Atrophic Dermatofibrosarcoma Protuberans with Eosinophilic Infiltration
Abstract
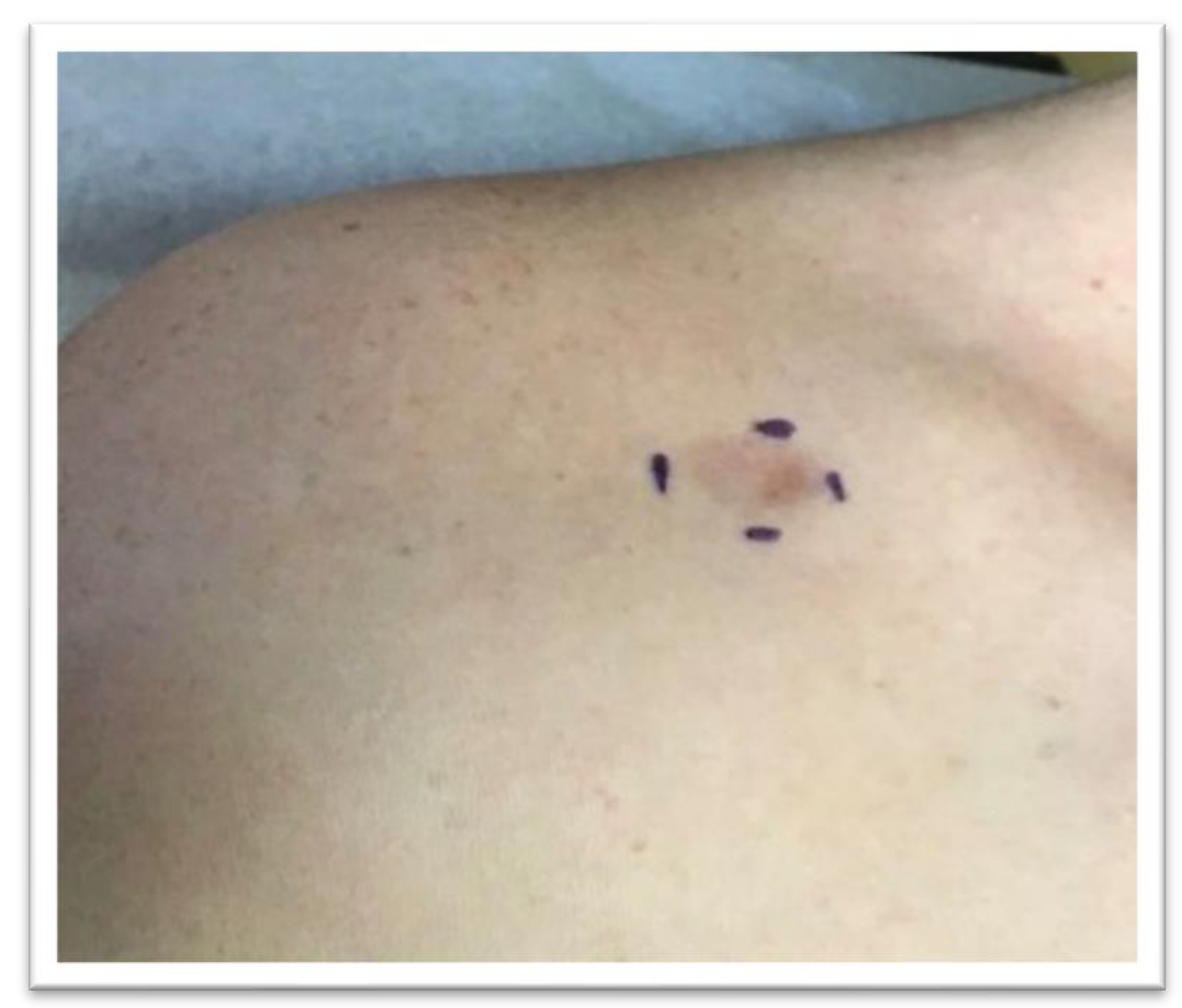
:1. Introduction
2. Material and Method
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aragão, S.S.D.C.; Leite, E.J.D.S.; Cardoso, A.E.C.; Houly, R.L.S. An unusual variant of atrophic dermatofibrosarcoma protuberans *. An. Bras. Dermatol. 2018, 93, 282–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elafram, R.; Ben Romdhane, M.; Khessairi, N.; Sghaier, M.; Annabi, H. Dermatofibrosarcoma protuberans of the hallux: A case report with review of the literature. Int. J. Surg. Case Rep. 2022, 96, 107325, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.A.; Jambusaria-Pahlajani, A.; Karia, P.S.; Elenitsas, R.; Zhang, P.D.; Schmults, C.D. A systematic review of outcome data for dermatofibrosarcoma protuberans with and without fibrosarcomatous change. J. Am. Acad. Dermatol. 2014, 71, 781–786. [Google Scholar] [CrossRef]
- Kallen, M.E.; Hornick, J.L. The 2020 WHO classification: What’s new in soft tissue tumor pathology? Am. J. Surg. Pathol. 2021, 45, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Kreicher, K.L.; Kurlander, D.E.; Gittleman, H.R.; Barnholtz-Sloan, J.S.; Bordeaux, J.S. Incidence and Survival of Primary Dermatofibrosarcoma Protuberans in the United States. Dermatol. Surg. 2016, 42 (Suppl. 1), S24–S31. [Google Scholar] [CrossRef] [PubMed]
- Criscione, V.D.; Weinstock, M.A. Descriptive epidemiology of dermatofibrosarcoma protuberans in the United States, 1973 to 2002. J. Am. Acad. Dermatol. 2007, 56, 968–973, Epub. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.B.; Helwig, E.B. Dermatofibrosarcoma protuberans. A study of 115 cases. Cancer 1962, 15, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Akay, B.N.; Unlu, E.; Erdem, C.; Heper, A.O. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol. Pract. Concept. 2015, 5, 71–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, W.C.; Abramovits, W.; Gonzalez-Sevra, A.; Souchon, E.; Schwartz, R.A.; Little, W.P. Dermatofibrosarcoma non-protuberans: Description and report of five cases of a morpheaform variant of dermatofibrosarcoma. J. Surg. Oncol. 1985, 28, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, N.; Misago, N.; Shinogi, T.; Inoue, T.; Miura, Y.; Narisawa, Y. Atrophic dermatofibrosarcoma protuberans with diffuse eosinophilic infiltrate. J. Dermatol. 2006, 33, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Al Barwani, A.S.; Taif, S.; Al Mazrouai, R.A.; Al Muzahmi, K.S.; Alrawi, A. Dermatofibrosarcoma Protuberans: Insights into a Rare Soft Tissue Tumor. J. Clin. Imaging Sci. 2016, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Hanabusa, M.; Kamo, R.; Harada, T.; Ishii, M. Dermatofibrosarcoma protuberans with atrophic appearance at early stage of the tumor. J. Dermatol. 2007, 34, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Zelger, B.W.; Ofner, D.; Zelger, B.G. Atrophic variants of dermatofibroma and dermatofibrosarcoma protuberans. Histopathology 1995, 26, 519–527. [Google Scholar] [CrossRef]
- Souiki, T.; Belhaj, A.; Abderrhim, A.A.; Alami, B.; Tahiri, L.; Chbani, L.; Ibn Majdoub, K.; Toughrai, I.; Mazaz, K. Dermatofibrosarcoma protuberans of the anterior abdominal wall: Case report and literature review. J. Surg. Case Rep. 2022, 2022, rjac272. [Google Scholar] [CrossRef] [PubMed]
- Wiesmueller, F.; Agaimy, A.; Perrakis, A.; Arkudas, A.; Horch, R.E.; Grützmann, R.; Vassos, N. Dermatofibrosarcoma protuberans: Surgical management of a challenging mesenchymal tumor. World J. Surg. Oncol. 2019, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Patel, K.U.; López-Terrada, D.; Fang, H. Atrophic dermatofibrosarcoma protuberans: Report of a case demonstrated by detecting COL1A1-PDGFB rearrangement. Diagn. Pathol. 2012, 7, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPeak, C.J.; Cruz, T.; Nicastri, A.D. Dermatofibrosarcoma protuberans: An analysis of 86 cases–five with metastasis. Ann. Surg. 1967, 166, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Marone, G.; Schiavoni, G. Eosinophils: The unsung heroes in cancer? OncoImmunology 2017, 7, e1393134. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahboob, A.; Turgeon, C.; Qasim, S.; Usmani, A. Atrophic Dermatofibrosarcoma Protuberans with Eosinophilic Infiltration. Dermatopathology 2022, 9, 379-384. https://doi.org/10.3390/dermatopathology9040044
Mahboob A, Turgeon C, Qasim S, Usmani A. Atrophic Dermatofibrosarcoma Protuberans with Eosinophilic Infiltration. Dermatopathology. 2022; 9(4):379-384. https://doi.org/10.3390/dermatopathology9040044
Chicago/Turabian StyleMahboob, Anber, Claire Turgeon, Syeda Qasim, and Arif Usmani. 2022. "Atrophic Dermatofibrosarcoma Protuberans with Eosinophilic Infiltration" Dermatopathology 9, no. 4: 379-384. https://doi.org/10.3390/dermatopathology9040044





