Effects of Nonnutritive Sweeteners on Body Composition Changes during Pubertal Growth
Abstract
:1. Introduction
2. Materials and Methods
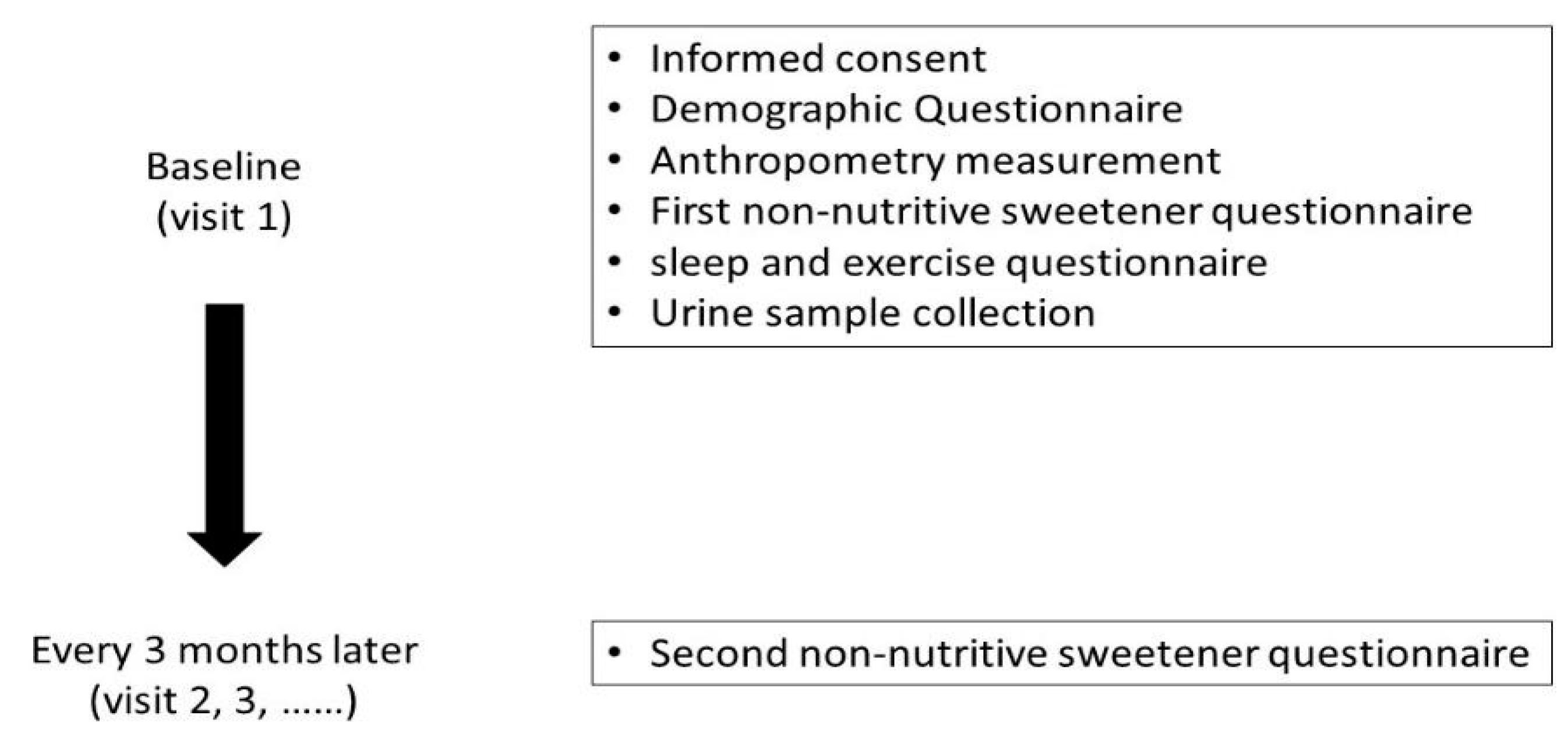
2.1. Study Design and Data Collection
2.2. Exposure Assessment
2.3. Establishment of the Semiquantitative NNS-FFQ
2.4. Covariate Assessment
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Association between NNS Consumption and Body Composition
3.3. Association between NNSs and Body Composition in Different Sexes
3.4. Association between NNSs and Body Composition in Different Tanner Stages
3.5. Association between NNSs and Body Composition in Obese or Normal-Weight Children
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nittari, G.; Scuri, S.; Sagaro, G.G.; Petrelli, F.; Grappasonni, I. Epidemiology of Obesity in Children and Adolescents. In Teamwork in Healthcare; Michael, S.F., Stanislaw, P.S., Eds.; IntechOpen: London, UK, 2020; Chapter 10. [Google Scholar]
- Health Promotion Administration, Ministry of Health and Welfare. 2016 Annual Report of Health Promotion Administration; Health Promotion Administration, Ministry of Health and Welfare: Taipei, Taiwan, 2018.
- Krebs, N.F.; Jacobson, M.S. Prevention of pediatric overweight and obesity. Pediatrics 2003, 112, 424–430. [Google Scholar]
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J. Dietary sugars intake and cardiovascular health: A scientific statement from the American Heart Association. Circulation 2009, 120, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Breda, J.; Jewell, J.; Keller, A. The Importance of the World Health Organization Sugar Guidelines for Dental Health and Obesity Prevention. Caries Res. 2019, 53, 149–152. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Rother, K.I. Nonnutritive Sweeteners in Weight Management and Chronic Disease: A Review. Obesity 2018, 26, 635–640. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Lee, Y.Y. Neurophysiological symptoms and aspartame: What is the connection? Nutr. Neurosci. 2018, 21, 306–316. [Google Scholar] [CrossRef]
- Sylvetsky Meni, A.C.; Swithers, S.E.; Rother, K.I. Positive association between artificially sweetened beverage consumption and incidence of diabetes. Diabetologia 2015, 58, 2455–2456. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Bertrand, K.A.; Birmann, B.M.; Sampson, L.; Willett, W.C.; Feskanich, D. Consumption of artificial sweetener–and sugar-containing soda and risk of lymphoma and leukemia in men and women. Am. J. Clin. Nutr. 2012, 96, 1419–1428. [Google Scholar] [CrossRef]
- Archibald, A.J.; Dolinsky, V.W.; Azad, M.B. Early-Life Exposure to Non-Nutritive Sweeteners and the Developmental Origins of Childhood Obesity: Global Evidence from Human and Rodent Studies. Nutrients 2018, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Berkey, C.S.; Rockett, H.R.H.; Field, A.E.; Gillman, M.W.; Colditz, G.A. Sugar-added beverages and adolescent weight change. Obes. Res. 2004, 12, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Mander, A.P.; Jones, L.R.; Emmett, P.M.; Jebb, S.A. Is sugar-sweetened beverage consumption associated with increased fatness in children? Nutrition 2007, 23, 557–563. [Google Scholar] [CrossRef]
- Laverty, A.A.; Magee, L.; Monteiro, C.A.; Saxena, S.; Millett, C. Sugar and artificially sweetened beverage consumption and adiposity changes: National longitudinal study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Broyles, S.T.; Champagne, C.M.; Chaput, J.-P.; Fogelholm, M.; Hu, G.; Kuriyan, R.; Kurpad, A.; Lambert, E.V.; Maia, J.; et al. Relationship between Soft Drink Consumption and Obesity in 9–11 Years Old Children in a Multi-National Study. Nutrients 2016, 8, 770. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Jin, Y.; Mathieu, K.; DiPietro, L.; Rother, K.I.; Talegawkar, S.A. Low-Calorie Sweeteners: Disturbing the Energy Balance Equation in Adolescents? Obesity 2017, 25, 2049–2054. [Google Scholar] [CrossRef] [PubMed]
- O′Connor, T.M.; Yang, S.J.; Nicklas, T.A. Beverage intake among preschool children and its effect on weight status. Pediatrics 2006, 118, e1010-8. [Google Scholar] [CrossRef] [PubMed]
- Kral, T.V.; Stunkard, A.J.; Berkowitz, R.I.; Stallings, V.A.; Moore, R.H.; Faith, M.S. Beverage consumption patterns of children born at different risk of obesity. Obesity 2008, 16, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Taljaard, C.; Covic, N.; Van Graan, A.E.; Kruger, H.S.; Smuts, C.; Baumgartner, J.; Kvalsvig, J.D.; Wright, H.H.; Van Stuijvenberg, M.E.; Jerling, J. Effects of a multi-micronutrient-fortified beverage, with and without sugar, on growth and cognition in South African schoolchildren: A randomised, double-blind, controlled intervention. Br. J. Nutr. 2013, 110, 2271–2284. [Google Scholar] [CrossRef]
- Rodearmel, S.J.; Wyatt, H.R.; Stroebele, N.; Smith, S.M.; Ogden, L.G.; Hill, J.O. Small changes in dietary sugar and physical activity as an approach to preventing excessive weight gain: The America on the Move family study. Pediatrics 2007, 120, e869-79. [Google Scholar] [CrossRef]
- Williams, C.L.; Strobino, B.A.; Brotanek, J. Weight control among obese adolescents: A pilot study. Int. J. Food Sci. Nutr. 2007, 58, 217–230. [Google Scholar] [CrossRef]
- De Ruyter, J.C.; Olthof, M.R.; Seidell, J.C.; Katan, M.B. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N. Engl. J. Med. 2012, 367, 1397–1406. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Feldman, H.A.; Chomitz, V.R.; Antonelli, T.A.; Gortmaker, S.L.; Osganian, S.K.; Ludwig, D.S. A randomized trial of sugar-sweetened beverages and adolescent body weight. N. Engl. J. Med. 2012, 367, 1407–1416. [Google Scholar] [CrossRef]
- Azad, M.B.; Sharma, A.K.; de Souza, R.; Dolinsky, V.W.; Becker, A.B.; Mandhane, P.J.; Turvey, S.; Subbarao, P.; Lefebvre, D.L.; Sears, M.R.; et al. Association Between Artificially Sweetened Beverage Consumption During Pregnancy and Infant Body Mass Index. JAMA Pediatr. 2016, 170, 662–670. [Google Scholar] [CrossRef]
- Zhu, Y.; Olsen, S.F.; Mendola, P.; Halldorsson, T.I.; Rawal, S.; Hinkle, S.N.; Yeung, E.H.; Chavarro, J.E.; Grunnet, L.G.; Granström, C.; et al. Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: A prospective cohort study. Int. J. Epidemiol. 2017, 46, 1499–1508. [Google Scholar] [CrossRef]
- Von Poser Toigo, E.; Huffell, A.; Mota, C.; Bertolini, D.; Pettenuzzo, L.; Dalmaz, C. Metabolic and feeding behavior alterations provoked by prenatal exposure to aspartame. Appetite 2015, 87, 168–174. [Google Scholar] [CrossRef]
- Collison, K.S.; Makhoul, N.J.; Zaidi, M.Z.; Saleh, S.M.; Andres, B.; Inglis, A.; Al-Rabiah, R.; Al-Mohanna, F.A. Gender dimorphism in aspartame-induced impairment of spatial cognition and insulin sensitivity. PLoS ONE 2012, 7, e31570. [Google Scholar] [CrossRef]
- Parlee, S.D.; Simon, B.R.; Scheller, E.L.; Alejandro, E.U.; Learman, B.S.; Krishnan, V.; Bernal-Mizrachi, E.; MacDougald, O.A. Administration of saccharin to neonatal mice influences body composition of adult males and reduces body weight of females. Endocrinology 2014, 155, 1313–1326. [Google Scholar] [CrossRef]
- Chu, Y.-Y.; Chen, Y.-H.; Hsieh, R.-H.; Hsia, S.-M.; Wu, H.-T.; Chen, Y.-C. Development and Validation of the Chinese Version Non-Nutritive Sweetener Food Frequency Questionnaire with Urinary Biomarker in Children and Adolescents. Public Health Nutr. 2022, 25, 1–23. [Google Scholar] [CrossRef]
- Emmanuel, M.; Bokor, B.R. Tanner Stages. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Prader, A. Testicular size: Assessment and clinical importance. Triangle Sandoz J. Med. Sci. 1966, 7, 240–243. [Google Scholar]
- Chen, W.; Chang, M.H. New growth charts for Taiwanese children and adolescents based on World Health Organization standards and health-related physical fitness. Pediatr. Neonatol. 2010, 51, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-S.; Hsieh, R.-H.; Tung, Y.-T.; Chen, Y.-H.; Yang, C.; Chen, Y.C. Evaluation of a Technological Image-Based Dietary Assessment Tool for Children during Pubertal Growth: A Pilot Study. Nutrients 2019, 11, 2527. [Google Scholar] [CrossRef] [PubMed]
- Luque, V.; Closa-Monasterolo, R.; Rubio-Torrents, C.; Zaragoza-Jordana, M.; Ferré, N.; Gispert-Llauradó, M.; Escribano, J. Bioimpedance in 7-year-old children: Validation by dual X-ray absorptiometry—Part 1: Assessment of whole body composition. Ann. Nutr. Metab. 2014, 64, 113–121. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, P.; Bakker, M.; Zwitser, R.J.; Michel, N.; Hofman, A.; Francis, T.; Partchev, I. The Estimation of Item Response Models with the lmer Function from the lme4 Package in R. J. Stat. Softw. 2011, 39, 1–28. [Google Scholar] [CrossRef]
- World Health Organization. Evaluation of Certain Food Additives: Sixty-Fifth Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Forshee, R.A.; Storey, M.L. Total beverage consumption and beverage choices among children and adolescents. Int. J. Food Sci. Nutr. 2003, 54, 297–307. [Google Scholar] [CrossRef]
- Blum, J.W.; Jacobsen, D.J.; Donnelly, J.E. Beverage consumption patterns in elementary school aged children across a two-year period. J. Am. Coll. Nutr. 2005, 24, 93–98. [Google Scholar] [CrossRef]
- Hasnain, S.R.; Singer, M.R.; Bradlee, M.L.; Moore, L.L. Beverage intake in early childhood and change in body fat from preschool to adolescence. Child. Obes. 2014, 10, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Giammattei, J.; Blix, G.; Marshak, H.H.; Wollitzer, A.O.; Pettitt, D.J. Television watching and soft drink consumption: Associations with obesity in 11- to 13-year-old schoolchildren. Arch. Pediatr. Adolesc. Med. 2003, 157, 882–886. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Jin, Y.; Clark, E.J.; Welsh, J.A.; Rother, K.I.; Talegawkar, S.A. Consumption of Low-Calorie Sweeteners among Children and Adults in the United States. J. Acad. Nutr. Diet. 2017, 117, 441–448.e2. [Google Scholar] [CrossRef] [PubMed]
- Palmnäs, M.S.A.; Cowan, T.E.; Bomhof, M.R.; Su, J.; Reimer, R.A.; Vogel, H.J.; Hittel, D.S.; Shearer, J. Low-dose aspartame consumption differentially affects gut microbiota-host metabolic interactions in the diet-induced obese rat. PLoS ONE 2014, 9, e109841. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, M.; Park, J.; Kim, E. Aspartame downregulates 3T3-L1 differentiation. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 851–857. [Google Scholar] [CrossRef]
- Fernando, H.A.; Chandramouli, C.; Rosli, D.; Lam, Y.L.; Yong, S.T.; Yaw, H.P.; Ton, S.H.; Kadir, K.A.; Sainsbury, A. Glycyrrhizic acid can attenuate metabolic deviations caused by a high-sucrose diet without causing water retention in male Sprague-Dawley rats. Nutrients 2014, 6, 4856–4871. [Google Scholar] [CrossRef]
- Ghorbanlou, M.; Rostamkhani, S.; Shokri, S.; Mahmazi, S.; Fallah, R.; Moradi, F.; Nejatbakhsh, R. Possible ameliorating effects of Glycyrrhiza Glabra (Licorice) on the sperm parameters in rats under high fat diet. Endocr. Regul. 2020, 54, 22–30. [Google Scholar] [CrossRef]
- Blakemore, S.J.; Burnett, S.; Dahl, R.E. The role of puberty in the developing adolescent brain. Hum. Brain. Mapp. 2010, 31, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Clegg, D.J. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J. Steroid Biochem. Mol. Biol. 2010, 122, 65–73. [Google Scholar] [CrossRef]
- Masubuchi, Y.; Nakagawa, Y.; Ma, J.; Sasaki, T.; Kitamura, T.; Yamamoto, Y.; Kurose, H.; Kojima, I.; Shibata, H. A novel regulatory function of sweet taste-sensing receptor in adipogenic differentiation of 3T3-L1 cells. PLoS ONE 2013, 8, e54500. [Google Scholar] [CrossRef] [PubMed]
- Higgins, K.A.; Mattes, R.D. A randomized controlled trial contrasting the effects of 4 low-calorie sweeteners and sucrose on body weight in adults with overweight or obesity. Am. J. Clin. Nutr. 2019, 109, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Nagasawa, M.; Yamada, S.; Hara, A.; Mogami, H.; Nikolaev, V.O.; Lohse, M.J.; Shigemura, N.; Ninomiya, Y.; Kojima, I. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE 2009, 4, e5106. [Google Scholar] [CrossRef]
- Abudula, R.; Matchkov, V.V.; Jeppesen, P.B.; Nilsson, H.; Aalkjaer, C.; Hermansen, K. Rebaudioside A directly stimulates insulin secretion from pancreatic beta cells: A glucose-dependent action via inhibition of ATP-sensitive K+-channels. Diabetes Obes. Metab. 2008, 10, 1074–1085. [Google Scholar] [CrossRef]
- Moran, A.W.; Al-Rammahi, M.A.; Arora, D.K.; Batchelor, D.J.; Coulter, E.A.; Daly, K.; Ionescu, C.; Bravo, D.; Shirazi-Beechey, S.P. Expression of Na+/glucose co-transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. Br. J. Nutr. 2010, 104, 637–646. [Google Scholar] [CrossRef]
- Stearns, A.; Balakrishnan, A.; Rhoads, D.B.; Tavakkolizadeh, A. Rapid upregulation of sodium-glucose transporter SGLT1 in response to intestinal sweet taste stimulation. Ann. Surg. 2010, 251, 865–871. [Google Scholar] [CrossRef]
- Nettleton, J.E.; Reimer, R.A.; Shearer, J. Reshaping the gut microbiota: Impact of low calorie sweeteners and the link to insulin resistance? Physiol. Behav. 2016, 164, 488–493. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]

| Characteristics | Girls | Boys | p Value | ||
|---|---|---|---|---|---|
| n | 1239 | 654 | |||
| Age (years) | 9.69 | 1.82 (SD) | 11.78 | 1.93 (SD) | <0.01 |
| Birth weight (g) | 2922.14 | 563.43 (SD) | 3012.23 | 532.96 (SD) | <0.01 |
| Breastfed | 857 | 83.69% | 415 | 80.74% | 0.17 |
| Poor sleep quality | 219 | 20.64% | 139 | 25.93% | 0.02 |
| Total calorie intake (kcal) | 1494.79 | 426.99 (SD) | 1792.37 | 462.59 (SD) | <0.01 |
| Parental education | 0.36 | ||||
| Senior high school or below | 82 | 7.97% | 34 | 6.63% | |
| College | 591 | 57.43% | 285 | 55.56% | |
| Graduate school or higher | 356 | 34.60% | 194 | 37.82% | |
| Family income NTD | 0.03 | ||||
| <70,000 | 119 | 11.69% | 39 | 7.68% | |
| 70,000–100,000 | 374 | 36.74% | 182 | 35.83% | |
| >100,000 | 525 | 51.57% | 287 | 56.50% | |
| Physical activity METD | 0.03 | ||||
| Mild (<3 kcal/kg/h) | 345 | 55.83% | 100 | 29.33% | |
| Moderate (3~6 kcal/kg/h) | 147 | 23.79% | 90 | 26.39% | |
| Vigorous (>6 kcal/kg/h) | 126 | 20.39% | 151 | 44.28% | |
| Tanner stage | 0.02 | ||||
| Tanner I and II | 647 | 77.67% | 394 | 83.12% | |
| Tanner III–V | 186 | 22.33% | 80 | 16.88% | |
| NNS consumption | |||||
| Acesulfame-K | 198 | 21.50% | 155 | 33.99% | <0.01 |
| Aspartame | 314 | 34.09% | 198 | 43.42% | <0.01 |
| Sucralose | 348 | 37.79% | 201 | 44.08% | 0.03 |
| Glycyrrhizin | 322 | 34.96% | 144 | 31.58% | 0.24 |
| Stevioside | 119 | 12.92% | 70 | 15.35% | 0.25 |
| Sorbitol | 477 | 51.79% | 212 | 46.49% | 0.07 |
| Added sugar | 615 | 66.78% | 300 | 65.79% | 0.78 |
| Absolute intake (mg)/(%ADI) | (mg) | (%ADI) | (mg) | (%ADI) | |
| Acesulfame-K | 0.0007 | 0.0040 | 0.0023 | 0.0094 | <0.01 |
| Aspartame | 0.0004 | 0.0021 | 0.0010 | 0.0050 | 0.01 |
| Sucralose | 0.0021 | 0.0091 | 0.0040 | 0.0129 | 0.02 |
| Glycyrrhizin | 0.0009 | 0.0029 | 0.0007 | 0.0023 | 0.06 |
| Stevioside | 0.0010 | 0.0040 | 0.0023 | 0.0094 | 0.11 |
| Sorbitol | 0.0003 | 0.0008 | 0.0004 | 0.0015 | 0.48 |
| Added sugar | 0.0037 | 0.0076 | 0.0036 | 0.0065 | 0.65 |
| Body mass index kg/m2 (z-score) | 0.19 | 1.34 | 0.39 | 1.47 | <0.01 |
| Obesity | 140 | 11.41% | 86 | 13.35% | 0.25 |
| Fat mass (%) | 20.13 | 8.49 | 18.66 | 11.66 | 0.01 |
| Fat-free mass (%) | 79.87 | 8.48 | 81.34 | 11.67 | 0.01 |
| Waist circumference (cm) | 60.14 | 21.58 | 66.87 | 30.06 | <0.01 |
| Waist-to-hip ratio (%) | 0.84 | 0.33 | 0.85 | 0.33 | 0.36 |
| Waist-to-height ratio (%) | 0.44 | 0.15 | 0.46 | 0.93 | 0.06 |
| Sweetener | Exposure Amount * | n | BMI-z Score | Fat Mass % | Fat-Free Mass % | Waist-to-Height Ratio | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p Value | β | 95% CI | p Value | β | 95% CI | p Value | β | 95% CI | p Value | |||||||
| Acesulfame-K | 0 | 944 | ref | - | - | - | ref | - | - | - | ref | - | - | - | ref | - | - | - |
| T1 | 109 | −0.05 | −0.19 | 0.09 | 0.48 | 0.25 | −0.89 | 1.39 | 0.67 | −0.25 | −1.39 | 0.89 | 0.67 | <0.01 | −0.02 | 0.02 | 0.84 | |
| T2 | 112 | −0.17 | −0.30 | −0.04 | 0.01 | −1.02 | −2.06 | 0.03 | 0.06 | 1.02 | −0.03 | 2.07 | 0.06 | −0.01 | −0.03 | 0.01 | 0.40 | |
| T3 | 115 | −0.11 | −0.24 | 0.02 | 0.09 | −0.11 | −1.16 | 0.94 | 0.84 | 0.10 | −0.95 | 1.15 | 0.86 | −0.02 | −0.04 | <0.01 | 0.13 | |
| Aspartame | 0 | 816 | ref | - | - | - | ref | - | - | - | ref | - | - | - | ref | - | - | - |
| T1 | 157 | 0.01 | −0.09 | 0.10 | 0.91 | −0.17 | −0.98 | 0.63 | 0.68 | 0.16 | −0.65 | 0.97 | 0.70 | <0.01 | −0.02 | 0.02 | 0.75 | |
| T2 | 167 | −0.20 | −0.31 | −0.10 | <0.01 | −1.08 | −1.95 | −0.21 | 0.02 | 1.09 | 0.22 | 1.97 | 0.01 | 0.01 | −0.01 | 0.03 | 0.42 | |
| T3 | 166 | −0.16 | −0.27 | −0.06 | <0.01 | −1.21 | −2.04 | −0.38 | <0.01 | 1.20 | 0.36 | 2.03 | 0.01 | 0.01 | −0.01 | 0.03 | 0.30 | |
| Sucralose | 0 | 778 | ref | - | - | - | ref | - | - | - | ref | - | - | - | ref | - | - | - |
| T1 | 183 | −0.06 | −0.15 | 0.04 | 0.27 | 0.05 | −0.75 | 0.85 | 0.90 | −0.03 | −0.83 | 0.78 | 0.95 | 0.01 | −0.01 | 0.03 | 0.25 | |
| T2 | 173 | −0.13 | −0.24 | −0.03 | 0.01 | −1.20 | −2.02 | −0.37 | <0.01 | 1.18 | 0.36 | 2.01 | <0.01 | <0.01 | −0.01 | 0.02 | 0.69 | |
| T3 | 174 | −0.14 | −0.24 | −0.04 | 0.01 | −0.62 | −1.42 | 0.19 | 0.13 | 0.62 | −0.19 | 1.43 | 0.13 | −0.01 | −0.03 | <0.01 | 0.11 | |
| Glycyrrhizin | 0 | 860 | ref | - | - | - | ref | - | - | - | ref | - | - | - | ref | - | - | - |
| T1 | 147 | −0.04 | −0.14 | 0.06 | 0.44 | −0.81 | −1.64 | 0.01 | 0.05 | 0.82 | <0.01 | 1.64 | 0.05 | <0.01 | −0.02 | 0.02 | 1.00 | |
| T2 | 145 | −0.03 | −0.13 | 0.06 | 0.50 | −0.28 | −1.07 | 0.51 | 0.48 | 0.30 | −0.49 | 1.10 | 0.45 | <0.01 | −0.02 | 0.02 | 0.89 | |
| T3 | 164 | −0.18 | −0.27 | −0.08 | <0.01 | −1.26 | −2.05 | −0.47 | <0.01 | 1.27 | 0.48 | 2.06 | <0.01 | −0.01 | −0.03 | 0.01 | 0.23 | |
| Stevioside | 0 | 1088 | ref | - | - | - | ref | - | - | - | ref | - | - | - | ref | - | - | - |
| T1 | 63 | 0.05 | −0.10 | 0.19 | 0.53 | 0.14 | −1.04 | 1.32 | 0.82 | −0.18 | −1.36 | 1.00 | 0.77 | <0.01 | −0.03 | 0.03 | 0.92 | |
| T2 | 61 | −0.18 | −0.34 | −0.03 | 0.02 | −1.24 | −2.49 | <0.01 | 0.05 | 1.26 | 0.01 | 2.50 | 0.05 | 0.01 | −0.02 | 0.04 | 0.40 | |
| T3 | 62 | −0.06 | −0.24 | 0.11 | 0.46 | −0.90 | −2.28 | 0.48 | 0.20 | 0.85 | −0.53 | 2.23 | 0.23 | −0.01 | −0.03 | 0.02 | 0.69 | |
| Sorbitol | 0 | 661 | ref | - | - | - | ref | - | - | - | ref | - | - | - | ref | - | - | - |
| T1 | 199 | 0.08 | −0.04 | 0.19 | 0.18 | 0.58 | −0.33 | 1.50 | 0.21 | −0.59 | −1.50 | 0.33 | 0.21 | 0.01 | −0.01 | 0.03 | 0.17 | |
| T2 | 227 | 0.01 | −0.09 | 0.10 | 0.92 | −0.11 | −0.89 | 0.67 | 0.78 | 0.12 | −0.66 | 0.91 | 0.76 | <0.01 | −0.01 | 0.02 | 0.66 | |
| T3 | 222 | −0.12 | −0.21 | −0.02 | 0.02 | −0.87 | −1.67 | −0.08 | 0.03 | 0.87 | 0.08 | 1.67 | 0.03 | −0.01 | −0.03 | 0.01 | 0.26 | |
| Added sugar | 0 | 453 | ref | - | - | - | ref | - | - | - | ref | - | - | - | ref | - | - | - |
| T1 | 289 | 0.07 | −0.01 | 0.16 | 0.11 | 0.69 | −0.01 | 1.38 | 0.05 | −0.68 | −1.38 | 0.02 | 0.06 | 0.01 | −0.01 | 0.03 | 0.20 | |
| T2 | 286 | −0.06 | −0.14 | 0.03 | 0.22 | −0.23 | −0.94 | 0.49 | 0.54 | 0.24 | −0.48 | 0.96 | 0.51 | <0.01 | −0.01 | 0.02 | 0.65 | |
| T3 | 303 | −0.08 | −0.17 | 0.01 | 0.09 | −0.26 | −1.01 | 0.49 | 0.50 | 0.28 | −0.47 | 1.03 | 0.47 | −0.01 | −0.02 | 0.01 | 0.54 | |
| Sweetener | Exposure Amount * | n | Fat Mass % | Fat-Free Mass % | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | Girls | Boys | p ** | Girls | Boys | p ** | |||||||||||||||
| Girls | Boys | β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | |||||||||
| Acesulfame-K | 0 | 936 | 258 | 678 | ref | - | - | - | ref | - | - | - | - | ref | - | - | - | ref | - | - | - | - |
| T1 | 143 | 47 | 96 | 0.46 | −0.79 | 1.71 | 0.47 | −0.41 | −2.66 | 1.84 | 0.72 | 0.30 | −0.47 | −1.72 | 0.78 | 0.46 | 0.41 | −1.84 | 2.67 | 0.72 | 0.29 | |
| T2 | 135 | 53 | 82 | −1.21 | −2.31 | −0.10 | 0.03 | −1.20 | −3.39 | 0.98 | 0.28 | 0.67 | 1.21 | 0.11 | 2.32 | 0.03 | 1.19 | −1.00 | 3.38 | 0.29 | 0.68 | |
| T3 | 127 | 67 | 60 | −1.20 | −2.54 | 0.14 | 0.08 | 0.34 | −1.37 | 2.05 | 0.70 | 0.25 | 1.17 | −0.17 | 2.51 | 0.09 | −0.34 | −2.05 | 1.37 | 0.70 | 0.27 | |
| Aspartame | 0 | 837 | 235 | 602 | ref | - | - | - | ref | - | - | - | - | ref | - | - | - | ref | - | - | - | - |
| T1 | 213 | 54 | 159 | −0.71 | −1.54 | 0.12 | 0.09 | 0.60 | −1.29 | 2.49 | 0.54 | 0.10 | 0.68 | −0.15 | 1.51 | 0.11 | −0.57 | −2.46 | 1.33 | 0.56 | 0.12 | |
| T2 | 192 | 77 | 115 | −1.33 | −2.30 | −0.37 | 0.01 | −1.14 | −2.82 | 0.55 | 0.19 | 0.72 | 1.36 | 0.39 | 2.32 | 0.01 | 1.13 | −0.56 | 2.81 | 0.19 | 0.70 | |
| T3 | 195 | 85 | 110 | −1.45 | −2.42 | −0.47 | <0.01 | −1.27 | −2.75 | 0.22 | 0.10 | 1.00 | 1.42 | 0.45 | 2.40 | <0.01 | 1.26 | −0.23 | 2.75 | 0.10 | 0.98 | |
| Sucralose | 0 | 804 | 231 | 573 | ref | - | - | - | ref | - | - | - | - | ref | - | - | - | ref | - | - | - | - |
| T1 | 224 | 69 | 155 | −0.08 | −1.00 | 0.85 | 0.87 | −0.53 | −2.02 | 0.96 | 0.49 | 0.60 | 0.10 | −0.83 | 1.03 | 0.83 | 0.56 | −0.93 | 2.05 | 0.46 | 0.59 | |
| T2 | 204 | 70 | 134 | −0.83 | −1.79 | 0.13 | 0.09 | −1.88 | −3.34 | −0.41 | 0.01 | 0.36 | 0.82 | −0.14 | 1.79 | 0.09 | 1.86 | 0.39 | 3.33 | 0.01 | 0.37 | |
| T3 | 196 | 78 | 118 | −1.16 | −2.08 | −0.23 | 0.01 | −0.12 | −1.64 | 1.40 | 0.87 | 0.18 | 1.16 | 0.24 | 2.09 | 0.01 | 0.13 | −1.39 | 1.65 | 0.87 | 0.19 | |
| Glycyrrhizin | 0 | 867 | 269 | 598 | ref | - | - | - | ref | - | - | - | - | ref | - | - | - | ref | - | - | - | - |
| T1 | 184 | 53 | 131 | −0.59 | −1.39 | 0.21 | 0.15 | −1.54 | −3.77 | 0.70 | 0.18 | 0.62 | 0.61 | −0.19 | 1.41 | 0.14 | 1.53 | −0.71 | 3.77 | 0.18 | 0.64 | |
| T2 | 190 | 52 | 138 | −0.58 | −1.38 | 0.22 | 0.16 | −0.06 | −1.98 | 1.87 | 0.95 | 0.32 | 0.62 | −0.18 | 1.42 | 0.13 | 0.04 | −1.89 | 1.97 | 0.97 | 0.29 | |
| T3 | 189 | 55 | 134 | −1.00 | −1.85 | −0.14 | 0.02 | −1.73 | −3.31 | −0.16 | 0.03 | 0.83 | 1.03 | 0.17 | 1.88 | 0.02 | 1.70 | 0.12 | 3.28 | 0.03 | 0.77 | |
| Stevioside | 0 | 1041 | 293 | 748 | ref | - | - | - | ref | - | - | - | - | ref | - | - | - | ref | - | - | - | - |
| T1 | 79 | 28 | 51 | −0.14 | −1.41 | 1.13 | 0.83 | 1.20 | −1.28 | 3.68 | 0.34 | 0.68 | 0.09 | −1.18 | 1.36 | 0.89 | −1.23 | −3.71 | 1.25 | 0.33 | 0.69 | |
| T2 | 70 | 31 | 39 | −1.52 | −2.91 | −0.13 | 0.03 | −0.55 | −2.90 | 1.80 | 0.65 | 0.40 | 1.50 | 0.11 | 2.89 | 0.04 | 0.62 | −1.74 | 2.98 | 0.61 | 0.44 | |
| T3 | 71 | 23 | 48 | 0.07 | −1.40 | 1.54 | 0.93 | −3.70 | −6.66 | −0.74 | 0.01 | 0.02 | −0.12 | −1.59 | 1.35 | 0.87 | 3.65 | 0.69 | 6.61 | 0.02 | 0.02 | |
| Sorbitol | 0 | 714 | 229 | 485 | ref | - | - | - | ref | - | - | - | - | ref | - | - | - | ref | - | - | - | - |
| T1 | 241 | 64 | 177 | 0.39 | −0.50 | 1.28 | 0.39 | 1.29 | −1.24 | 3.82 | 0.32 | 0.41 | −0.38 | −1.27 | 0.51 | 0.40 | −1.32 | −3.86 | 1.22 | 0.31 | 0.39 | |
| T2 | 258 | 67 | 191 | 0.04 | −0.77 | 0.85 | 0.92 | −0.50 | −2.22 | 1.22 | 0.57 | 0.69 | −0.03 | −0.84 | 0.78 | 0.95 | 0.51 | −1.21 | 2.23 | 0.56 | 0.70 | |
| T3 | 249 | 72 | 177 | −0.43 | −1.23 | 0.38 | 0.30 | −1.44 | −3.28 | 0.40 | 0.13 | 0.44 | 0.44 | −0.37 | 1.24 | 0.29 | 1.42 | −0.42 | 3.27 | 0.13 | 0.46 | |
| Added sugar | 0 | 554 | 169 | 385 | ref | - | - | - | ref | - | - | - | - | ref | - | - | - | ref | - | - | - | - |
| T1 | 331 | 103 | 228 | 0.68 | −0.02 | 1.38 | 0.06 | 0.20 | −1.50 | 1.89 | 0.82 | 0.65 | −0.67 | −1.37 | 0.03 | 0.06 | −0.19 | −1.89 | 1.51 | 0.82 | 0.65 | |
| T2 | 311 | 99 | 212 | −0.53 | −1.33 | 0.28 | 0.20 | 0.06 | −1.32 | 1.45 | 0.93 | 0.23 | 0.53 | −0.28 | 1.34 | 0.20 | −0.03 | −1.42 | 1.35 | 0.96 | 0.25 | |
| T3 | 331 | 109 | 222 | −0.30 | −1.18 | 0.58 | 0.51 | −0.40 | −1.75 | 0.96 | 0.57 | 0.77 | 0.31 | −0.57 | 1.19 | 0.49 | 0.42 | −0.94 | 1.77 | 0.55 | 0.79 | |
| Sweetener | Tanner Stage | n | Fat Mass % | Fat-Free Mass % | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | I and II | III–V | p ** | I and II | III–V | p ** | |||||||||||||||
| Exposure Amount * | I and II | III–V | β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | ||||||||
| Acesulfame-K | 0 | 781 | 694 | 87 | ref | - | - | - | ref | - | - | - | - | ref | - | - | - | ref | - | - | - | - |
| T1 | 122 | 106 | 16 | 0.33 | −0.98 | 1.64 | 0.62 | −0.65 | −3.77 | 2.48 | 0.69 | 0.67 | −0.34 | −1.65 | 0.97 | 0.61 | 0.64 | −2.47 | 3.76 | 0.69 | 0.66 | |
| T2 | 102 | 86 | 16 | −0.32 | −1.49 | 0.86 | 0.60 | −3.17 | −5.86 | −0.47 | 0.02 | 0.69 | 0.32 | −0.86 | 1.49 | 0.60 | 3.18 | 0.49 | 5.86 | 0.02 | 0.66 | |
| T3 | 110 | 94 | 16 | 0.43 | −0.72 | 1.58 | 0.46 | −2.00 | −5.32 | 1.33 | 0.24 | 0.13 | −0.44 | −1.59 | 0.71 | 0.45 | 1.97 | −1.35 | 5.28 | 0.25 | 0.14 | |
| Aspartame | 0 | 696 | 620 | 76 | ref | - | - | - | ref | - | - | - | - | ref | - | - | - | ref | - | - | - | - |
| T1 | 168 | 132 | 36 | 0.06 | −0.90 | 1.01 | 0.91 | −1.63 | −3.51 | 0.25 | 0.09 | 0.58 | −0.06 | −1.02 | 0.89 | 0.90 | 1.58 | −0.30 | 3.45 | 0.10 | 0.62 | |
| T2 | 161 | 134 | 27 | −1.03 | −2.03 | −0.03 | 0.04 | −2.51 | −4.75 | −0.27 | 0.03 | 0.94 | 1.05 | 0.05 | 2.05 | 0.04 | 2.50 | 0.27 | 4.73 | 0.03 | 0.93 | |
| T3 | 154 | 138 | 16 | −0.94 | −1.85 | −0.03 | 0.04 | −3.69 | −6.54 | −0.85 | 0.01 | 0.78 | 0.93 | 0.02 | 1.84 | 0.05 | 3.67 | 0.83 | 6.51 | 0.01 | 0.77 | |
| Sucralose | 0 | 652 | 575 | 77 | ref | - | - | - | ref | - | - | - | - | ref | - | - | - | ref | - | - | - | - |
| T1 | 200 | 169 | 31 | −0.30 | −1.21 | 0.60 | 0.51 | 1.27 | −0.81 | 3.35 | 0.23 | 0.15 | 0.33 | −0.58 | 1.24 | 0.48 | −1.23 | −3.30 | 0.84 | 0.25 | 0.15 | |
| T2 | 161 | 144 | 17 | −0.76 | −1.64 | 0.13 | 0.09 | −3.48 | −6.25 | −0.72 | 0.01 | 0.10 | 0.74 | −0.14 | 1.63 | 0.10 | 3.46 | 0.71 | 6.21 | 0.01 | 0.11 | |
| T3 | 169 | 143 | 26 | −0.07 | −0.98 | 0.84 | 0.88 | −1.78 | −3.88 | 0.33 | 0.10 | 0.46 | 0.08 | −0.83 | 0.99 | 0.87 | 1.79 | −0.31 | 3.88 | 0.10 | 0.44 | |
| Glycyrrhizin | 0 | 708 | 622 | 86 | ref | - | - | - | ref | - | - | - | - | ref | - | - | - | ref | - | - | - | - |
| T1 | 165 | 142 | 23 | −0.76 | −1.70 | 0.17 | 0.11 | −1.43 | −3.57 | 0.72 | 0.19 | 0.50 | 0.78 | −0.16 | 1.72 | 0.10 | 1.41 | −0.73 | 3.55 | 0.20 | 0.50 | |
| T2 | 147 | 131 | 16 | −0.20 | −1.07 | 0.67 | 0.65 | 0.43 | −2.08 | 2.93 | 0.74 | 0.77 | 0.22 | −0.65 | 1.09 | 0.62 | −0.38 | −2.87 | 2.12 | 0.77 | 0.77 | |
| T3 | 140 | 127 | 13 | −1.00 | −1.88 | −0.12 | 0.03 | −1.61 | −4.16 | 0.95 | 0.22 | 0.19 | 1.00 | 0.12 | 1.88 | 0.03 | 1.63 | −0.91 | 4.18 | 0.21 | 0.18 | |
| Stevioside | 0 | 890 | 796 | 94 | ref | - | - | - | ref | - | - | - | - | ref | - | - | - | ref | - | - | - | - |
| T1 | 62 | 54 | 8 | 0.70 | −0.57 | 1.97 | 0.28 | −1.74 | −6.83 | 3.34 | 0.50 | 0.47 | −0.74 | −2.01 | 0.54 | 0.26 | 1.66 | −3.41 | 6.73 | 0.52 | 0.45 | |
| T2 | 63 | 53 | 10 | −0.55 | −1.88 | 0.78 | 0.42 | −4.03 | −8.13 | 0.06 | 0.05 | 0.14 | 0.57 | −0.77 | 1.90 | 0.40 | 4.01 | −0.07 | 8.09 | 0.06 | 0.14 | |
| T3 | 61 | 51 | 10 | −1.30 | −2.91 | 0.31 | 0.11 | −0.09 | −3.34 | 3.15 | 0.96 | 0.29 | 1.27 | −0.35 | 2.88 | 0.12 | −0.03 | −3.27 | 3.21 | 0.99 | 0.27 | |
| Sorbitol | 0 | 593 | 521 | 72 | ref | - | - | - | ref | - | - | - | - | ref | - | - | - | ref | - | - | - | - |
| T1 | 199 | 169 | 30 | 1.21 | 0.16 | 2.26 | 0.02 | −0.63 | −3.23 | 1.97 | 0.63 | 0.25 | −1.22 | −2.28 | −0.17 | 0.02 | 0.66 | −1.93 | 3.26 | 0.62 | 0.26 | |
| T2 | 193 | 173 | 20 | −0.05 | −0.93 | 0.83 | 0.91 | −0.29 | −2.71 | 2.13 | 0.82 | 0.94 | 0.06 | −0.82 | 0.94 | 0.89 | 0.30 | −2.11 | 2.71 | 0.81 | 0.94 | |
| T3 | 201 | 171 | 30 | −0.62 | −1.52 | 0.29 | 0.18 | −2.39 | −4.80 | 0.02 | 0.05 | 0.19 | 0.62 | −0.29 | 1.53 | 0.18 | 2.42 | 0.01 | 4.82 | 0.05 | 0.17 | |
| Added sugar | 0 | 496 | 441 | 55 | ref | - | - | - | ref | - | - | - | - | ref | - | - | - | ref | - | - | - | - |
| T1 | 272 | 227 | 45 | 0.53 | −0.26 | 1.32 | 0.19 | −0.19 | −2.09 | 1.72 | 0.85 | 0.75 | −0.53 | −1.32 | 0.26 | 0.19 | 0.21 | −1.69 | 2.11 | 0.83 | 0.74 | |
| T2 | 240 | 200 | 40 | −0.05 | −0.88 | 0.78 | 0.90 | −1.84 | −3.68 | 0.01 | 0.05 | 0.17 | 0.07 | −0.77 | 0.90 | 0.87 | 1.86 | 0.02 | 3.70 | 0.05 | 0.18 | |
| T3 | 249 | 216 | 33 | −0.09 | −0.96 | 0.78 | 0.84 | −0.88 | −2.88 | 1.11 | 0.39 | 0.79 | 0.12 | −0.75 | 0.99 | 0.79 | 0.88 | −1.11 | 2.87 | 0.39 | 0.82 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, Y.-H.; Lin, C.-Y.; Hsu, S.-Y.; Chen, Y.-H.; Wu, H.-T.; Huang, S.-W.; Chen, Y.-C. Effects of Nonnutritive Sweeteners on Body Composition Changes during Pubertal Growth. Nutrients 2023, 15, 2319. https://doi.org/10.3390/nu15102319
Chien Y-H, Lin C-Y, Hsu S-Y, Chen Y-H, Wu H-T, Huang S-W, Chen Y-C. Effects of Nonnutritive Sweeteners on Body Composition Changes during Pubertal Growth. Nutrients. 2023; 15(10):2319. https://doi.org/10.3390/nu15102319
Chicago/Turabian StyleChien, Yu-Hsin, Chia-Yuan Lin, Shih-Yuan Hsu, Yue-Hwa Chen, Hung-Tsung Wu, Shiu-Wen Huang, and Yang-Ching Chen. 2023. "Effects of Nonnutritive Sweeteners on Body Composition Changes during Pubertal Growth" Nutrients 15, no. 10: 2319. https://doi.org/10.3390/nu15102319
APA StyleChien, Y.-H., Lin, C.-Y., Hsu, S.-Y., Chen, Y.-H., Wu, H.-T., Huang, S.-W., & Chen, Y.-C. (2023). Effects of Nonnutritive Sweeteners on Body Composition Changes during Pubertal Growth. Nutrients, 15(10), 2319. https://doi.org/10.3390/nu15102319







