Single-Center Clinico-Pathological Case Study of 19 Patients with Cutaneous Adverse Reactions Following COVID-19 Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Histopathology
3. Results
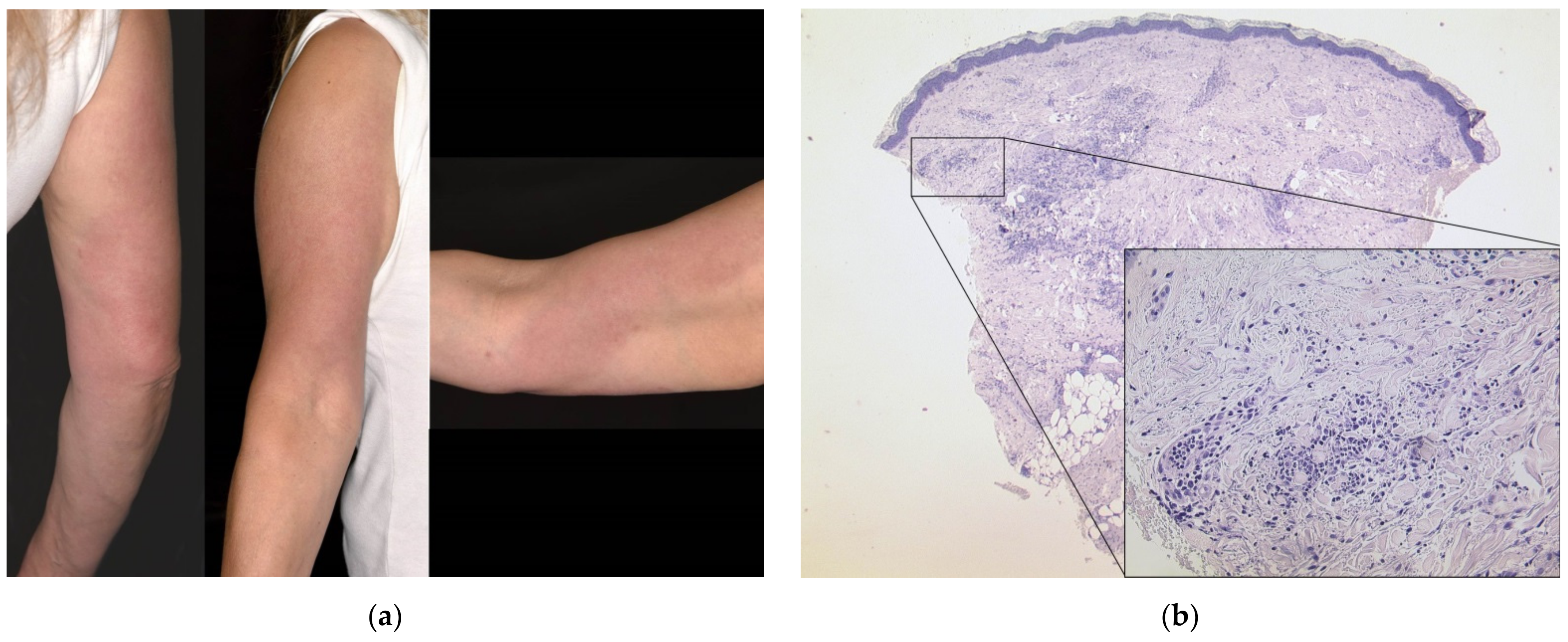
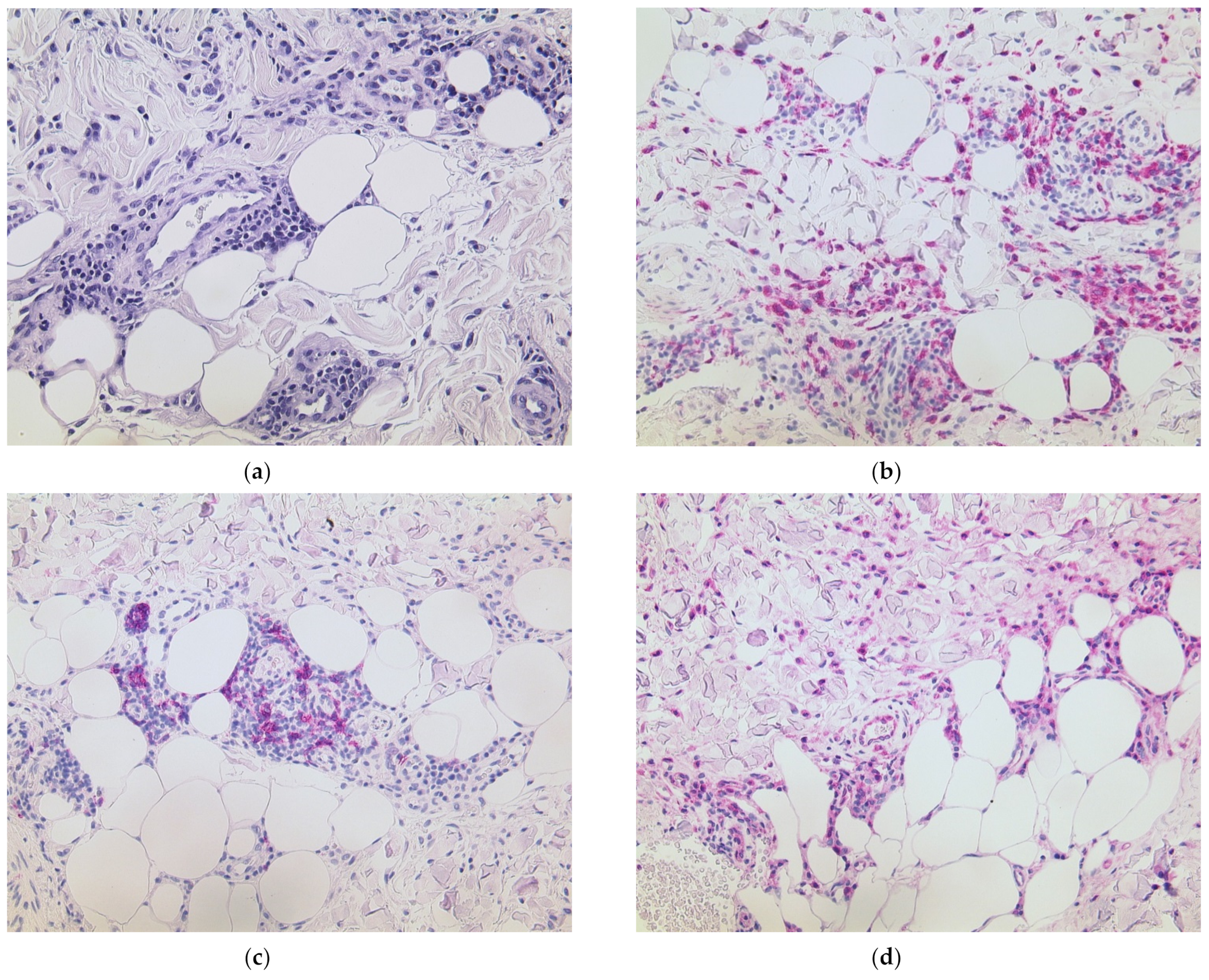
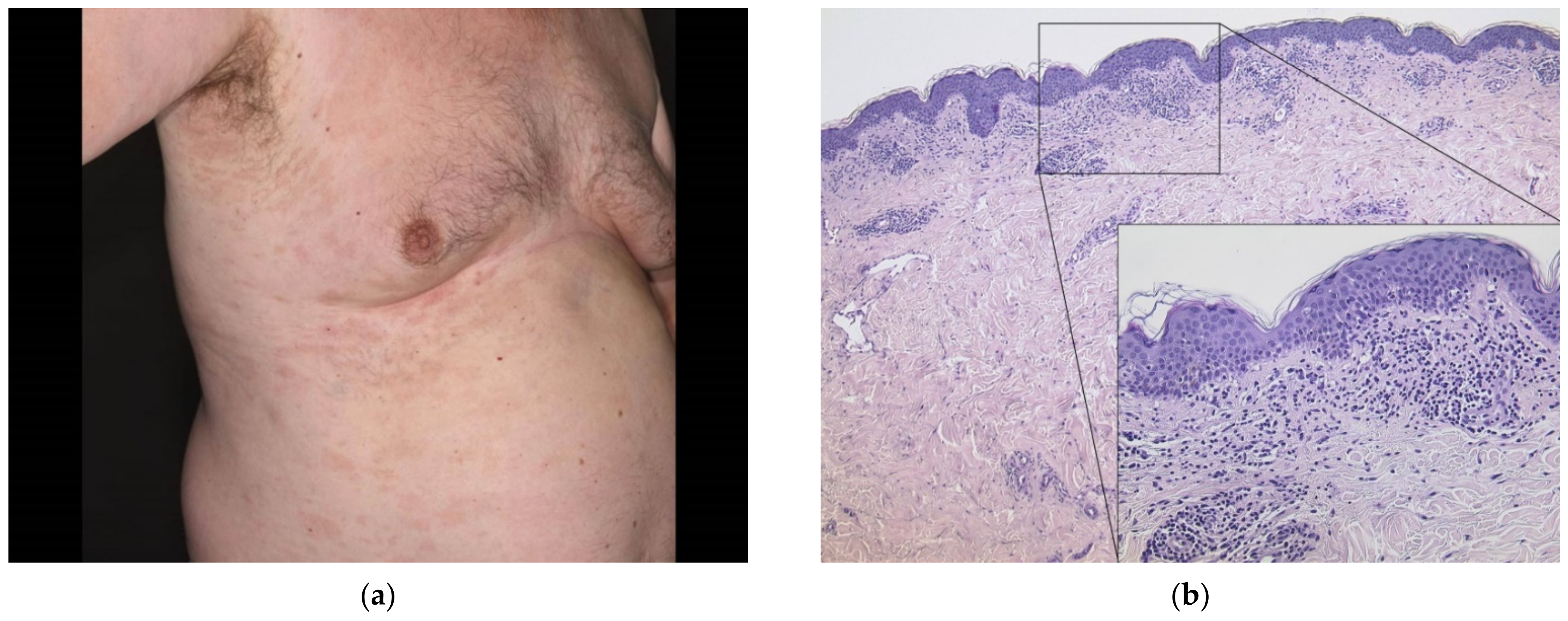
3.1. Delayed Large Local Reaction (“COVID Arm”) with Erythema Nodosum
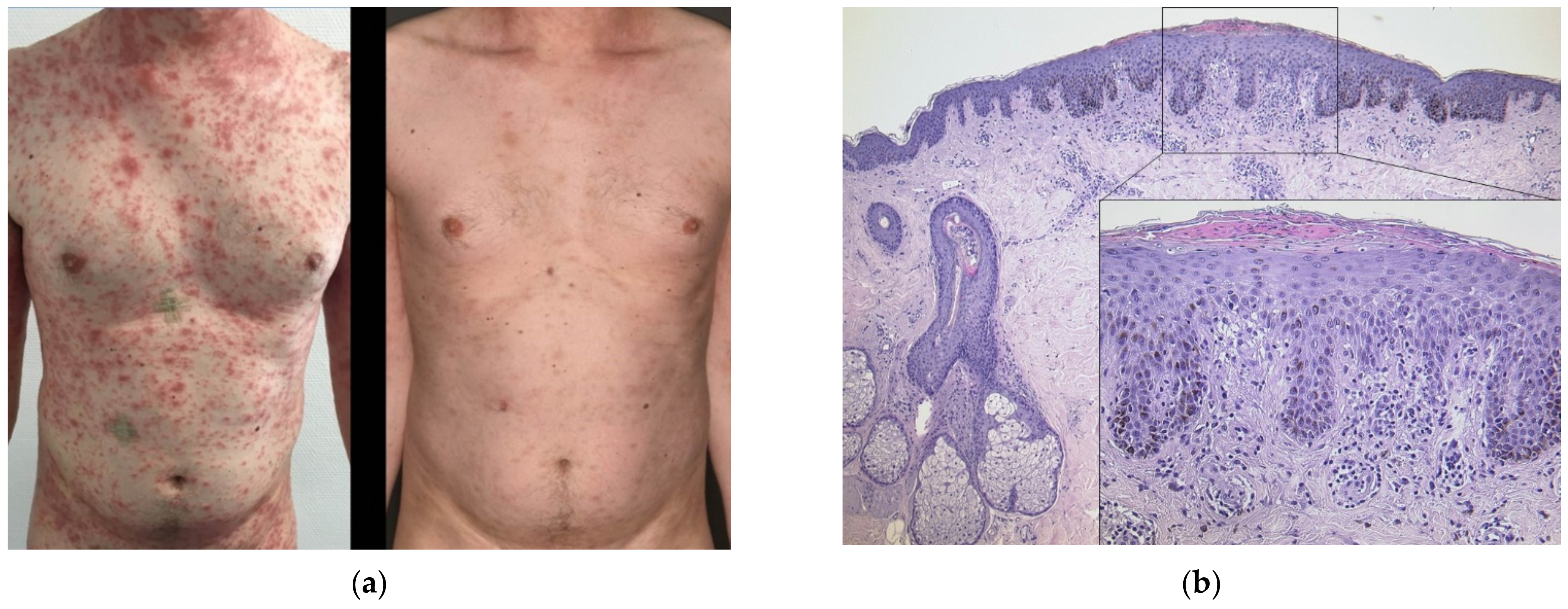
3.2. Generalized Psoriasiform Eruption in an Atopic Patient
3.3. Hematogenous Contact Dermatitis
3.4. Flare of Psoriasis
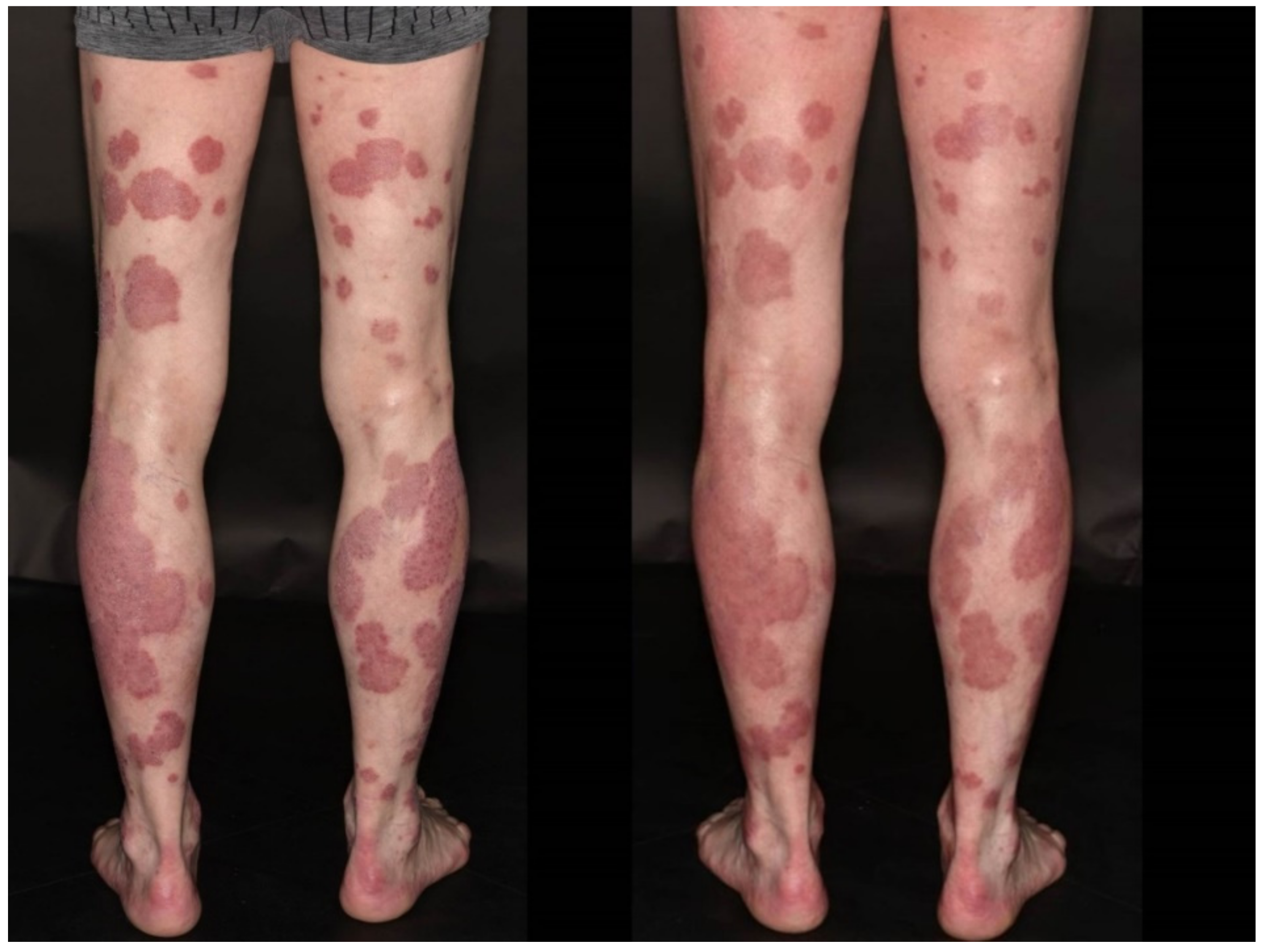
3.5. Pityriasis Rosea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef]
- Català, A.; Muñoz-Santos, C.; Galván-Casas, C.; Roncero Riesco, M.; Revilla Nebreda, D.; Solá-Truyols, A.; Giavedoni, P.; Llamas-Velasco, M.; González-Cruz, C.; Cubiró, X.; et al. Cutaneous reactions after SARS-COV-2 vaccination: A cross-sectional Spanish nationwide study of 405 cases. Br. J. Dermatol. 2021. [Google Scholar] [CrossRef]
- Galván Casas, C.; Català, A.; Carretero Hernández, G.; Rodríguez-Jiménez, P.; Fernández-Nieto, D.; Rodríguez-Villa Lario, A.; Navarro Fernández, I.; Ruiz-Villaverde, R.; Falkenhain-López, D.; Llamas Velasco, M.; et al. Classification of the cutaneous manifestations of COVID-19: A rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 2020, 183, 71–77. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Freeman, E.E.; Saff, R.R.; Robinson, L.B.; Wolfson, A.R.; Foreman, R.K.; Hashimoto, D.; Banerji, A.; Li, L.; Anvari, S.; et al. Delayed Large Local Reactions to mRNA-1273 Vaccine against SARS-CoV-2. N. Engl. J. Med. 2021, 384, 1273–1277. [Google Scholar] [CrossRef]
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef]
- Larson, V.; Seidenberg, R.; Caplan, A.; Brinster, N.K.; Meehan, S.A.; Kim, R.H. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination. J. Cutan. Pathol. 2021. [Google Scholar] [CrossRef]
- Bundesministerium für Gesundheit. Impfdashboard. Available online: https://impfdashboard.de/ (accessed on 24 June 2021).
- Niebel, D.; Ralser-Isselstein, V.; Jaschke, K.; Braegelmann, C.; Bieber, T.; Wenzel, J. Exacerbation of subacute cutaneous lupus erythematosus following vaccination with BNT162b2 mRNA vaccine. Dermatol. Ther. 2021, e15017. [Google Scholar] [CrossRef]
- Niebel, D.; Wilhelmi, J.; de Vos, L.; Ziob, J.; Jaschke, K.; Bieber, T.; Wenzel, J.; Braegelmann, C. Annular plaques mimicking Rowell’s syndrome in the course of covid-19 mRNA vaccines—An overlooked phenomenon? J. Dermatol. 2021. under review. [Google Scholar]
- Niebel, D.; Wenzel, J. Immunpathologie von kutanen Medikamentennebenwirkungen. Pathologe 2018, 39, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, A.E.; Stein, S.L. Cutaneous reactions to vaccinations. Clin. Dermatol. 2015, 33, 327–332. [Google Scholar] [CrossRef]
- Bae, S.; Lee, Y.W.; Lim, S.Y.; Lee, J.H.; Lim, J.S.; Lee, S.; Park, S.; Kim, S.K.; Lim, Y.J.; Kim, E.O.; et al. Adverse Reactions Following the First Dose of ChAdOx1 nCoV-19 Vaccine and BNT162b2 Vaccine for Healthcare Workers in South Korea. J. Korean Med. Sci. 2021, 36, e115. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, G.; Bogdanov, I.; Kazandjieva, J.; Tsankov, N. Cutaneous adverse effects of the available COVID-19 vaccines. Clin. Dermatol. 2021, 39, 523–531. [Google Scholar] [CrossRef]
- Corbeddu, M.; Diociaiuti, A.; Vinci, M.R.; Santoro, A.; Camisa, V.; Zaffina, S.; El Hachem, M. Transient cutaneous manifestations after administration of Pfizer-BioNTech COVID-19 Vaccine: An Italian single-centre case series. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e483–e485. [Google Scholar] [CrossRef] [PubMed]
- Farinazzo, E.; Ponis, G.; Zelin, E.; Errichetti, E.; Stinco, G.; Pinzani, C.; Gambelli, A.; de Manzini, N.; Toffoli, L.; Moret, A.; et al. Cutaneous adverse reactions after m-RNA COVID-19 vaccine: Early reports from North-East Italy. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e548–e551. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Nieto, D.; Hammerle, J.; Fernandez-Escribano, M.; Moreno-Del Real, C.M.; Garcia-Abellas, P.; Carretero-Barrio, I.; Solano-Solares, E.; de-la-Hoz-Caballer, B.; Jimenez-Cauhe, J.; Ortega-Quijano, D.; et al. Skin manifestations of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers. ‘COVID-arm’: A clinical and histological characterization. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e425–e427. [Google Scholar] [CrossRef] [PubMed]
- Gringeri, M.; Mosini, G.; Battini, V.; Cammarata, G.; Guarnieri, G.; Carnovale, C.; Clementi, E.; Radice, S. Preliminary evidence on the safety profile of BNT162b2 (Comirnaty): New insights from data analysis in EudraVigilance and adverse reaction reports from an Italian health facility. Hum. Vaccin. Immunother. 2021, 1–3. [Google Scholar] [CrossRef]
- Niebel, D.; Novak, N.; Wilhelmi, J.; Ziob, J.; Wilsmann-Theis, D.; Bieber, T.; Wenzel, J.; Braegelmann, C. Cutaneous adverse reactions to covid-19 vaccines—Insights from an immuno-dermatological perspective. Vaccines 2021, 9, 944. [Google Scholar] [CrossRef]
- Krajewski, P.K.; Matusiak, Ł.; Szepietowski, J.C. Psoriasis flare-up associated with second dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e632–e634. [Google Scholar] [CrossRef]
- Onsun, N.; Kaya, G.; Işık, B.G.; Güneş, B. A generalized pustular psoriasis flare after CoronaVac COVID-19 vaccination: Case report. Health Promot. Perspect. 2021, 11, 261–262. [Google Scholar] [CrossRef]
- Jovanović, M.; Poljacki, M.; Vujanović, L.; Duran, V. Acute febrile neutrophilic dermatosis (Sweet’s syndrome) after influenza vaccination. J. Am. Acad. Dermatol. 2005, 52, 367–369. [Google Scholar] [CrossRef]
- Leasure, A.C.; Cowper, S.; McNiff, J.; Cohen, J.M. Generalized eczematous reactions to the Pfizer-BioNTech COVID-19 vaccine. J. Eur. Acad. Dermatol. Venereol. 2021. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; de Gregorio, C.; Velissaris, D.; Petalas, K.; Brinia, A.; Assimakopoulos, S.F.; Gogos, C.; Kouni, S.N.; Kounis, G.N.; et al. Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations. Vaccines 2021, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Fishman, M.; Wattenberg, D.; Gordon, M.; Lebwohl, M. “COVID arm”: A reaction to the Moderna vaccine. JAAD Case Rep. 2021, 10, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, H.; Paik, S.S.; Moon, J.-Y.; Yoon, H.J.; Kim, S.-H. Delayed cutaneous reaction to ChAdOx1 nCoV-19 vaccine: Is it an ‘AstraZeneca arm’? J. Eur. Acad. Dermatol. Venereol. 2021. [Google Scholar] [CrossRef]
- Johnston, M.S.; Galan, A.; Watsky, K.L.; Little, A.J. Delayed Localized Hypersensitivity Reactions to the Moderna COVID-19 Vaccine: A Case Series. JAMA Dermatol. 2021, 157, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Sipfle DO, N.; Bridwell Md, R.E.; Roper DO, J. Erythema nodosum-like rash in a COVID-19 patient: A case report. Am. J. Emerg. Med. 2021, 40, 227.e1–227.e2. [Google Scholar] [CrossRef]

| # | Sex | Age | Dose | Vaccine | Onset | Clinical | Histological | Comorbidity | Diagnosis | Management | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 73 | 1 | BNT162b2 | 10 d | Ill-defined erythematous plaques on the trunk | NA | CLE | Flare of CLE | Prednisolone 1 mg/kg tapered over three weeks | Excellent; no flare with second dose BNT162b2 [8] |
| 2 | M | 41 | 1 | BNT162b2 | 5 d | Generalized erythematous annular plaques | Patchy lymphocytic infiltrate, slight interface dermatitis | MCTD | Drug-induced CLE | Prednisolone 1 mg/kg tapered over three weeks; continuation of hydroxychloroquine 200 mg 2 x daily; methotrexate 15 mg s.c. | Excellent, no flare with second dose [9] |
| 3 | F | 50 | 1 | BNT162b2 | 30 d | Periorbital erythema and edema, V-sign | NA | None | Dermatomyositis | Prednisolone 1 mg/kg tapered over six weeks | No flare with second dose; diagnostic workup to exclude malignancy pending |
| 4 | F | 54 | 1 | BNT162b2 | 1 d | Upper arm and shoulder erythematous and edematous, forearm swollen | Dense lymphocytic infiltrate with numerous histiocytes and neutrophils, septal panniculitis | None | “COVID arm”, protracted development of erythema nodosum | Prednisolone 1 mg/kg tapered over three weeks | Second dose with BNT162b2 two weeks delayed; skin improved, protracted course of arthritic pain → diagnosis of rheumatoid arthritis |
| 5 | M | 39 | 1 | BNT162b2 | 21 d | Generalized eczematous plaques | Psoriasiform acanthosis, spongiosis, eosinophilia | Atopic diathesis | Psoriasiform flare of AD | Prednisolone 0.5 mg/kg tapered over a week; TCS 2 x daily, UVB311nm | Excellent; no flare with second dose |
| 6 | M | 77 | 1 | BNT162b2 | 3 d | Localized eczematous and urticarial plaques | Parakeratosis, dermal edema with eosinophils, interface dermatitis | Atopic diathesis | Hematogenous contact dermatitis | TCS 2 x daily | Protracted course with flare after second dose |
| 7 | F | 55 | 2 | BNT162b2 | 7 d | Grouped pruritic papulovesicles | NA | Atopic diathesis, chronic spontaneous urticaria | Vesicular reaction | Topical fusidine ointment 2 x daily | Excellent |
| 8 | M | 62 | 2 | BNT162b2 | 20 d | Generalized erythemato-squamous plaques | NA | Psoriasis vulgaris | Flare of psoriasis | Cignoline, TCS 2 x daily, UVB311nm, tildrakizumab | Excellent |
| 9 | M | 76 | 2 | BNT162b2 | 2 d | Petechial annular plaques on the lower extremities | NA | IgG/IgM cutaneous immune complex vasculitis | Flare of immune complex vasculitis | Continued use of dapsone, prednisolone 10 mg p.o. for a week, 5 mg p.o. maintenance | Excellent |
| 10 | F | 30 | 1 | BNT162b2 | 10 d | Generalized non-scaling erythematous plaques | Edematous papillary dermis with admixed neutrophils | None | Neutrophilic drug eruption | Prednisolone 1 mg/kg tapered over six weeks | Protracted course with fatigue and nausea; second dose adjourned |
| 11 | F | 15 | 1 | BNT162b2 | 2 d | Generalized hives | NA | None | Acute urticaria | Antihistamines | Second dose scheduled with BNT162b2 after six weeks, no flare with second dose |
| 12 | M | 68 | 1 | BNT162b2 | 2 d | Scaling erythematous plaques on extremities | Spongiotic dermatitis, dermal edema with eosinophils | Stasis dermatitis | Hematogenous contact dermatitis | TCS 2 x daily | Excellent; second dose adjourned (wish of patient), allergological diagnostics anticipated |
| 13 | F | 40 | 2 | BNT162b2 | 8 d | Generalized non-pruritic scaling plaques | NA | None | Pityriasis rosea | TCS 2 x daily | Excellent |
| 14 | F | 67 | 2 | BNT162b2 | 12 d | Pruritic scaling erythema in light-exposed areas | Parakeratosis, spongiosis, eosinophils and neutrophils | Chronic hand eczema | Hematogenous contact dermatitis with flare of chronic hand eczema | Prednisolone 10 mg p.o. for a week, ciclosporine raised from 50 to 100 mg p.o., TCS 2 x daily | Steady improvement over weeks, chronic lesions persistent |
| 15 | F | 49 | 2 | AZD1222/BNT162b2 | 3 d | Grouped pruritic papulovesicles | NA | None | Vesicular reaction | TCS 2 x daily | Excellent |
| 16 | F | 22 | 1 | mRNA-1273 | 10 d | Elevated erythematous annular papules and plaques without scaling | Superficial and deep lymphocytic infiltrate with interface dermatitis | None | Drug-induced CLE | Prednisolone 1 mg/kg tapered over three weeks; etoricoxib 90 mg p.o. | Recurrent joint pain and fever, hospitalization, second dose adjourned [9] |
| 17 | F | 20 | 2 | mRNA-1273 | 9 d | Generalized pruritic exanthema | Superficial and deep lymphocytic infiltrate with interface dermatitis | None | Drug-induced CLE | Prednisolone 10 mg p.o., antihistamines, TCS 2 x daily | Excellent |
| 18 | F | 38 | 1 | AZD1222 | 1 d | Generalized hives | NA | None | Acute urticaria | Antihistamines | Second dose scheduled with BNT162b2 after three months (pending) |
| 19 | M | 63 | 1 | AZD1222 | 22 d | Pale erythematous maculae along Langer lines | Interface dermatitis and erythrocyte extravasation | Atopic diathesis | Pityriasis rosea | TCS on demand, emollients, “watchful waiting” | Second dose scheduled with BNT162b2 after three months (pending) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niebel, D.; Wenzel, J.; Wilsmann-Theis, D.; Ziob, J.; Wilhelmi, J.; Braegelmann, C. Single-Center Clinico-Pathological Case Study of 19 Patients with Cutaneous Adverse Reactions Following COVID-19 Vaccines. Dermatopathology 2021, 8, 463-476. https://doi.org/10.3390/dermatopathology8040049
Niebel D, Wenzel J, Wilsmann-Theis D, Ziob J, Wilhelmi J, Braegelmann C. Single-Center Clinico-Pathological Case Study of 19 Patients with Cutaneous Adverse Reactions Following COVID-19 Vaccines. Dermatopathology. 2021; 8(4):463-476. https://doi.org/10.3390/dermatopathology8040049
Chicago/Turabian StyleNiebel, Dennis, Joerg Wenzel, Dagmar Wilsmann-Theis, Jana Ziob, Jasmin Wilhelmi, and Christine Braegelmann. 2021. "Single-Center Clinico-Pathological Case Study of 19 Patients with Cutaneous Adverse Reactions Following COVID-19 Vaccines" Dermatopathology 8, no. 4: 463-476. https://doi.org/10.3390/dermatopathology8040049
APA StyleNiebel, D., Wenzel, J., Wilsmann-Theis, D., Ziob, J., Wilhelmi, J., & Braegelmann, C. (2021). Single-Center Clinico-Pathological Case Study of 19 Patients with Cutaneous Adverse Reactions Following COVID-19 Vaccines. Dermatopathology, 8(4), 463-476. https://doi.org/10.3390/dermatopathology8040049







