Epidural and Intrathecal Drug Delivery in Rats and Mice for Experimental Research: Fundamental Concepts, Techniques, Precaution, and Application
Abstract
:1. Introduction
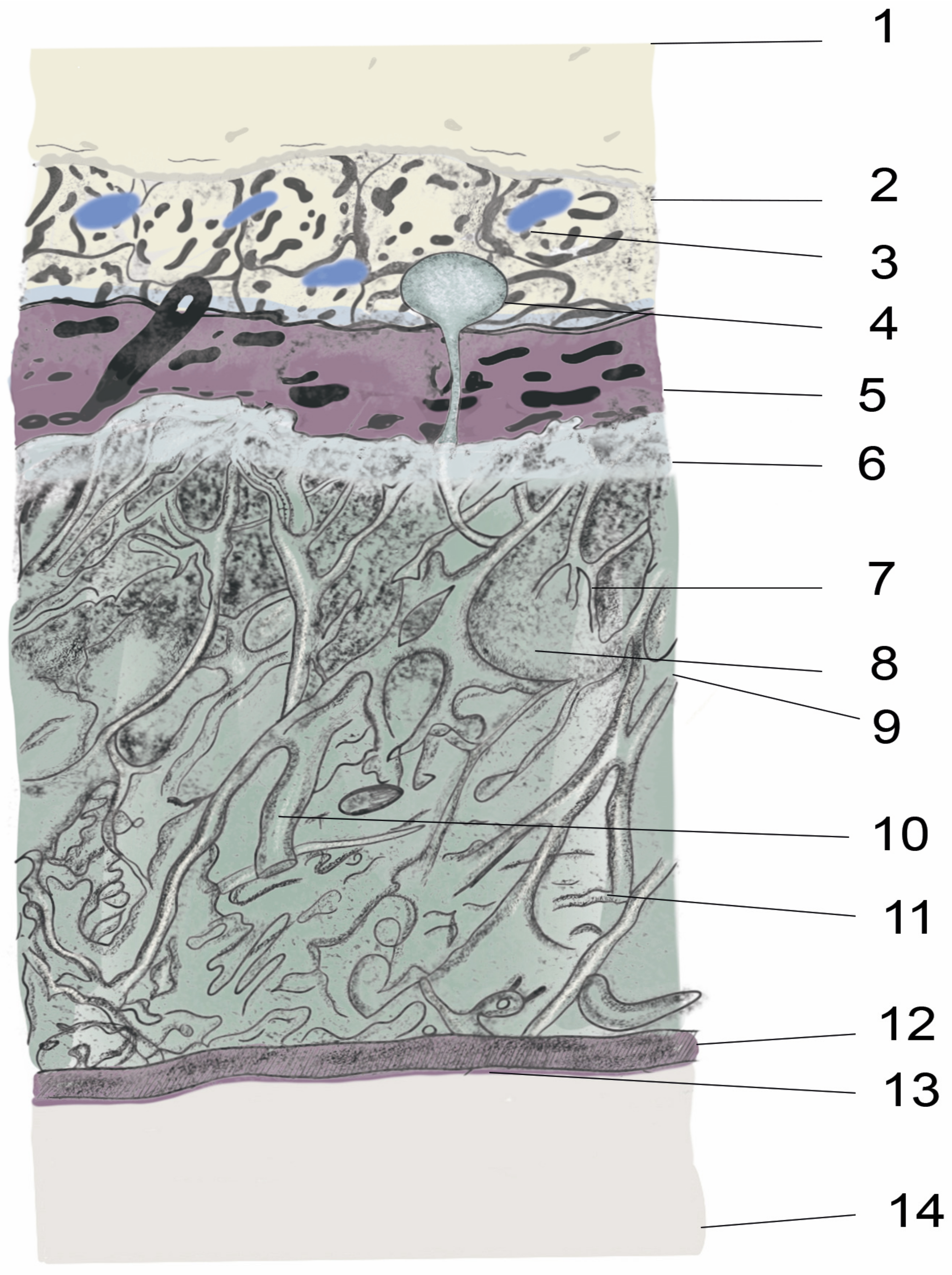
2. Anatomy and Physiology of the Spinal Meninges
2.1. Organs, Tissues from Skin to Spinal Cord
2.2. Epidural Space
2.3. Dura mater
2.4. Subdural Space
2.5. Arachnoid Mater
2.6. Subarachnoid Space/Intrathecal Space
2.7. Pia Mater
2.8. Cerebrospinal Fluid Production, Volume, and Circulation
2.8.1. CSF Production and Volume
2.8.2. CSF Circulation
3. Dorsal Root Ganglion (DRG)
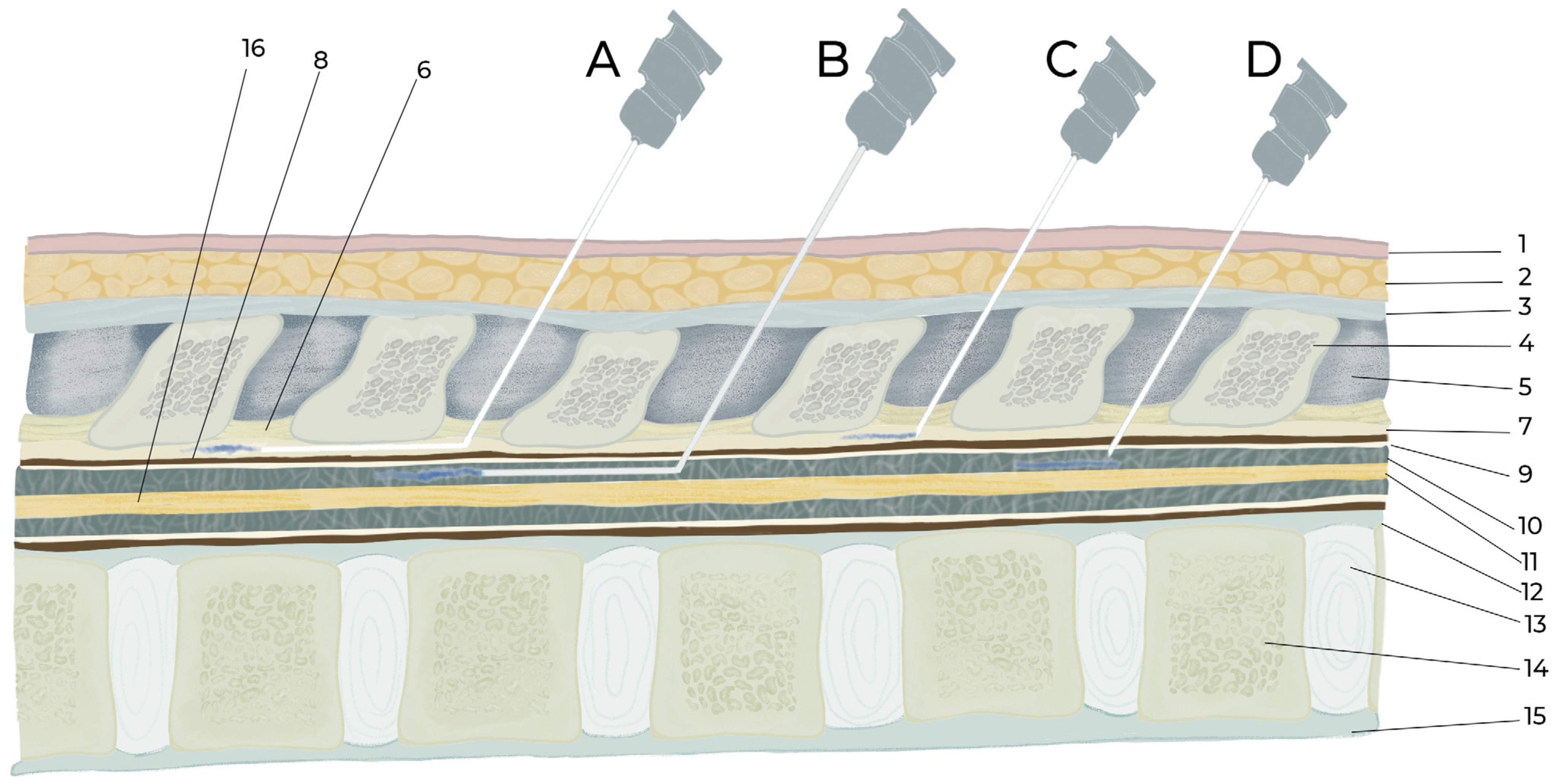
4. Epidural and Intrathecal Injection Procedures
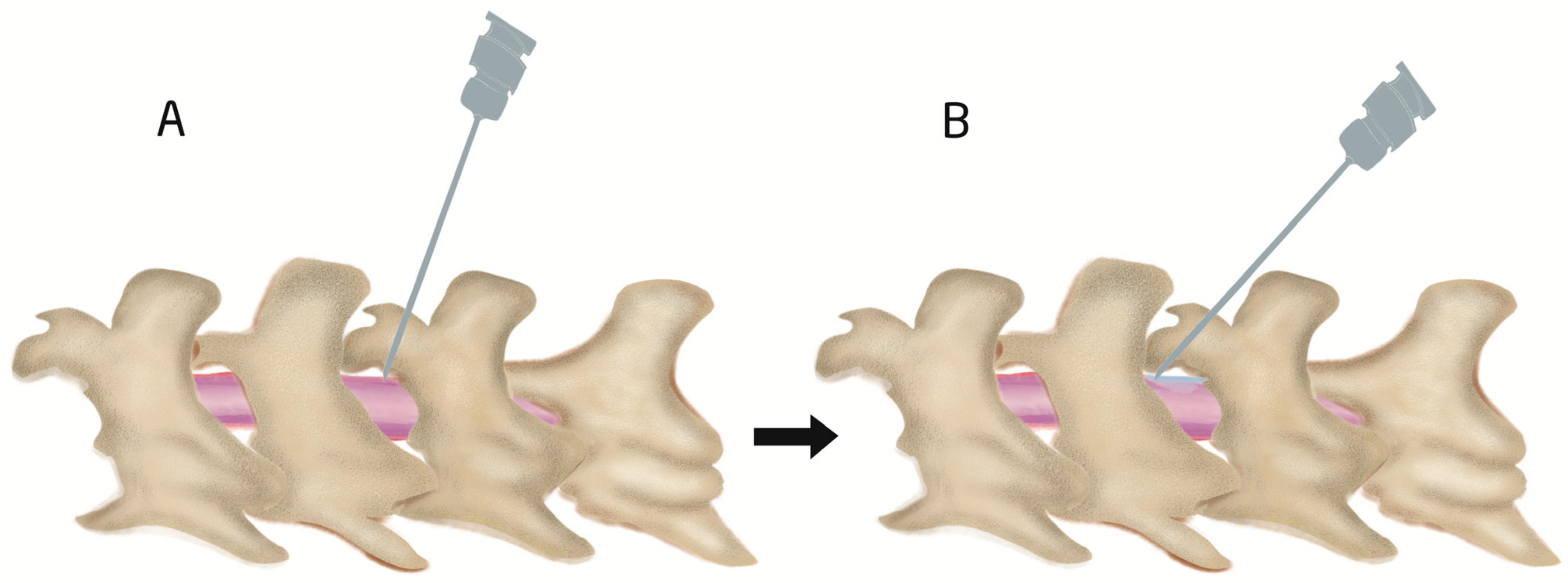
4.1. Procedure of Intrathecal Injection by Acute Needle Puncturing
4.2. Epidural and Intrathecal Catheterization
| Species | Injection Site | Volume | Syringe Size | Time of Injection | Time of Syringe Withdrawal | Number of Injections | Reference |
|---|---|---|---|---|---|---|---|
| C57BL/6 mice | L4–5 | 10 μL | 30-G needle to 50-μL Hamilton | 3 μL/min | The needle was removed 1 min after completion and was kept in Trendelenburg position 5 min more | Single | [17] |
| C57BL/6 mice | L5–6 | 10 μL | 30-G 0.5-in needle | - | - | Three injections, 24-h intervals | [7] |
| Kunming mice | L5–L6 | 10 μL | Delivered for more than 30 s | Syringe maintained for an additional 15 s to ensure diffusion before removal | Single | [6] | |
| C57BL/6J mice | L5–6 | 5 μL | 30-G in 10-μL Hamilton | - | Three injections at two-day intervals | [5] | |
| FVB/NJ mice | L5–L6 | 8 μL | 27-G needle 25-μL Hamilton syringe | 1 μL/4 s | 1 min after finishing delivery | Single | [16] |
| Mice | L5–L6 | 5 μL | 30 G | 1 μL/6 s | 15 s | Single | [47] |
| C57BL/6 mice | top of the foramen magnum | 20 μL | 25-G, 1-mL syringe | Slowly | After 2 min | Three injections at 7 days intervals | [46] |
| CDI mice | L5–6 | 10 μL | 30-G needle | - | - | Single | [62] |
| Mice | 20 μL | 30-G 1/2 in 50 μL Hamilton | Injections were delivered as a bolus within 5 s | Single | [63] | ||
| SD rat | L2–3 | 0.2 mL or 2 mL | 1-mL syringe | 1-mL syringe | After injection, rats placed upside-down at a 45° angle for 15 min | Single | [54] |
| SD rat | L5–6 | 30 μL | 31 G | - | Single | [44] | |
| Wistar rats | L4–5 | 15 μL | 26 G | 3 μL/min | - | Two injections, 24-h intervals | [45] |
| Wistar rats | L6–S1 | 0.02 mL/kg, average of 0.05 mL per rat | 25 G | 1 mL/min, average: 3 s/injection | 1 mL/min, average: 3 s /injection | Single | [15] |
| Wistar rats | L3–4 or L4–5 | 25 G | 1 min | Single | [53] |
| Species | Site of Insertion | Catheter Size and Total Length | Inserted Length | Dead Space and Filling Agent | Injected Volume | Reference |
|---|---|---|---|---|---|---|
| Lumbar | ||||||
| SD rat | L4–5 | PE-10 (0.6 mm diameter) | 1–2 cm | 20 µL, saline | 10 μL | [11] |
| SD rats | L4–5 | PE-10 tube, 12 cm | 2 cm | - | - | [43] |
| SD rats | L5–6 | PE-10 (0.6 mm diameter), 10 cm | 4 cm | - | 10 μL | [59] |
| SD rats | L5–6 | PE-10 (0.6 mm diameter), 15 cm | 3 cm | 4.5 µL, saline (7 µL) | 10 μL | [14] |
| SD rat | L2 laminectomy, tip located between L3 and L5 | SUBL-14 | L3–L5 | 10 µL | 25 or 50 μL | [64] |
| Rats | T13–L1 | PE-5 catheters (outside diameter: 0.36 mm) | L2–L5 | 6 µL, PBS | 20 µL | [60] |
| Atlanto-occipital | ||||||
| SD rat | Atlanto-occipital | ALZET catheter (PU-10 28G | 8 cm caudally to reach lumbar enlargement | 10 μL, sterile saline | 20 μL | [18] |
| SD rat | Atlanto-occipital | PE-10 | 8.5 cm caudally to reach lumbar enlargement | 10 μL, sterile saline | 10 μL | [65] |
| Mice | Atlanto-occipital | -ALZET IT mice catheter -O’Buckley IT catheter | 2.5 cm | [13] |
| Species | Site of Insertion | Catheter Size and Total Length | Inserted Length | Dead Space and Filling Agent | Injected Volume | Reference |
|---|---|---|---|---|---|---|
| SD rat | L4–5 | PE-10 (0.6 mm diameter) | 1–2 cm | 20 µL, saline | 10 μL | [11] |
| SD rat | T13–L1 | PE-10 | ~3.0 cm until L5–6 | 100 µL of hyaluronic acid, 0.9% saline | 100 µL of hyaluronic acid, 0.9% saline | [2] |
| SD art | T13–L1 | PE-10 catheter | ~3.0 cm until L5–6 | 10 µL of sa | 160 µL | [3] |
| Mice | T11–T12 | PU-10catheter | 1 cm | - | 50 µL | [4] |
5. Uses and Application of Epidural and Intrathecal Injection
| Species | Method of Drugs Administration | Disease Model | Types of Agents Injected | Purpose of Injection | Concentration | Injected Volume and Vehicle | Reference |
|---|---|---|---|---|---|---|---|
| SD rat | ITc | Resiniferatoxin-induced postherpetic neuralgia | -Amiloride, a potent ASIC3 inhibitor -7,8-DHF, TrkB agonist, 3 mg/kg | -To evaluate involvement of ASIC3 and TrkB signaling in pain in dorsal root ganglia | 100-μg amiloride daily for 7 days -3-mg/kg, TrkB agonist for 7 days | 10 μL | [43] |
| SD rat | ITc | Spinal nerve ligation-induced pain model | Phosphodiesterase 4B-specific siRNA | -To reduce neuroinflammation | 2 μg | [11] | |
| SD rat | ITinj | Chronic pancreatis model | Cognate receptor C–X–C chemokine receptor type 4 (CXCR4) inhibitor | -to reduce pancreatic pain | 5 μg/10 μL daily for one week | 10 μL | [19] |
| SD rat | ITc | Freund’s complete adjuvant-induced rheumatoid arthritis | Crocin | -To reduce rheumatoid arthritis-induced pain | 100 mg/kg | 20 μL | [72] |
| SD rat | ITc | Bone cancer pain model | Genetically engineered human bone marrow stem cells | -To reduce bone cancer pain | 6 × 106 cells | 10 μL | [68] |
| SD rat | ITinj | Neuropathic pain | Adipose tissue-derived stem cells (ASCs) | -To relieve neuropathic pain | 1 × 106 cells | 30 μL DMEM | [44] |
| SD rat | EDc | Foraminal stenosis-induced pain | Hyaluronic acid (HA) | -To relieve neuropathic pain | 100 µL of HA | 100 µL of HA | [2] |
| SD rat | EDc | Healthy rats | Gabapentin | -To evaluate safety and toxicity | 30 mg | 300 μL | [12] |
| SD rat | EDc | Lumbar foraminal stenosis-induced pain | Polydeoxyribonucleotide | -To evaluate analgesic effect | 0.1 mg/kg | 160 µL | [3] |
| Wistar Rat | ITc | Spinal cord ischemia | human umbilical cord blood stem cells | To improve spinal cord function | 1 × 104 HUCBSCs | 10 μL | [67] |
| SD rat | ITc | Spinal cord injury model | Embryonic Stem Cell-Derived Spinal GABAergic Neural Precursor Cells | To reduce central neuropathic pain and motor function | 1 × 106 cells | - | [57] |
| Wistar rat | ITinj | Chronic DRG compression-induced pain model | Bone marrow stromal cell | -To reduce neuropathic Pain | 1 × 106 cells | 15 μL | [45] |
| CD1 mice | ITinj | CCI-induced neuropathic pain model | Bone marrow stromal cell | -To reduce neuropathic pain | 1 or 2.5 × 105 cells | 10 μL | [62] |
| Rat | ITc | Spinal cord injury-induced spasticity | -Potassium-chloride cotransporter KCC2 - BDNF | -To evaluate the involvement of KCC2 and BDNF in spasticity | 20 μg 10 μg | 20 μL | [60] |
| Rat | ITc | Phasic andincisional pain | Gentamycin, Streptomycin, Neomycin | -To evaluate Antinociceptive potency of aminoglycoside antibiotics | 5 μg, 15 μg, 15 μg, respectively | 10 μL | [74] |
| Rat | ITc | Diabetes-induced neuropathic pain | Insulin | -To evaluate Antinociceptive potency of insulin | 0.2 U | 10 μL | [69] |
| Rats, Mice | ITc | Diabetes-induced neuropathic pain | Sirtuin 1 agonist, SRT1720 | -To reduce neuropathic pain | 0.8 μg in rats, 1.4 μg in mice | 10 μL | [73] |
| Rat | ITc | Spared nerve injury (SNI) | TMEM16A, U0126 inhibitors | -To find out the mechanisms of neuropathic pain | 10 μg, 10 μg | 10 μL | [76] |
| Mice | ITinj | Chemotherapy (Paclitaxel)-induced neuropathic pain | -Artesunate | -To reduce chemotherapy-induced neuropathic pain | 100 μg | 5 μL | [75] |
| Mice | Neuropathic Pain | Decursin | -To reduce pain | [5] | |||
| Mice | ITinj | Spontaneous pain | Capsaicin | To induce spontaneous pain | 0.5 µg in 10 μL | [71] | |
| Mice | ITinj | PAR-2 activator trypsin-induced scratching behavior | -gastrin-releasing peptide (GRP) -Opioids | -To reduce scratching behavior | 1 nmol/5 μL | 5 μL | [78] |
| Mice | ITinj | Morphine-induced pruritis | Morphine | -To induce scratching behavior | 0.3 nmol | 5 μL | [42] |
| Mice | ITinj | Chronic post-ischemia neuropathic pain model | Human mesenchymal stem cells | -To reduce pain behavior | 2 × 105 cells | 5 μL | [77] |
| Mice | ITinj | Collagen-induced arthritis | ERK1/2 inhibitor (U0126), Tramadol, and NMDAR antagonist D-2-amino-5-phosphonovaleric acid | -To reduce pain behavior | 1.6 µg, 250 µg, 0.5 µg, respectively | 5 μL | [24] |
| Mice | ITc | Acetic acid-induced writhing test | Neomycin, gentamicin | -To evaluate antinociceptive effects | 0.5–20.0 µg, 5–40 µg, respectively | 10 μL | [70] |
6. Limitation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fowler, M.J.; Cotter, J.D.; Knight, B.E.; Sevick-Muraca, E.M.; Sandberg, D.I.; Sirianni, R.W. Intrathecal drug delivery in the era of nanomedicine. Adv. Drug Deliv. Rev. 2020, 165–166, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Nahm, F.S.; Lee, P.B.; Choe, G.Y.; Lim, Y.J.; Kim, Y.C. Therapeutic effect of epidural hyaluronic acid in a rat model of foraminal stenosis. J. Pain Res. 2017, 10, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ju, J.; Choi, E.; Nahm, F.S.; Choe, G.Y.; Lee, P.B. Effect of epidural polydeoxyribonucleotide in a rat model of lumbar foraminal stenosis. Korean J. Pain 2021, 34, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Agbigbe, O.; Nigro, N.; Yakobi, G.; Shapiro, J.; Ginosar, Y. Use of high-resolution thermography as a validation measure to confirm epidural anesthesia in mice: A cross-over study. Int. J. Obstet. Anesth. 2021, 46, 102981. [Google Scholar] [CrossRef] [PubMed]
- Son, D.B.; Choi, W.; Kim, M.; Go, E.J.; Jeong, D.; Park, C.K.; Kim, Y.H.; Lee, H.; Suh, J.W. Decursin Alleviates Mechanical Allodynia in a Paclitaxel-Induced Neuropathic Pain Mouse Model. Cells 2021, 10, 547. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, Q.; Jiao, J.; Wang, G.; Gu, Z.; Huang, D.; Wang, Z. Intrathecal injection of dexmedetomidine ameliorates chronic neuropathic pain via the modulation of MPK3/ERK1/2 in a mouse model of chronic neuropathic pain. Neurol. Res. 2019, 41, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Njoo, C.; Heinl, C.; Kuner, R. In vivo SiRNA transfection and gene knockdown in spinal cord via rapid noninvasive lumbar intrathecal injections in mice. J. Vis. Exp. 2014, 85, e51229. [Google Scholar] [CrossRef]
- Bottros, M.M.; Christo, P.J. Current perspectives on intrathecal drug delivery. J. Pain Res. 2014, 7, 615–626. [Google Scholar] [CrossRef]
- Bhaskar, A.K. Interventional management of cancer pain. Curr. Opin. Support. Palliat. Care 2012, 6, 1–9. [Google Scholar] [CrossRef]
- House, L.M.; Barrette, K.; Mattie, R.; McCormick, Z.L. Cervical Epidural Steroid Injection: Techniques and Evidence. Phys. Med. Rehabil. Clin. N. Am. 2018, 29, 1–17. [Google Scholar] [CrossRef]
- Ji, Q.; Di, Y.; He, X.; Liu, Q.; Liu, J.; Li, W.; Zhang, L. Intrathecal injection of phosphodiesterase 4B-specific siRNA attenuates neuropathic pain in rats with L5 spinal nerve ligation. Mol. Med. Rep. 2016, 13, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Kim, Y.C.; Lim, Y.J.; Lee, C.J.; Lee, P.B.; Lee, S.C.; Sim, W.S.; Choi, Y.L. The neurological safety of epidural gabapentin in rats: A light microscopic examination. Anesth. Analg. 2005, 101, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, F.A.; Ciszek, B.P.; O’Buckley, S.C.; Nackley, A.G. Novel intrathecal and subcutaneous catheter delivery systems in the mouse. J. Neurosci. Methods 2016, 264, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Storkson, R.V.; Kjorsvik, A.; Tjolsen, A.; Hole, K. Lumbar catheterization of the spinal subarachnoid space in the rat. J. Neurosci. Methods 1996, 65, 167–172. [Google Scholar] [CrossRef]
- Thomas, A.A.; Detilleux, J.; Sandersen, C.F.; Flecknell, P.A. Minimally invasive technique for intrathecal administration of morphine in rats: Practicality and antinociceptive properties. Lab Anim. 2017, 51, 479–489. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Tian, Y.; Xu, Z.; Guo, Y. Direct Intrathecal Injection of Recombinant Adeno-associated Viruses in Adult Mice. J. Vis. Exp. 2019, 144, e58565. [Google Scholar] [CrossRef]
- Bey, K.; Ciron, C.; Dubreil, L.; Deniaud, J.; Ledevin, M.; Cristini, J.; Blouin, V.; Aubourg, P.; Colle, M.A. Efficient CNS targeting in adult mice by intrathecal infusion of single-stranded AAV9-GFP for gene therapy of neurological disorders. Gene Ther. 2017, 24, 325–332. [Google Scholar] [CrossRef]
- Kaya, C.; Atalay, Y.O.; Meydan, B.C.; Ustun, Y.B.; Koksal, E.; Caliskan, S. Evaluation of the neurotoxic effects of intrathecal administration of (S)-(+)-Ketoprofen on rat spinal cords: Randomized controlled experimental study. Braz. J. Anesthesiol. 2019, 69, 403–412. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Liu, X.; Miao, X.; Li, D.; Wang, S.; Xu, G.Y. Up-regulation of CXCR4 expression contributes to persistent abdominal pain in rats with chronic pancreatitis. Mol. Pain 2017, 13, 1744806917697979. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, M.; Pei, L. Comparison of three methods of drug delivery in the rat lumbar spinal subarachnoid space. Anat. Rec. 2012, 295, 1212–1220. [Google Scholar] [CrossRef]
- Macpherson, D.; Quondamatteo, F.; Broom, M. Update on applied epidural anatomy. BJA Educ. 2022, 22, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Reina, M.A.; de Leon-Casasola, O.A.; Lopez, A.; De Andres, J.; Martin, S.; Mora, M. An in vitro study of dural lesions produced by 25-gauge Quincke and Whitacre needles evaluated by scanning electron microscopy. Reg. Anesth. Pain Med. 2000, 25, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Braehler, M. Use of test dose allows early detection of subdural local anesthetic injection with lumbar plexus block. J. Clin. Anesth. 2017, 37, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, K.; Miao, J.; Zhao, P.; Lv, M.; Li, J.; Fu, X.; Luo, X.; Zhu, P. The spinal NR2BR/ERK2 pathway as a target for the central sensitization of collagen-induced arthritis pain. PLoS ONE 2018, 13, e0201021. [Google Scholar] [CrossRef]
- Adeeb, N.; Deep, A.; Griessenauer, C.J.; Mortazavi, M.M.; Watanabe, K.; Loukas, M.; Tubbs, R.S.; Cohen-Gadol, A.A. The intracranial arachnoid mater: A comprehensive review of its history, anatomy, imaging, and pathology. Child’s Nerv. Syst. 2013, 29, 17–33. [Google Scholar] [CrossRef]
- Vandenabeele, F.; Creemers, J.; Lambrichts, I. Ultrastructure of the human spinal arachnoid mater and dura mater. J. Anat. 1996, 189, 417–430. [Google Scholar]
- Belov, V.; Appleton, J.; Levin, S.; Giffenig, P.; Durcanova, B.; Papisov, M. Large-Volume Intrathecal Administrations: Impact on CSF Pressure and Safety Implications. Front. Neurosci. 2021, 15, 604197. [Google Scholar] [CrossRef]
- Ghannam, J.Y.; Al Kharazi, K.A. Neuroanatomy, Cranial Meninges; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Miller, A.D.; Subramanian, A.; Viljoen, H.J. Theoretically proposed optimal frequency for ultrasound induced cartilage restoration. Theor. Biol. Med. Model. 2017, 14, 21. [Google Scholar] [CrossRef]
- Ceylan, D.; Tatarli, N.; Abdullaev, T.; Seker, A.; Yildiz, S.D.; Keles, E.; Konya, D.; Bayri, Y.; Kilic, T.; Cavdar, S. The denticulate ligament: Anatomical properties, functional and clinical significance. Acta Neurochir. 2012, 154, 1229–1234. [Google Scholar] [CrossRef]
- Brinker, T.; Stopa, E.; Morrison, J.; Klinge, P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 2014, 11, 10. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Chiba, Y.; Murakami, R.; Miyai, Y.; Matsumoto, K.; Kamada, M.; Nonaka, W.; Uemura, N.; Yanase, K.; Ueno, M. Metabolites and Biomarker Compounds of Neurodegenerative Diseases in Cerebrospinal Fluid. Metabolites 2022, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Pei, M.C.; Bo, B.S.; Zhao, X.X.; Cang, J.; Fang, F.; Liang, Z.F. Whole-brain mapping of mouse CSF flow via HEAP-METRIC phase-contrast MRI. Magn. Reason. Med. 2022, 87, 2851–2861. [Google Scholar] [CrossRef] [PubMed]
- Murtha, L.A.; Yang, Q.; Parsons, M.W.; Levi, C.R.; Beard, D.J.; Spratt, N.J.; McLeod, D.D. Cerebrospinal fluid is drained primarily via the spinal canal and olfactory route in young and aged spontaneously hypertensive rats. Fluids Barriers CNS 2014, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Sleigh, J.N.; Weir, G.A.; Schiavo, G. A simple, step-by-step dissection protocol for the rapid isolation of mouse dorsal root ganglia. BMC Res. Notes 2016, 9, 82. [Google Scholar] [CrossRef]
- Berta, T.; Qadri, Y.; Tan, P.H.; Ji, R.R. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin. Ther. Targets 2017, 21, 695–703. [Google Scholar] [CrossRef]
- Hogan, Q.H. Labat lecture: The primary sensory neuron: Where it is, what it does, and why it matters. Reg. Anesth. Pain Med. 2010, 35, 306–311. [Google Scholar] [CrossRef]
- Chang, M.F.; Hsieh, J.H.; Chiang, H.; Kan, H.W.; Huang, C.M.; Chellis, L.; Lin, B.S.; Miaw, S.C.; Pan, C.L.; Chao, C.C.; et al. Effective gene expression in the rat dorsal root ganglia with a non-viral vector delivered via spinal nerve injection. Sci. Rep. 2016, 6, 35612. [Google Scholar] [CrossRef]
- Joukal, M.; Klusakova, I.; Dubovy, P. Direct communication of the spinal subarachnoid space with the rat dorsal root ganglia. Ann. Anat. 2016, 205, 9–15. [Google Scholar] [CrossRef]
- Sakka, L.; Coll, G.; Chazal, J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 309–316. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W. Influence of intrathecal injection with dexmedetomidine on the behavioral ability and analgesic effects on rats with neuropathic pain and expression of protein kinase C in the spinal dorsal horn. Exp. Ther. Med. 2018, 16, 3835–3840. [Google Scholar] [CrossRef]
- Ye, Y.S.; Pan, A.Z.; Zhen, Y.; Kang, M.R.; Zhang, B.; Yi, W.M. Antipruritic effects of electroacupuncture on morphine-induced pruritus model mice through the TLR2/4-MyD88-NF-kappaB pathway. Neuroreport 2019, 30, 331–337. [Google Scholar] [CrossRef]
- Wei, X.; Wang, L.; Hua, J.; Jin, X.H.; Ji, F.; Peng, K.; Zhou, B.; Yang, J.; Meng, X.W. Inhibiting BDNF/TrkB.T1 receptor improves resiniferatoxin-induced postherpetic neuralgia through decreasing ASIC3 signaling in dorsal root ganglia. J. Neuroinflamm. 2021, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Jwa, H.S.; Kim, Y.H.; Lee, J.; Back, S.K.; Park, C.K. Adipose Tissue-Derived Stem Cells Alleviate Cold Allodynia in a Rat Spinal Nerve Ligation Model of Neuropathic Pain. Stem Cells Int. 2020, 2020, 8845262. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Zhang, Y.; Yue, S.; Chen, H.; Qu, Y.; Wei, H.; Jia, X. Intrathecal injection of bone marrow stromal cells attenuates neuropathic pain via inhibition of P2X4R in spinal cord microglia. J. Neuroinflamm. 2019, 16, 271. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.K.; Yan, Q.J.; Vyshkina, T.; Sahabi, S.; Liu, X.; Sadiq, S.A. Clinical and pathological effects of intrathecal injection of mesenchymal stem cell-derived neural progenitors in an experimental model of multiple sclerosis. J. Neurol. Sci. 2012, 313, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Xue, Q.; Zhang, F.; Luo, Y.; Zhu, H.; Zhang, X.; Zhang, H.; Ding, W.; Yu, B. Spinal SIRT1 activation attenuates neuropathic pain in mice. PLoS ONE 2014, 9, e100938. [Google Scholar] [CrossRef]
- Crowley, S.T.; Fukushima, Y.; Uchida, S.; Kataoka, K.; Itaka, K. Enhancement of Motor Function Recovery after Spinal Cord Injury in Mice by Delivery of Brain-Derived Neurotrophic Factor mRNA. Mol. Ther. Nucleic Acids 2019, 17, 465–476. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Gao, J.; Jin, X.; Ji, F.; Yang, J. A modified procedure for lumbar intrathecal catheterization in rats. Neurol. Res. 2016, 38, 725–732. [Google Scholar] [CrossRef]
- Chen, Y.; Mazur, C.; Luo, Y.; Sun, L.; Zhang, M.; McCampbell, A.; Tomassy, G.S. Intrathecal Delivery of Antisense Oligonucleotides in the Rat Central Nervous System. J. Vis. Exp. 2019, 152, e60274. [Google Scholar] [CrossRef]
- Wang, H.C.; Cheng, K.I.; Chen, P.R.; Tseng, K.Y.; Kwan, A.L.; Chang, L.L. Glycine receptors expression in rat spinal cord and dorsal root ganglion in prostaglandin E2 intrathecal injection models. BMC Neurosci. 2018, 19, 72. [Google Scholar] [CrossRef]
- Mazur, C.; Fitzsimmons, B.; Kamme, F.; Nichols, B.; Powers, B.; Wancewicz, E. Development of a simple, rapid, and robust intrathecal catheterization method in the rat. J. Neurosci. Methods 2017, 280, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Quintana, E.; Judas; Kallenbach, K. Intrathecal injection of human umbilical cord blood stem cells attenuates spinal cord ischaemic compromise in rats CONFERENCE DISCUSSION. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 762. [Google Scholar]
- Kim, H.; Na, D.L.; Lee, N.K.; Kim, A.R.; Lee, S.; Jang, H. Intrathecal Injection in A Rat Model: A Potential Route to Deliver Human Wharton’s Jelly-Derived Mesenchymal Stem Cells into the Brain. Int. J. Mol. Sci. 2020, 21, 1272. [Google Scholar] [CrossRef] [PubMed]
- Hubler, M.; Litz, R.J.; von Kummer, R.; Albrecht, D.M. Intrathecal air following spinal anaesthesia. Anaesthesia 2002, 57, 307–308. [Google Scholar] [CrossRef]
- Kawamata, T.; Omote, K.; Kawamata, M.; Iwasaki, H.; Namiki, A. Antinociceptive interaction of intrathecal alpha2-adrenergic agonists, tizanidine and clonidine, with lidocaine in rats. Anesthesiology 1997, 87, 436–448. [Google Scholar] [CrossRef]
- Hwang, I.; Hahm, S.C.; Choi, K.A.; Park, S.H.; Jeong, H.; Yea, J.H.; Kim, J.; Hong, S. Intrathecal Transplantation of Embryonic Stem Cell-Derived Spinal GABAergic Neural Precursor Cells Attenuates Neuropathic Pain in a Spinal Cord Injury Rat Model. Cell Transpl. 2016, 25, 593–607. [Google Scholar] [CrossRef]
- Ouchi, K.; Sekine, J.; Koga, Y.; Nakao, S.; Sugiyama, K. Establishment of an animal model of sedation using epidural anesthesia that uses the tail-flick test for evaluating local anesthetic effects in rats. Exp. Anim. 2013, 62, 137–144. [Google Scholar] [CrossRef]
- Hou, J.; Xia, Z.; Xiao, X.; Wan, X.; Zhao, B. Neurotoxicity of intrathecal injections of dexmedetomidine into the rat spinal dorsal horn. Neural Regen. Res. 2012, 7, 1765–1770. [Google Scholar]
- Boulenguez, P.; Liabeuf, S.; Bos, R.; Bras, H.; Jean-Xavier, C.; Brocard, C.; Stil, A.; Darbon, P.; Cattaert, D.; Delpire, E.; et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010, 16, 302–307. [Google Scholar] [CrossRef]
- Jasmin, L.; Ohara, P.T. Long-term intrathecal catheterization in the rat. J. Neurosci. Methods 2001, 110, 81–89. [Google Scholar] [CrossRef]
- Chen, G.; Park, C.K.; Xie, R.G.; Ji, R.R. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-beta secretion. J. Clin. Investig. 2015, 125, 3226–3240. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, O.B.; Kovacs, K.J.; Larson, A.A. Chronic daily intrathecal injections of a large volume of fluid increase mast cells in the thalamus of mice. Brain Res. 2005, 1056, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.A.; Ducrocq, G.P.; Kim, J.S.; Kaufman, M.P. Intrathecal injection of brilliant blue G, a P2X7 antagonist, attenuates the exercise pressor reflex in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R223–R232. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.H.; Park, K.D.; Lee, H.G.; Kim, W.M.; An, T.H.; Kim, Y.O.; Huang, L.J.; Hua, C.J. Additive antinociception between intrathecal sildenafil and morphine in the rat formalin test. J. Korean Med. Sci. 2008, 23, 1033–1038. [Google Scholar] [CrossRef]
- Schaffer, D.P.H.; de Araujo, N.; Otero, A.R.; Dorea Neto, F.A.; Barbosa, V.F.; Martins Filho, E.F.; Oria, A.P. Cardiorespiratory effects of epidural anesthesia using lidocaine with morphine or dexmedetomidine in capuchin monkeys (Sapajus sp.) undergoing bilateral tubal ligation surgery, anesthetized with isoflurane. J. Med. Primatol. 2017, 46, 311–319. [Google Scholar] [CrossRef]
- Judas, G.I.; Ferreira, S.G.; Simas, R.; Sannomiya, P.; Benicio, A.; da Silva, L.F.; Moreira, L.F. Intrathecal injection of human umbilical cord blood stem cells attenuates spinal cord ischaemic compromise in rats. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 757–762. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, Y.; Li, H.; Zhang, D.; Sun, Q. Antinociceptive Effect of Intrathecal Injection of Genetically Engineered Human Bone Marrow Stem Cells Expressing the Human Proenkephalin Gene in a Rat Model of Bone Cancer Pain. Pain Res. Manag. 2017, 2017, 7346103. [Google Scholar] [CrossRef]
- Kou, Z.Z.; Li, C.Y.; Hu, J.C.; Yin, J.B.; Zhang, D.L.; Liao, Y.H.; Wu, Z.Y.; Ding, T.; Qu, J.; Li, H.; et al. Alterations in the neural circuits from peripheral afferents to the spinal cord: Possible implications for diabetic polyneuropathy in streptozotocin-induced type 1 diabetic rats. Front. Neural Circuits 2014, 8, 6. [Google Scholar] [CrossRef]
- Dogrul, A.; Yesilyurt, O. Effects of intrathecally administered aminoglycoside antibiotics, calcium-channel blockers, nickel and calcium on acetic acid-induced writhing test in mice. Gen. Pharmacol. 1998, 30, 613–616. [Google Scholar] [CrossRef]
- Han, Q.; Kim, Y.H.; Wang, X.; Liu, D.; Zhang, Z.J.; Bey, A.L.; Lay, M.; Chang, W.; Berta, T.; Zhang, Y.; et al. SHANK3 Deficiency Impairs Heat Hyperalgesia and TRPV1 Signaling in Primary Sensory Neurons. Neuron 2016, 92, 1279–1293. [Google Scholar] [CrossRef]
- Wang, J.F.; Xu, H.J.; He, Z.L.; Yin, Q.; Cheng, W. Crocin Alleviates Pain Hyperalgesia in AIA Rats by Inhibiting the Spinal Wnt5a/beta-Catenin Signaling Pathway and Glial Activation. Neural Plast. 2020, 2020, 4297483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ding, X.; Zhou, Z.; Qiu, Z.; Shi, N.; Zhou, S.; Du, L.; Zhu, X.; Wu, Y.; Yin, X.; et al. Sirtuin 1 alleviates diabetic neuropathic pain by regulating synaptic plasticity of spinal dorsal horn neurons. Pain 2019, 160, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Prado, W.A.; Machado Filho, E.B. Antinociceptive potency of aminoglycoside antibiotics and magnesium chloride: A comparative study on models of phasic and incisional pain in rats. Braz. J. Med. Biol. Res. 2002, 35, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kang, J.; Xu, Y.; Li, N.; Jiao, Y.; Wang, C.; Wang, C.; Wang, G.; Yu, Y.; Yuan, J.; et al. Artesunate Alleviates Paclitaxel-Induced Neuropathic Pain in Mice by Decreasing Metabotropic Glutamate Receptor 5 Activity and Neuroinflammation in Primary Sensory Neurons. Front. Mol. Neurosci. 2022, 15, 902572. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Kong, L.; Xu, Z.; Cao, N.; Tang, X.; Gao, R.; Zhang, J.; Deng, S.; Tan, C.; Zhang, M.; et al. The Role of TMEM16A/ERK/NK-1 Signaling in Dorsal Root Ganglia Neurons in the Development of Neuropathic Pain Induced by Spared Nerve Injury (SNI). Mol. Neurobiol. 2021, 58, 5772–5789. [Google Scholar] [CrossRef]
- Yoo, S.H.; Lee, S.H.; Lee, S.; Park, J.H.; Lee, S.; Jin, H.; Park, H.J. The effect of human mesenchymal stem cell injection on pain behavior in chronic post-ischemia pain mice. Korean J. Pain 2020, 33, 23–29. [Google Scholar] [CrossRef]
- Maciel, I.S.; Azevedo, V.M.; Pereira, T.C.; Bogo, M.R.; Souza, A.H.; Gomez, M.V.; Campos, M.M. The spinal inhibition of N-type voltage-gated calcium channels selectively prevents scratching behavior in mice. Neuroscience 2014, 277, 794–805. [Google Scholar] [CrossRef]
- Jaumard, N.V.; Leung, J.; Gokhale, A.J.; Guarino, B.B.; Welch, W.C.; Winkelstein, B.A. Relevant Anatomic and Morphological Measurements of the Rat Spine. Spine 2015, 40, E1084–E1092. [Google Scholar] [CrossRef]
- Toossi, A.; Bergin, B.; Marefatallah, M.; Parhizi, B.; Tyreman, N.; Everaert, D.G.; Rezaei, S.; Seres, P.; Gatenby, J.C.; Perlmutter, S.I.; et al. Comparative neuroanatomy of the lumbosacral spinal cord of the rat, cat, pig, monkey, and human. Sci. Rep. 2021, 11, 1955. [Google Scholar] [CrossRef]
- Weller, R.O.; Sharp, M.M.; Christodoulides, M.; Carare, R.O.; Mollgard, K. The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathol. 2018, 135, 363–385. [Google Scholar] [CrossRef]
- Schwaid, A.G.; Krasowka-Zoladek, A.; Chi, A.; Cornella-Taracido, I. Comparison of the Rat and Human Dorsal Root Ganglion Proteome. Sci. Rep. 2018, 8, 13469. [Google Scholar] [CrossRef] [PubMed]


| Epidural | Intrathecal | |
|---|---|---|
| Content | The epidural space contains fat, the dural sac, spinal nerves, blood vessels, and connective tissue. | The subarachnoid space consists of the cerebrospinal fluid (CSF), major blood vessels, and cisterns. |
| Site of drug delivery | Epidural space. | Intrathecal space, subarachnoid space, or superficial potential space of spinal cord. |
| Confirmation of proper injection |
|
|
| Blood–brain barrier | Mostly avoids absorptive problems made by the blood–brain barrier. | Avoids absorptive problems made by the blood–brain barrier. |
| Onset of action | After epidural drug administration, drug diffuses through the dura mater into the CSF, which is a significant barrier that needs to be passed, and thus, onset of action is slow. | As the drug is delivered directly into the CSF, the onset of action is fast and instantaneous. |
| Involvement of systemic circulation | Despite of diffusion of drug through dura mater, some portions of drug also reachss systemic circulation through epidural blood vessels. | There is no involvement of systemic circulation, only restricted within the CSF, circulating in the spinal canal and the brain ventricles. |
| Dose | Usually 10× that of intrathecal dose, depending on drugs. | Usually 10× lower than epidural dose, depending on drugs. |
| Occurrence of side effects | Comparatively higher occurrence of side effects due to systemic involvement. | Comparatively lower occurrence of side effects as there is no systemic involvement. |
| Pain relief | More suitable for short term. | Better for long term. |
| Uses | Analgesia, anesthesia. | Analgesia, anesthesia, spasticity, chemotherapy, stem cell therapy, antibiotic, protein therapy, etc. |
| Species | Volume (μL) | Production (μL/min) | Turnover (Times/24 h) |
|---|---|---|---|
| Mus musculus | 35–40 | 0.32–0.35 | 12–14 |
| Rattus norvegicus | 150 | 1.7–2.8 | 9–12 |
| Homo sapiens | 100,000–200,000 | 350–370 | 3–5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Lee, J.Y.; Kim, Y.H.; Park, C.-K. Epidural and Intrathecal Drug Delivery in Rats and Mice for Experimental Research: Fundamental Concepts, Techniques, Precaution, and Application. Biomedicines 2023, 11, 1413. https://doi.org/10.3390/biomedicines11051413
Rahman MM, Lee JY, Kim YH, Park C-K. Epidural and Intrathecal Drug Delivery in Rats and Mice for Experimental Research: Fundamental Concepts, Techniques, Precaution, and Application. Biomedicines. 2023; 11(5):1413. https://doi.org/10.3390/biomedicines11051413
Chicago/Turabian StyleRahman, Md. Mahbubur, Ji Yeon Lee, Yong Ho Kim, and Chul-Kyu Park. 2023. "Epidural and Intrathecal Drug Delivery in Rats and Mice for Experimental Research: Fundamental Concepts, Techniques, Precaution, and Application" Biomedicines 11, no. 5: 1413. https://doi.org/10.3390/biomedicines11051413
APA StyleRahman, M. M., Lee, J. Y., Kim, Y. H., & Park, C.-K. (2023). Epidural and Intrathecal Drug Delivery in Rats and Mice for Experimental Research: Fundamental Concepts, Techniques, Precaution, and Application. Biomedicines, 11(5), 1413. https://doi.org/10.3390/biomedicines11051413







