Pseudomalignancies in Children: Histological Clues, and Pitfalls to Be Avoided
Abstract
1. Introduction
2. Lymphocytic Infiltrates
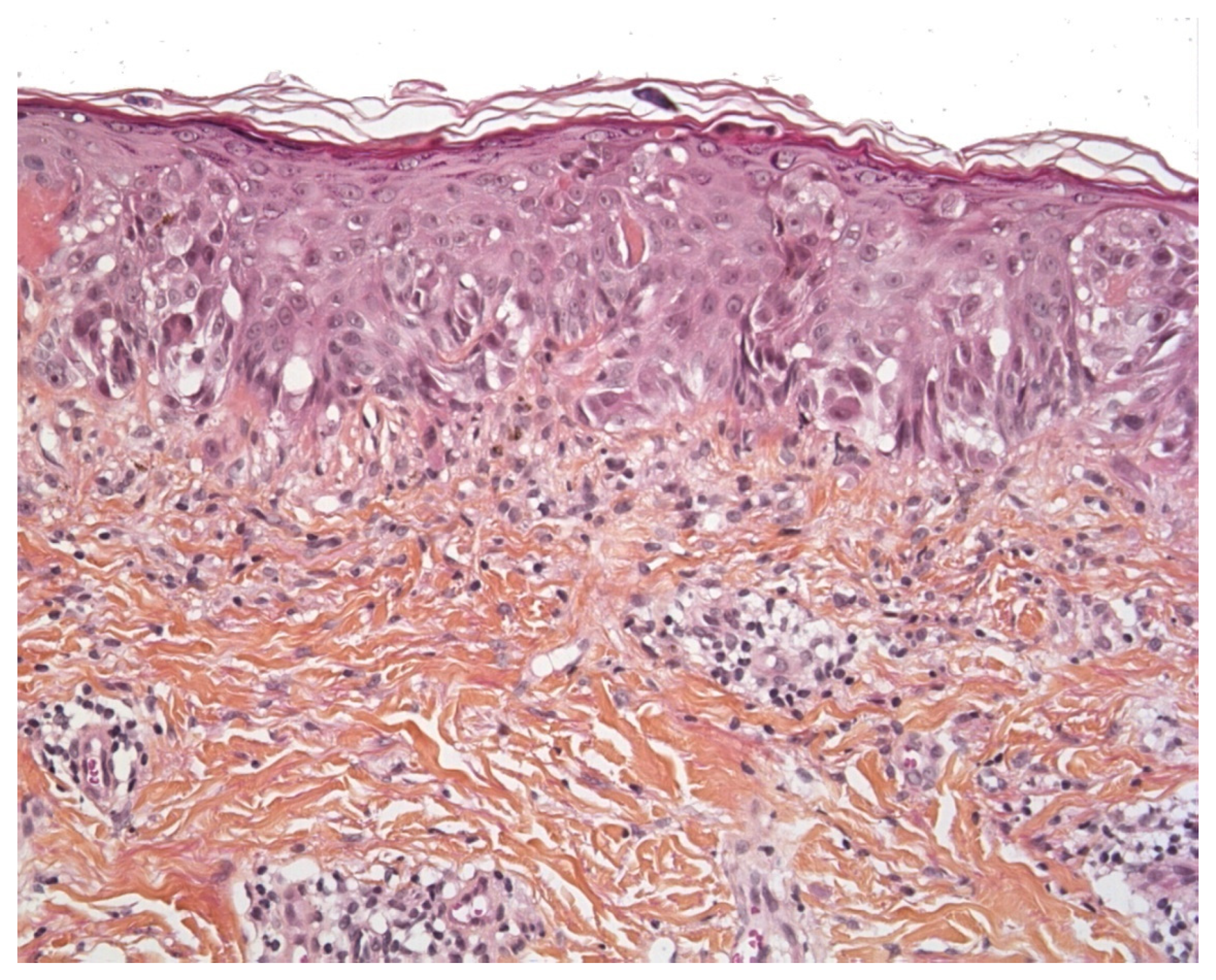
2.1. Vitiligo
2.2. Immunodeficiencies Rashes
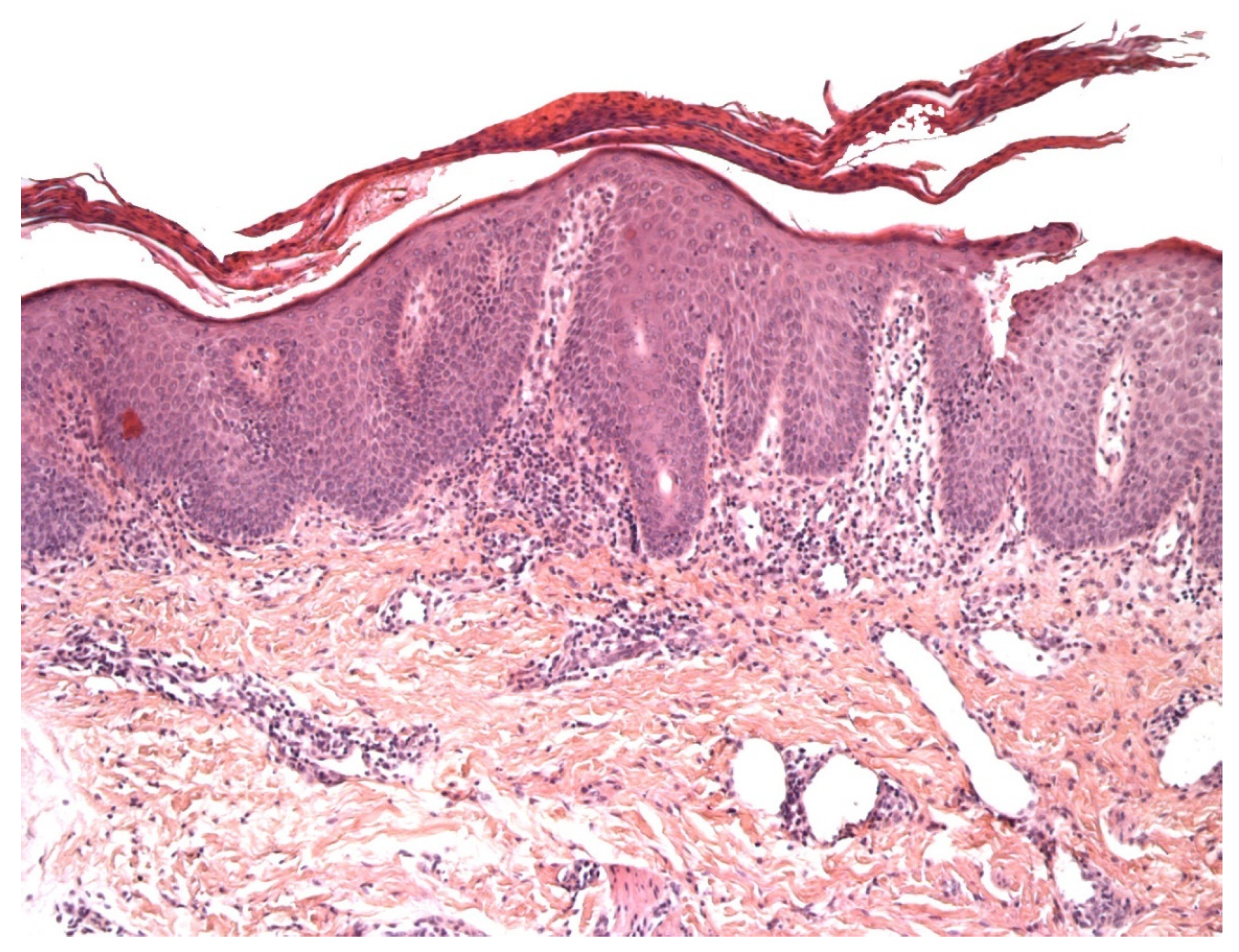
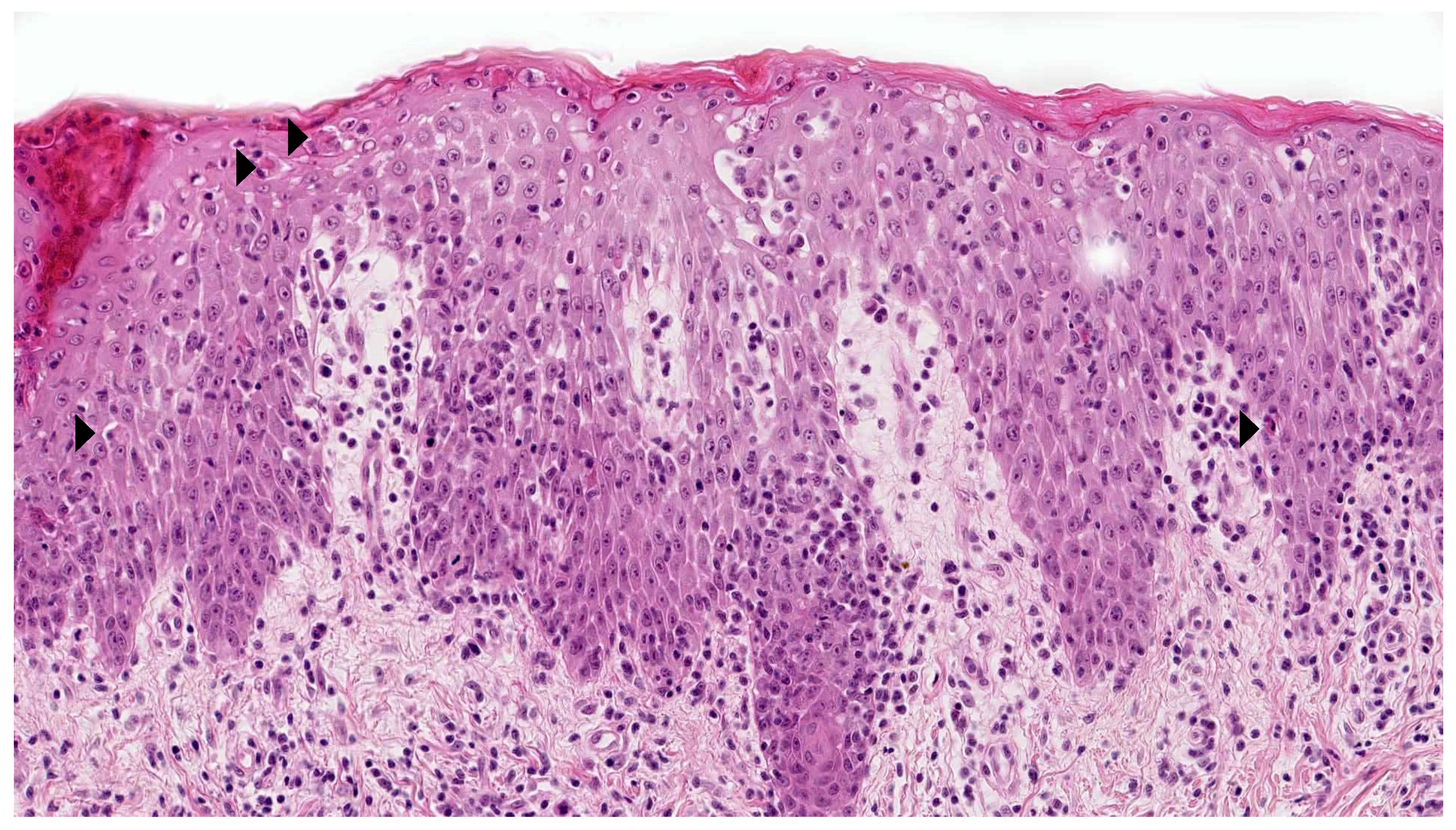
2.3. Pityriasis Lichenoides
2.4. Scabies Nodules and Insect Bite Reactions
2.5. Lymphoplasmacytic Plaque
2.6. Acral Pseudo-Lymphomatous Angiokeratoma of Children
3. Histiocytic Infiltrates
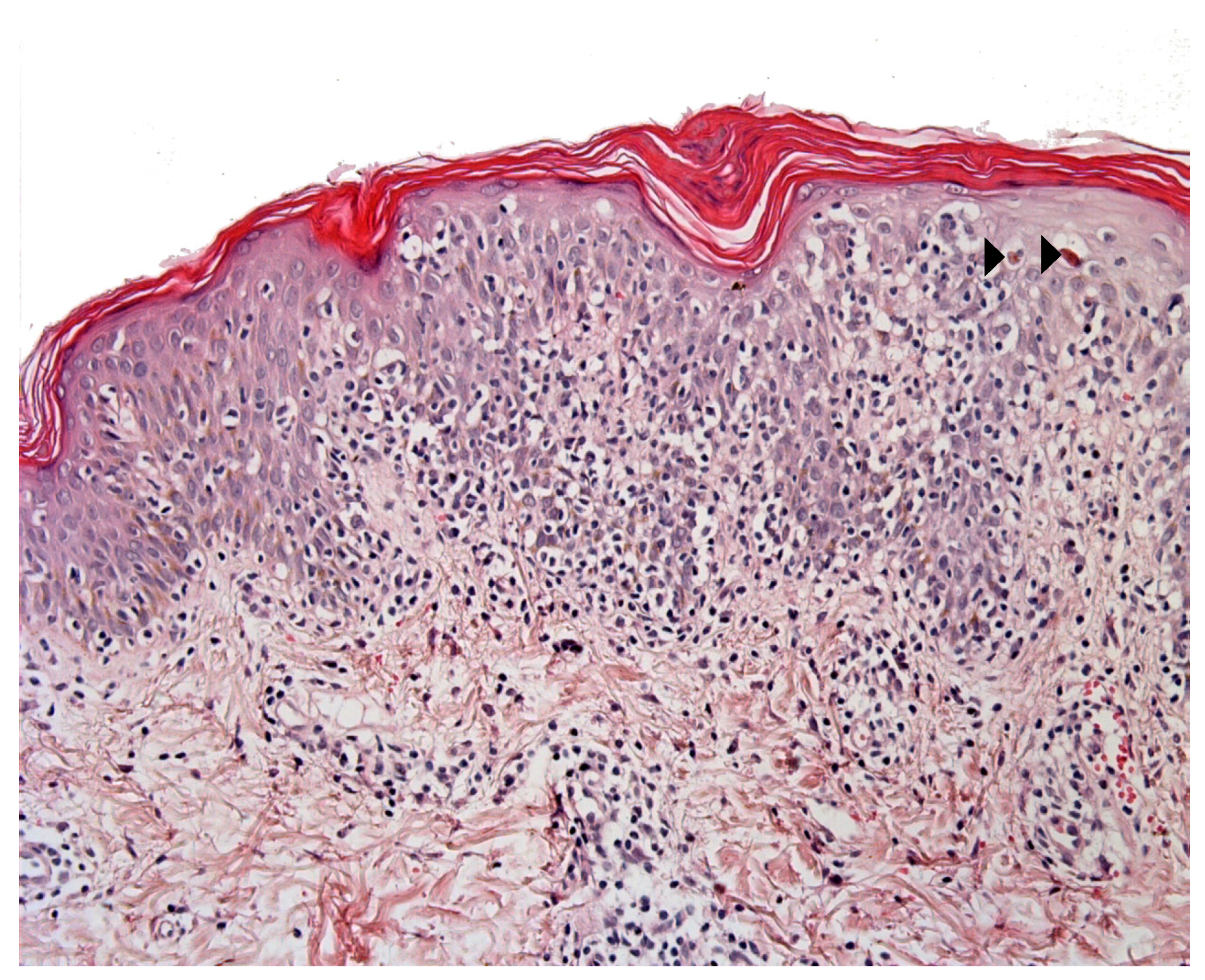
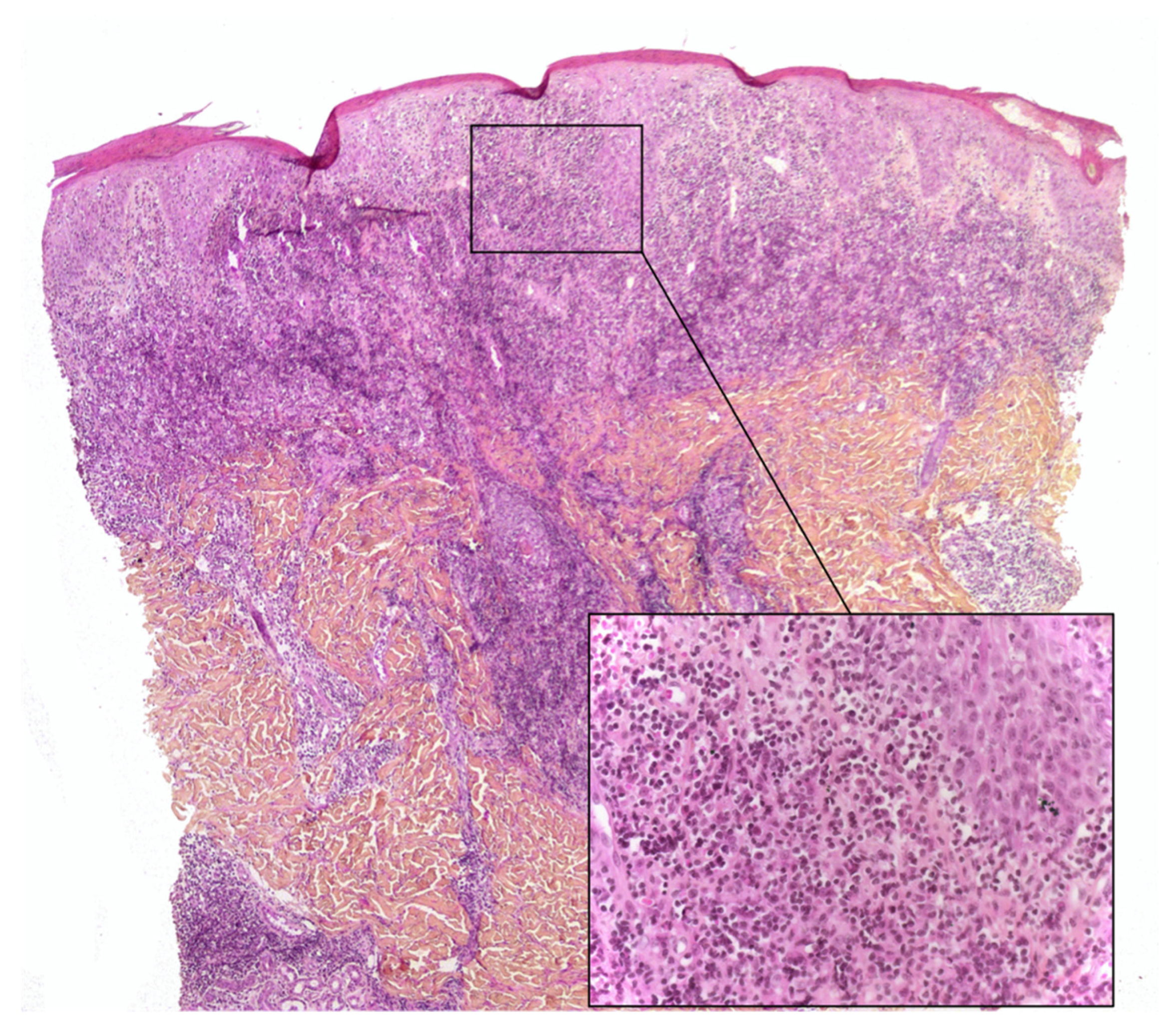
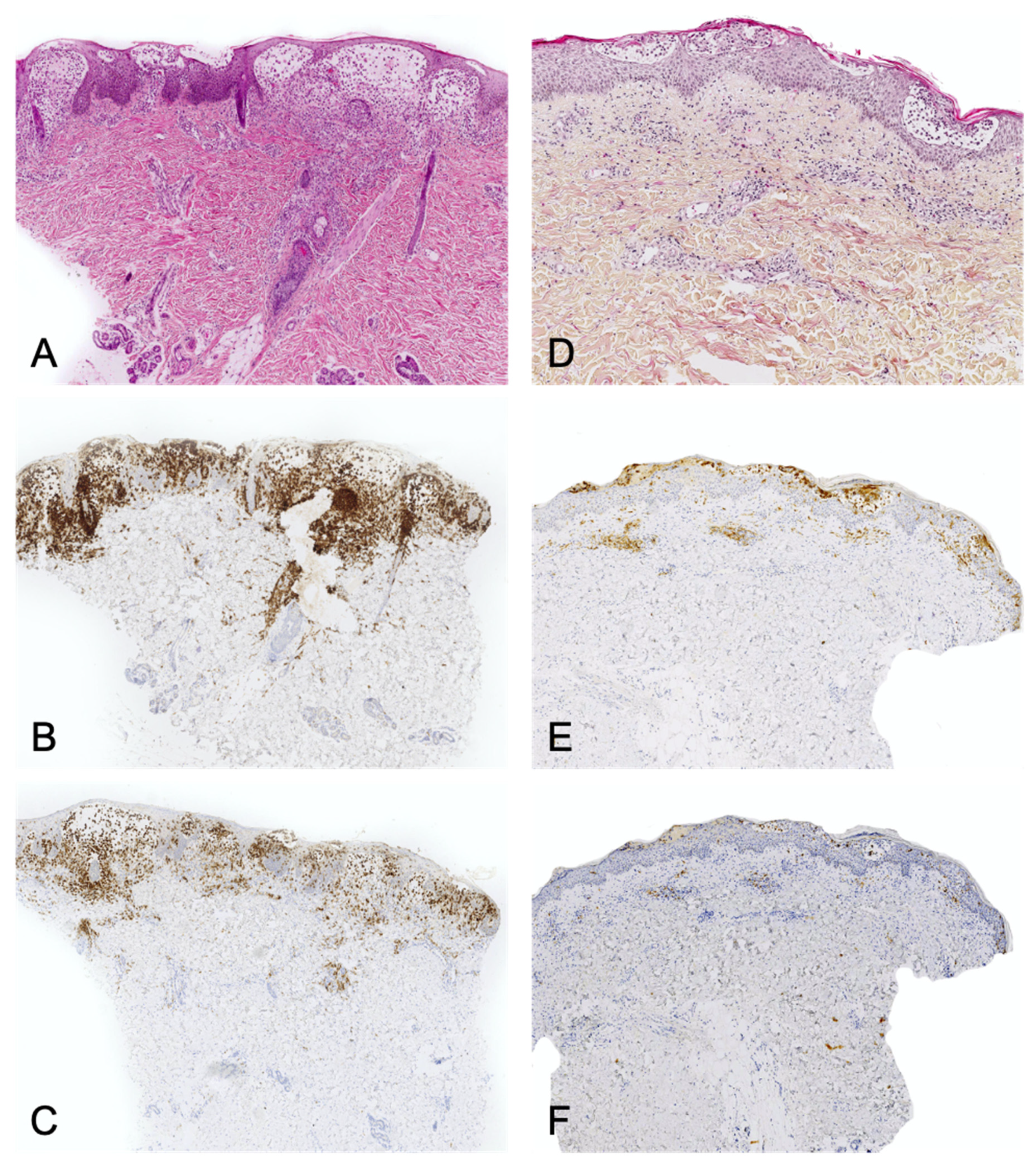
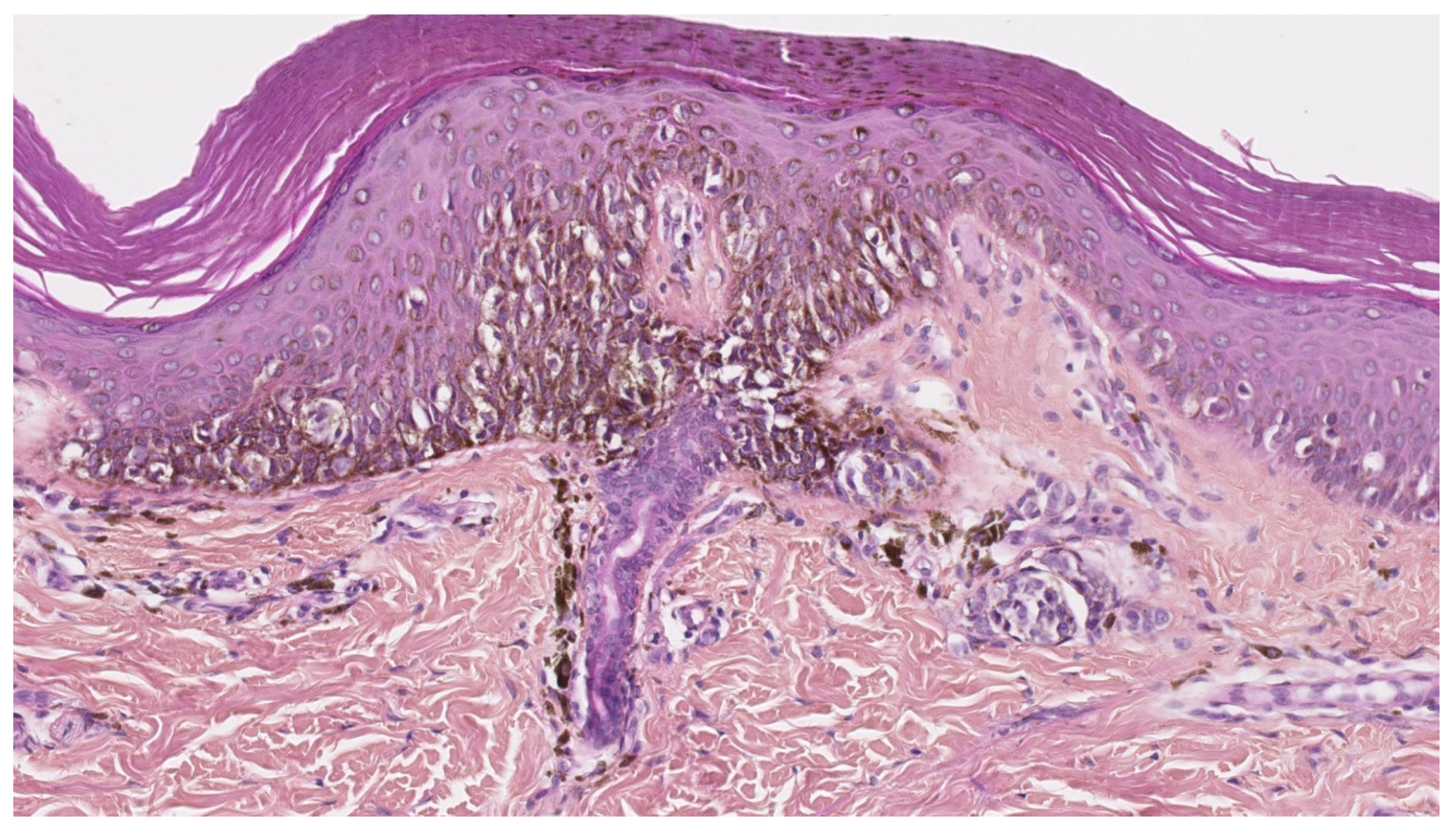
3.1. CD1a+ Dendritic Cell Hyperplasia
- The infiltrate’s architecture: in CD1a+ DCH, cells are mostly located around the vessels in the dermis and do not accumulate in the papillary dermis as in LCH;
- The infiltrate’s polymorphism: the CD1a+ cells are rarely predominant and are mixed with other cell types;
- Immunoreactivity: importantly, the cells are not true Langerhans cells because they do not usually express CD207 (Langerin); in fact, they are CD1a+/CD207− dendritic cells that lack Birbeck granules [27,33]. CD207 is a very sensitive, specific marker of Birbeck granules, which are located in the cytoplasm of Langerhans cells. Along with CD207, the presence of markers of MAPK pathway activation (involved in the pathogenesis of LCH [34]) can also help the pathologist to distinguish between CD1a+ DCH and LCH. In particular, cyclin D1 and pERK are expressed in all cases of LCH but are not expressed (or only weakly) in cases of “dermatitis” with CD1a+ DCH [35]. The CD1a+/CD207− dendritic cells probably come from bone marrow (as do Langerhans cells and interdigitated DCs) and might be indeterminate cells or immature Langerhans cell precursors.
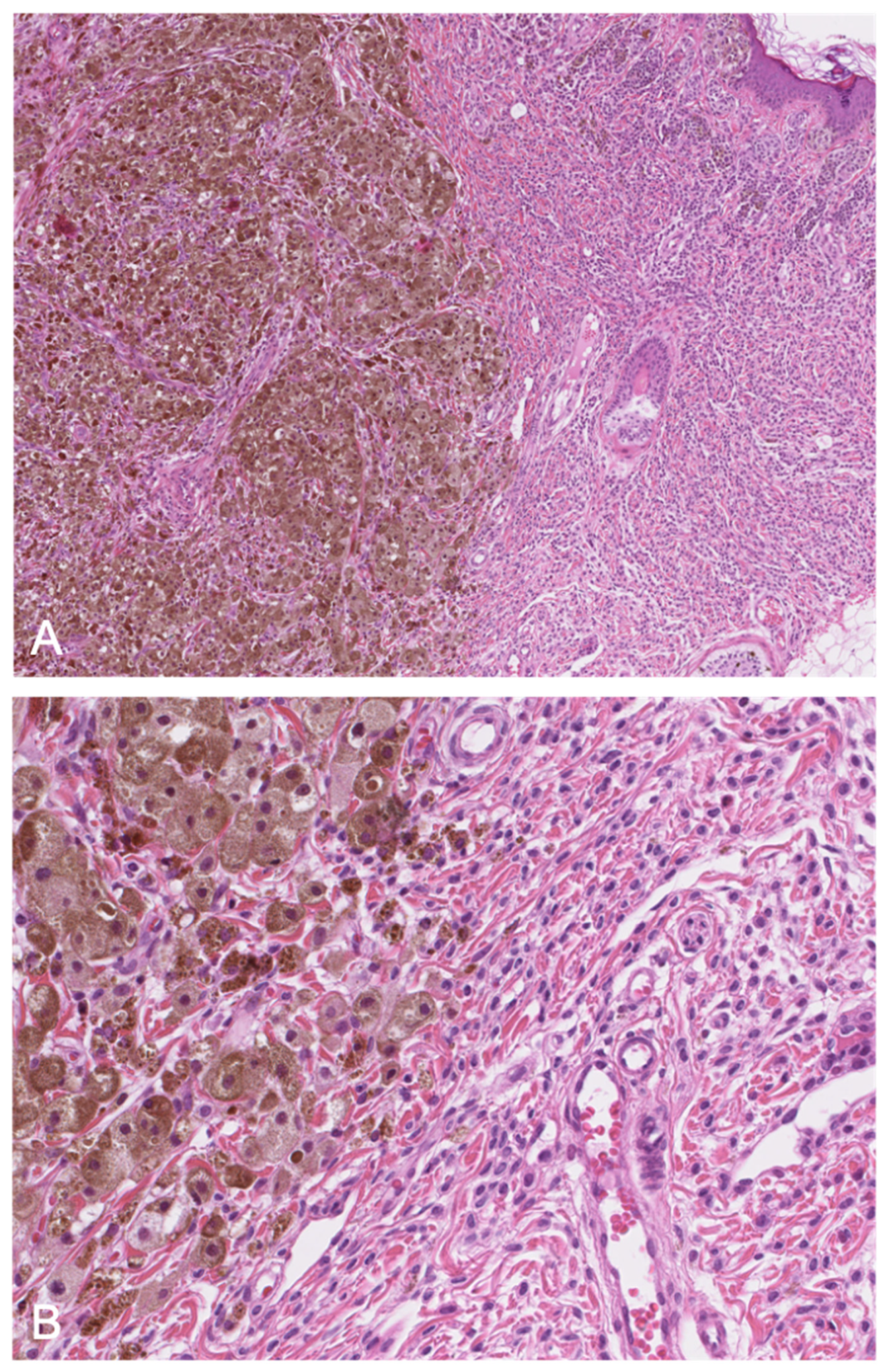
3.2. Juvenile Xanthogranuloma
4. Melanocytic Disorders
4.1. Melanocytic Lesions in Newborns
4.2. Melanocytic Lesions Associated with a Congenital Melanocytic Nevus
4.3. Juvenile Nevi
4.4. Spitz Nevus and Atypical Spitz Tumor
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Connors, R.C.; Ackerman, A.B. Histologic pseudomalignancies of the skin. Arch. Dermatol. 1976, 112, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Mitteldorf, C.; Kempf, W. Cutaneous pseudolymphoma-A review on the spectrum and a proposal for a new classification. J. Cutan. Pathol. 2020, 47, 76–97. [Google Scholar] [CrossRef]
- Ezzedine, K.; Eleftheriadou, V.; Whitton, M.; van Geel, N. Vitiligo. Lancet Lond. Engl. 2015, 386, 74–84. [Google Scholar] [CrossRef]
- Castano, E.; Glick, S.; Wolgast, L.; Naeem, R.; Sunkara, J.; Elston, D.; Jacobson, M. Hypopigmented mycosis fungoides in childhood and adolescence: A long-term retrospective study. J. Cutan. Pathol. 2013, 40, 924–934. [Google Scholar] [CrossRef]
- Werner, B.; Brown, S.; Ackerman, A.B. Hypopigmented Mycosis Fungoides? Is Not Always Mycosis Fungoides! Am. J. Dermatopathol. 2005, 27, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Furlan, F.C.; Pereira, B.A.D.P.; Da Silva, L.F.; Sanches, J.A. Loss of melanocytes in hypopigmented mycosis fungoides: A study of 18 patients. J. Cutan. Pathol. 2013, 41, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Leclerc-Mercier, S.; Bodemer, C.; Bourdon-Lanoy, E.; Larousserie, F.; Hovnanian, A.; Brousse, N.; Fraitag, S. Early skin biopsy is helpful for the diagnosis and management of neonatal and infantile erythrodermas. J. Cutan. Pathol. 2010, 37, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Menzinger, S.; Frassati-Biaggi, A.; Leclerc-Mercier, S.; Bodemer, C.; Molina, T.J.; Fraitag, S. Pityriasis Lichenoides: A Large Histopathological Case Series with a Focus on Adnexotropism. Am. J. Dermatopathol. 2020, 42, 1–10. [Google Scholar] [CrossRef]
- Dereure, O.; Levi, E.; Kadin, M.E. T-Cell clonality in pityriasis lichenoides et varioliformis acuta: A heteroduplex analysis of 20 cases. Arch. Dermatol. 2000, 136, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, J.M.; Kristal, L.; Chooback, L.; Honig, P.J.; Kramer, E.M.; Lessin, S.R. The Clonal Nature of Pityriasis Lichenoides. Arch. Dermatol. 2002, 138, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Kempf, W.; Kazakov, D.V.; Palmedo, G.; Fraitag, S.; Schaerer, L.; Kutzner, H. Pityriasis lichenoides et varioliformis acuta with numerous CD30(+) cells: A variant mimicking lymphomatoid papulosis and other cutaneous lymphomas. A clinicopathologic, immunohistochemical, and molecular biological study of 13 cases. Am. J. Surg. Pathol. 2012, 36, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, L.T.; Pieretti, M.; Chapman, S.F.; Horenstein, M.G. CD30-Positive Atypical Lymphoid Cells in Common Non-Neoplastic Cutaneous Infiltrates Rich in Neutrophils and Eosinophils. Am. J. Surg. Pathol. 2003, 27, 912–918. [Google Scholar] [CrossRef]
- Hwong, H.; Jones, D.; Prieto, V.G.; Schulz, C.; Duvic, M. Persistent Atypical Lymphocytic Hyperplasia Following Tick Bite in a Child: Report of a Case and Review of the Literature. Pediatr. Dermatol. 2001, 18, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, F.; Barranco, C.; Toll, A.; Pujol, R.M. CD30 antigen expression in cutaneous inflammatory infiltrates of scabies: A dynamic immunophenotypic pattern that should be distinguished from lymphomatoid papulosis. J. Cutan. Pathol. 2002, 29, 368–373. [Google Scholar] [CrossRef]
- Marín, N.D.; García, L.F. The role of CD30 and CD153 (CD30L) in the anti-mycobacterial immune response. Tuberculosis 2016, 102, 8–15. [Google Scholar] [CrossRef]
- Miquel, J.; Fraitag, S.; Hamel-Teillac, D.; Molina, T.; Brousse, N.; De Prost, Y.; Bodemer, C. Lymphomatoid papulosis in children: A series of 25 cases. Br. J. Dermatol. 2014, 171, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Moulonguet, I.; Hadj-Rabia, S.; Gounod, N.; Bodemer, C.; Fraitag, S. Tibial Lymphoplasmacytic Plaque: A New, Illustrative Case of a Recently and Poorly Recognized Benign Lesion in Children. Dermatology 2012, 225, 27–30. [Google Scholar] [CrossRef]
- Bierbrier, R.M.; Amdemichael, E.; Adam, D.N. Pretibial Lymphoplasmacytic Plaque in Children: Case Report and Review of the Literature. Am. J. Dermatopathol. 2019, 41, 300–302. [Google Scholar] [CrossRef]
- Tsilika, K.; Montaudié, H.; Castela, E.; Cardot-Leccia, N.; Passeron, T.; Lacour, J.-P. A case of lymphoplasmacytic plaque in children. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e171–e172. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/jdv.15408 (accessed on 21 March 2021). [CrossRef] [PubMed]
- Moulonguet, I.; Gantzer, A.; Bourdon-Lanoy, E.; Fraitag, S. A Pretibial Plaque in a Five and a Half year–Old Girl. Am. J. Dermatopathol. 2012, 34, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Kempf, W.; Kazakov, D.V.; Buechner, S.A.; Graf, M.; Zettl, A.; Zimmermann, D.R. Primary cutaneous marginal zone lymphoma in children: A report of 3 cases and review of the literature. Am. J. Dermatopathol. 2014, 36, 661–666. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fried, I.; Wiesner, T.; Cerroni, L. Pretibial Lymphoplasmacytic Plaque in Children. Arch. Dermatol. 2010, 146, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, Y.; Arakura, F.; Murata, H.; Koga, H.; Kawachi, S.; Nakazawa, K. Acral pseudolymphomatous angiokeratoma of children: A case report with immunohistochemical study of antipodoplanin antigen. Am. J. Dermatopathol. 2012, 34, e128–e132. [Google Scholar] [CrossRef] [PubMed]
- Lessa, P.P.; Jorge, J.C.F.; Ferreira, F.R.; Lira, M.L.D.A.; Mandelbaum, S.H. Acral pseudolymphomatous angiokeratoma: Case report and literature review. An. Bras. Dermatol. 2013, 88, 39–43. [Google Scholar] [CrossRef]
- Hagari, Y.; Hagari, S.; Kambe, N.; Kawaguchi, T.; Nakamoto, S.; Mihara, M. Acral pseudolymphomatous angiokeratoma of children: Immunohistochemical and clonal analyses of the infiltrating cells. J. Cutan. Pathol. 2002, 29, 313–318. [Google Scholar] [CrossRef]
- Evans, M.S.; Burkhart, C.N.; Bowers, E.V.; Culpepper, K.S.; Googe, P.B.; Magro, C.M. Solitary plaque on the leg of a child: A report of two cases and a brief review of acral pseudolymphomatous angiokeratoma of children and unilesional mycosis fungoides. Pediatr. Dermatol. 2018, 36, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Glusac, E.J. Langerhans cell hyperplasia in scabies: A mimic of Langerhans cell histiocytosis. J. Cutan. Pathol. 2007, 34, 716–720. [Google Scholar] [CrossRef]
- Hatter, A.D.; Zhou, X.; Honda, K.; Popkin, D.L. Langerhans Cell Hyperplasia from Molluscum Contagiosum. Am. J. Dermatopathol. 2015, 37, e93–e95. [Google Scholar] [CrossRef][Green Version]
- Kim, S.H.; Kim, D.H.; Gil Lee, K. Prominent Langerhans’ cell migration in the arthropod bite reactions simulating Langerhans’ cell histiocytosis. J. Cutan. Pathol. 2007, 34, 899–902. [Google Scholar] [CrossRef]
- Jokinen, C.H.; Wolgamot, G.M.; Wood, B.L.; Olerud, J.; Argenyi, Z.B. Lymphomatoid papulosis with CD1a+ dendritic cell hyperplasia, mimicking Langerhans cell histiocytosis. J. Cutan. Pathol. 2007, 34, 584–587. [Google Scholar] [CrossRef]
- Ezra, N.; Van Dyke, G.S.; Binder, S.W. CD30 positive anaplastic large-cell lymphoma mimicking Langerhans cell histiocytosis. J. Cutan. Pathol. 2009, 37, 787–792. [Google Scholar] [CrossRef]
- Burkert, K.L.; Huhn, K.; Whitaker Menezes, D.; Murphy, G.F. Langerhans cell microgranulomas (pseudo-pautrier abscesses): Morphologic diversity, diagnostic implications and pathogenetic mechanisms: Langerhans cell microgranulomas. J. Cutan. Pathol. 2002, 29, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Drut, R.; Peral, C.G.; Garone, A.; Rositto, A. Langerhans cell hyperplasia of the skin mimicking Langerhans cell histiocytosis: A report of two cases in children not associated with scabies. Fetal Pediatr. Pathol. 2010, 29, 231–238. [Google Scholar] [CrossRef]
- Tran, G.; Huynh, T.N.; Paller, A.S. Langerhans cell histiocytosis: A neoplastic disorder driven by Ras-ERK pathway mutations. J. Am. Acad. Dermatol. 2018, 78, 579–590.e4. [Google Scholar] [CrossRef]
- Shanmugam, V.; Craig, J.W.; Hornick, J.L.; Morgan, E.A.; Pinkus, G.S.; Pozdnyakova, O. Cyclin D1 Is Expressed in Neoplastic Cells of Langerhans Cell Histiocytosis but Not Reactive Langerhans Cell Proliferations. Am. J. Surg. Pathol. 2017, 41, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, F.; Caputo, R. Histiocytic syndromes: A review. J. Am. Acad. Dermatol. 1985, 13, 383–404. [Google Scholar] [CrossRef]
- World Health Organization. ID: gnd/1007857-5. WHO Classification of Skin Tumours. In World Health Organization Classification of Tumours, 4th ed.; Elder, D.E., Massi, D., Scolyer, R.A., Willemze, R., Eds.; International Agency for Research on Cancer: Lyon, France, 2018; p. 470. [Google Scholar]
- Stefanaki, C.; Chardalias, L.; Soura, E.; Katsarou, A.; Stratigos, A. Paediatric melanoma. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1604–1615. [Google Scholar] [CrossRef]
- Wood, B.A. Paediatric melanoma. Pathology 2016, 48, 155–165. [Google Scholar] [CrossRef]
- Busam, K.J.; Scolyer, R.A.; Gerami, P. Pathology of Melanocytic Tumors E-Book. 2018. Available online: https://nls.ldls.org.uk/welcome.html?ark:/81055/vdc_100063476428.0x000001 (accessed on 8 June 2021).
- Masson Regnault, M.; Mazereeuw-Hautier, J.; Fraitag, S. Les mélanomes d’apparition précoce (congénitaux, néonataux, du nourrisson): Revue systématique des cas de la littérature. Ann. Dermatol. Vénéréol. 2020, 147, 729–745. [Google Scholar] [CrossRef]
- Pavlova, O.; Fraitag, S.; Hohl, D. 5-Hydroxymethylcytosine Expression in Proliferative Nodules Arising within Congenital Nevi Allows Differentiation from Malignant Melanoma. J. Investig. Dermatol. 2016, 136, 2453–2461. [Google Scholar] [CrossRef]
- Yélamos, O.; Arva, N.C.; Obregon, R.; Yazdan, P.; Wagner, A.; Guitart, J.; Gerami, P. A Comparative Study of Proliferative Nodules and Lethal Melanomas in Congenital Nevi from Children. Am. J. Surg. Pathol. 2015, 39, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Kinsler, V.; O’Hare, P.; Bulstrode, N.; Calonje, J.; Chong, W.; Hargrave, D.; Jacques, T.; Lomas, D.; Sebire, N.; Slater, O. Melanoma in congenital melanocytic naevi. Br. J. Dermatol. 2017, 176, 1131–1143. [Google Scholar] [CrossRef]
- Lacoste, C.; Avril, M.-F.; Frassati-Biaggi, A.; Dupin, N.; Chrétien-Marquet, B.; Mahé, E. Malignant Melanoma Arising in Patients with a Large Congenital Melanocytic Naevus: Retrospective Study of 10 Cases with Cytogenetic Analysis. Acta Derm. Venereol. 2015, 95, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, S.; Akiyama, M.; Mashiko, M.; Shibaki, A.; Shimizu, H. Extensive proliferative nodules in a case of giant congenital naevus. Clin. Exp. Dermatol. 2007, 33, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Van Houten, A.H.; van Dijk, M.C.R.F.; Schuttelaar, M.-L.A. Proliferative nodules in a giant congenital melanocytic nevus-case report and review of the literature. J. Cutan. Pathol. 2010, 37, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.T.; Theos, A.; Kelly, D.R.; Busam, K.; Andea, A.A. Mitotically active proliferative nodule arising in a giant congenital melanocytic nevus: A diagnostic pitfall. Am. J. Dermatopathol. 2013, 35, e16–e21. [Google Scholar] [CrossRef] [PubMed]
- Vergier, B.; Laharanne, E.; Prochazkova-Carlotti, M.; De La Fouchardière, A.; Merlio, J.-P.; Kadlub, N.; Avril, M.-F.; Bodemer, C.; Lacoste, C.; Boralevi, F.; et al. Proliferative Nodules vs Melanoma Arising in Giant Congenital Melanocytic Nevi During Childhood. JAMA Dermatol. 2016, 152, 1147–1151. [Google Scholar] [CrossRef]
- Busam, K.J.; Shah, K.N.; Gerami, P.; Sitzman, T.; Jungbluth, A.A.; Kinsler, V. Reduced H3K27me3 Expression Is Common in Nodular Melanomas of Childhood Associated with Congenital Melanocytic Nevi but Not in Proliferative Nodules. Am. J. Surg. Pathol. 2017, 41, 396–404. [Google Scholar] [CrossRef]
- Bastian, B.C.; Xiong, J.; Frieden, I.J.; Williams, M.L.; Chou, P.; Busam, K. Genetic changes in neoplasms arising in congenital melanocytic nevi: Differences between nodular proliferations and melanomas. Am. J. Pathol. 2002, 161, 1163–1169. [Google Scholar] [CrossRef]
- Fan, Y.; Lee, S.; Wu, G.; Easton, J.; Yergeau, D.; Dummer, R. Telomerase Expression by Aberrant Methylation of the TERT Promoter in Melanoma Arising in Giant Congenital Nevi. J. Investig. Dermatol. 2016, 136, 339–342. [Google Scholar] [CrossRef]
- Busam, K.J.; Barnhill, R.L. Pagetoid Spitz nevus. Intraepidermal Spitz tumor with prominent pagetoid spread. Am. J. Surg. Pathol. 1995, 19, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Gianotti, R.; Cavicchini, S.; Salviato, T.; Zalaudek, I.; Argenziano, G. Spitz nevus, Spitz tumor, and spitzoid melanoma: A comprehensive clinicopathologic overview. Dermatol. Clin. 2013, 31, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Barnhill, R.L. The Spitzoid lesion: Rethinking Spitz tumors, atypical variants, “Spitzoid melanoma” and risk assessment. Mod. Pathol. 2006, 19 (Suppl. 2), S21–S33. [Google Scholar] [CrossRef]
- Dika, E.; Ravaioli, G.M.; Fanti, P.A.; Neri, I.; Patrizi, A. Spitz Nevi and Other Spitzoid Neoplasms in Children: Overview of Incidence Data and Diagnostic Criteria. Pediatr. Dermatol. 2017, 34, 25–32. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menzinger, S.; Fraitag, S. Pseudomalignancies in Children: Histological Clues, and Pitfalls to Be Avoided. Dermatopathology 2021, 8, 376-389. https://doi.org/10.3390/dermatopathology8030042
Menzinger S, Fraitag S. Pseudomalignancies in Children: Histological Clues, and Pitfalls to Be Avoided. Dermatopathology. 2021; 8(3):376-389. https://doi.org/10.3390/dermatopathology8030042
Chicago/Turabian StyleMenzinger, Sébastien, and Sylvie Fraitag. 2021. "Pseudomalignancies in Children: Histological Clues, and Pitfalls to Be Avoided" Dermatopathology 8, no. 3: 376-389. https://doi.org/10.3390/dermatopathology8030042
APA StyleMenzinger, S., & Fraitag, S. (2021). Pseudomalignancies in Children: Histological Clues, and Pitfalls to Be Avoided. Dermatopathology, 8(3), 376-389. https://doi.org/10.3390/dermatopathology8030042






