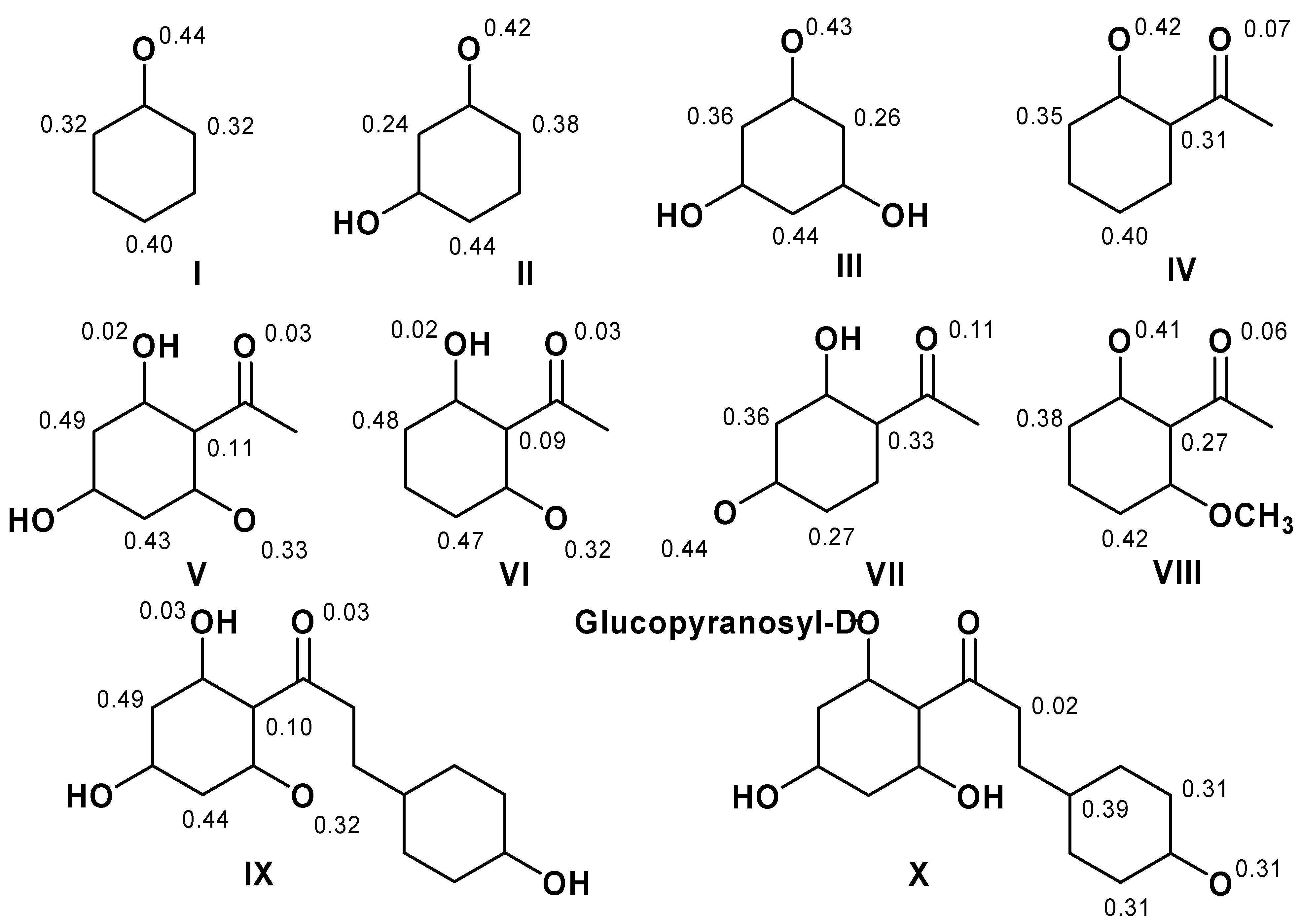
3. Computational Methods
4. Conclusions
Acknowledgements
References
- Hertog, M.G.L.; Hollman, P.C.H.; Van de Putte, B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines and fruit juices. J. Agric. Food Chem. 1993, 41, 1242–1246. [Google Scholar]
- Calliste, C.; Bail, J.L.; Trouillas, P.; Pouget, C.; Habrioux, G.; Chulia, A.; Duroux, J. Chalcones: Structural requirements for antioxidant, estrogenic and antiproliferative activities. Anticancer Res. 2001, 21, 3949–3956. [Google Scholar]
- Rezk, B.M.; Haenen, G.R.M.M.; van der Vijgh, W.J.F.; Bast, A. The antioxidant activity of phloretin: The disclosure of a new antioxidant pharmacophore in flavonoids. Biochem. Bioph. Res. Comm. 2002, 295, 9–13. [Google Scholar] [CrossRef]
- Mathiesen, L.; Malterud, K.E.; Sound, R.B. Hydrogen Bond Formation as Basis For Radical Scavenging Activity: A Structure-Activity Study of C-Methylated Dihydrochalcones from Myrica gale and Structurally Related Acetophenones. Free Radical Biol. Med. 1997, 22, 307–315. [Google Scholar] [CrossRef]
- Leopoldini, M.; Prieto Pitarch, I.; Russo, N.; Toscano, M. Structure, conformation and electronic properties of apigenin, luteolin and taxifolin antioxidants. A first principle theoretical study. J. Phys. Chem. B 2004, 108, 92–94. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds. H-atom versus electron transfer mechanism. J. Phys. Chem. B 2004, 108, 4916–4922. [Google Scholar]
- Lobo, L.T.; da Silva, G.A.; Ferreira, M.; da Silva, M.N.; Santos, A.S.; Arruda, A.C.; Guilhon, G.M.S.P.; Santos, L.S.; Borges, R.S.; Arruda, M.S.P. Dihydroflavonols from the leaves of Derris urucu (Leguminosae): Structural Elucidation and DPPH Radical-Scavenging Activity. J. Braz. Chem. Soc. 2009, 20, 1082–1088. [Google Scholar]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Density functional computations of the energetic and spectroscopic parameters of quercetin and its radicals in the gas phase and in solvent. Theor. Chem. Acc. 2004, 111, 210–216. [Google Scholar] [CrossRef]
- Janovic, S.V.; Steenken, S.; Simic, M.G.; Hara, Y. Flavonoids in Health and Disease; Rice-Evans, C., Packer, L., Eds.; Marcel Dekker: New York, NY, USA, 1998. [Google Scholar]
- Brown, J.E.; Khodr, H.; Hider, R.C.; Rice-Evans, C.A. Structural dependence of flavonoid interactions with Cu2+ ions: Implications for their antioxidant properties. Biochem. J. 1998, 330, 1173–1178. [Google Scholar]
- Van Acker, S.A.B.E.; Van den Berg, D.J.; Tromp, M.N.J.L.; Griffioen, D.H.; Van Bennekom, W.P.; Van der Vijgh, W.J.F.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radical Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, E.R.; Dilabio, G.A. Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Boots, A.W.; Li, H.; Schins, R.P.F.; Duffin, R.; Heemskerk, J.W.M.; Bast, A.; Haenen, G.R.M.M. The quercetin paradox. Toxicol. Appl. Pharmacol. 2007, 222, 89–96. [Google Scholar] [CrossRef]
- Kozlowski, D.; Troillas, P.; Calliste, C.; Marsal, P.; Lazzaroni, R.; Duroux, J.-L. Density functional theory study of the conformational, electronic, and antioxidant properties of natural chalcones. J. Phys. Chem. 2007, 111, 1138–1145. [Google Scholar]
- Santos, R.M.B.; Simões, J.A.M. Energetics of the O-H Bond in Phenol and Substituted Phenols: A Critical Evaluation of Literature Data. J. Phys. Chem. Ref. Data 1998, 27, 707–739. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S. Flavonoids as antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar] [CrossRef]
- Wang, L.F.; Zang, H.Y. Unexpected role of 5-OH in DPPH radical-scavenging activity of 4-thiaflavans. Revealed by theoretical calculations. Bioorg. Med. Chem. Lett. 2004, 14, 2609–2611. [Google Scholar] [CrossRef]
- Modak, B.; Contreras, L.; González-Nilo, F.; Torres, R. Structure-antioxidant activity relationships of flavonoids isolated from the resinous exudate of Heliotropium sinuatum. Bioorg. Med. Chem. Lett. 2005, 15, 309–312. [Google Scholar] [CrossRef]
- Burton, G.W.; Doba, T.; Gabe, E.J.; Hughes, L.; Lee, F.L.; Prasad, L.; Ingold, K.U. Autoxidation of biological molecules. 4. Maximizing the antioxidant activity of phenols. J. Am. Chem. Soc. 1985, 107, 7053–7065. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. Vitamin E: application of the principles of physical organic chemistry to the exploration of its structure and function. Acc. Chem. Res. 1986, 19, 194–201. [Google Scholar] [CrossRef]
- Spiteller, G. Linoleic acid peroxidation: the dominant lipid peroxidation process in low density lipoprotein—and its relationship to chronic diseases. Chem. Phys. Lipids 1998, 95, 105–162. [Google Scholar] [CrossRef]
- Mikulski, D.; Górniak, R.; Molski, M. A theoretical study of the structure–radical scavenging activity of trans-resveratrol analogues and cis-resveratrol in gas phase and water environment. Eur. J. Med. Chem. 2010, 45, 1015–1027. [Google Scholar] [CrossRef]
- Queiroz, A.N.; Gomes, B.A.Q.; Moraes, W.M., Jr.; Borges, R.S. A theoretical antioxidant pharmacophore for resveratrol. Eur. J. Med. Chem. 2009, 44, 1644–1649. [Google Scholar] [CrossRef]
- Diniz, J.E.M.; Borges, R.S.; Alves, C.N. A DFT study for paracetamol and 3,5-disubstituted analogues. J. Mol. Struct. (TheoChem) 2004, 673, 93–97. [Google Scholar] [CrossRef]
- Alves, C.N.; Borges, R.S.; da Silva, A.B.F. Density functional theory study of metabolic derivatives of the oxidation of paracetamol. Int. J. Quantum Chem. 2006, 106, 2617–2623. [Google Scholar] [CrossRef]
- Reis, M.; Lobato, B.; Lameira, J.; Santos, A.S.; Alves, C.N. A theoretical study of phenolic compounds with antioxidant properties. Eur. J. Med. Chem. 2007, 42, 440–446. [Google Scholar] [CrossRef]
- Freire, A.D.T.; Landivar, L.M.C.; Queiroz, A.N.; Borges, R.S. A Theoretical Study for Oxidative Metabolism of Salicylates. Journal of Computational and Theoretical Nanoscience. J. Comp. Theor. Nanosci. 2009, 6, 1140–1142. [Google Scholar] [CrossRef]
- Silva, E.R.; Queiroz, A.N.; Almeida, E.D.; Borges, R.S. A DFT Study of Aminophenol Stability. J. Comp. Theor. Nanosci. 2009, 6, 1694–1696. [Google Scholar] [CrossRef]
- Gomes, B.A.Q.; Queiroz, A.N.; Borges, R.B. Tautomerism and Radical-Scavenging Activity of Edaravone by DFT Methods. J. Comp. Theor. Nanosci. 2009, 6, 1637–1639. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A.; Stratmann, R.E.; Burant, J.C.; Dapprich, S.; Millam, J.M.; Daniels, A.D.; Kudin, K.N.; Strain, M.C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G.A.; Ayala, P.Y.; Cui, Q.; Morokuma, K.; Salvador, P.; Dannenberg, J.J.; Malick, D.K.; Rabuck, A.D.; Raghavachari, K.; Foresman, J.B.; Cioslowski, J.; Ortiz, J.V.; Baboul, A.G.; Stefanov, B.B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaroni, I.; Gomperts, R.; Martin, R.L.; Fox, D.J.; Keith, T.; Al-Laham, M.A.; Peng, C.Y.; Nanayakkara, A.; Challacombe, M.; Gill, P.M.W.; Johnson, B.; Chen, W.; Wong, M.W.; Andres, J.L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E.S.; Pople, J.A. Gaussian 03, Revision C.02; Gaussian, Inc: Wallingford, CT, USA, 2004. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar]
- Hehre, W.J.; Radom, L.; Schleyer, P.V.R.; Pople, J.A. Ab Initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Sample Availability: Contact the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bentes, A.L.A.; Borges, R.S.; Monteiro, W.R.; De Macedo, L.G.M.; Alves, C.N. Structure of Dihydrochalcones and Related Derivatives and Their Scavenging and Antioxidant Activity against Oxygen and Nitrogen Radical Species. Molecules 2011, 16, 1749-1760. https://doi.org/10.3390/molecules16021749
Bentes ALA, Borges RS, Monteiro WR, De Macedo LGM, Alves CN. Structure of Dihydrochalcones and Related Derivatives and Their Scavenging and Antioxidant Activity against Oxygen and Nitrogen Radical Species. Molecules. 2011; 16(2):1749-1760. https://doi.org/10.3390/molecules16021749
Chicago/Turabian StyleBentes, Alexandre L. A., Rosivaldo S. Borges, Waldinei R. Monteiro, Luiz G. M. De Macedo, and Cláudio N. Alves. 2011. "Structure of Dihydrochalcones and Related Derivatives and Their Scavenging and Antioxidant Activity against Oxygen and Nitrogen Radical Species" Molecules 16, no. 2: 1749-1760. https://doi.org/10.3390/molecules16021749
APA StyleBentes, A. L. A., Borges, R. S., Monteiro, W. R., De Macedo, L. G. M., & Alves, C. N. (2011). Structure of Dihydrochalcones and Related Derivatives and Their Scavenging and Antioxidant Activity against Oxygen and Nitrogen Radical Species. Molecules, 16(2), 1749-1760. https://doi.org/10.3390/molecules16021749





