Cystic Neutrophilic Granulomatous Mastitis Treatment with Consecutive Dapsone and Adalimumab
Abstract
:1. Introduction
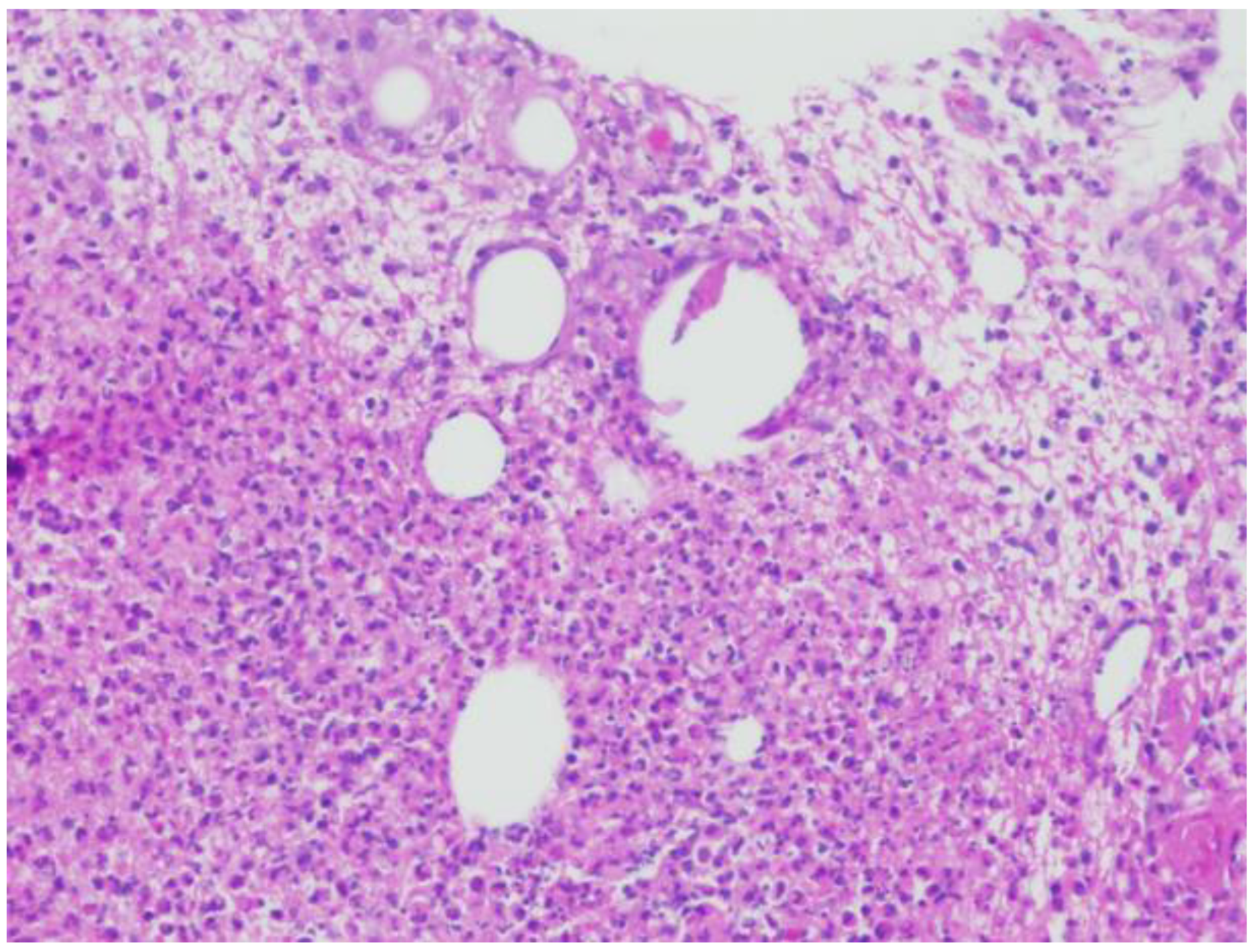
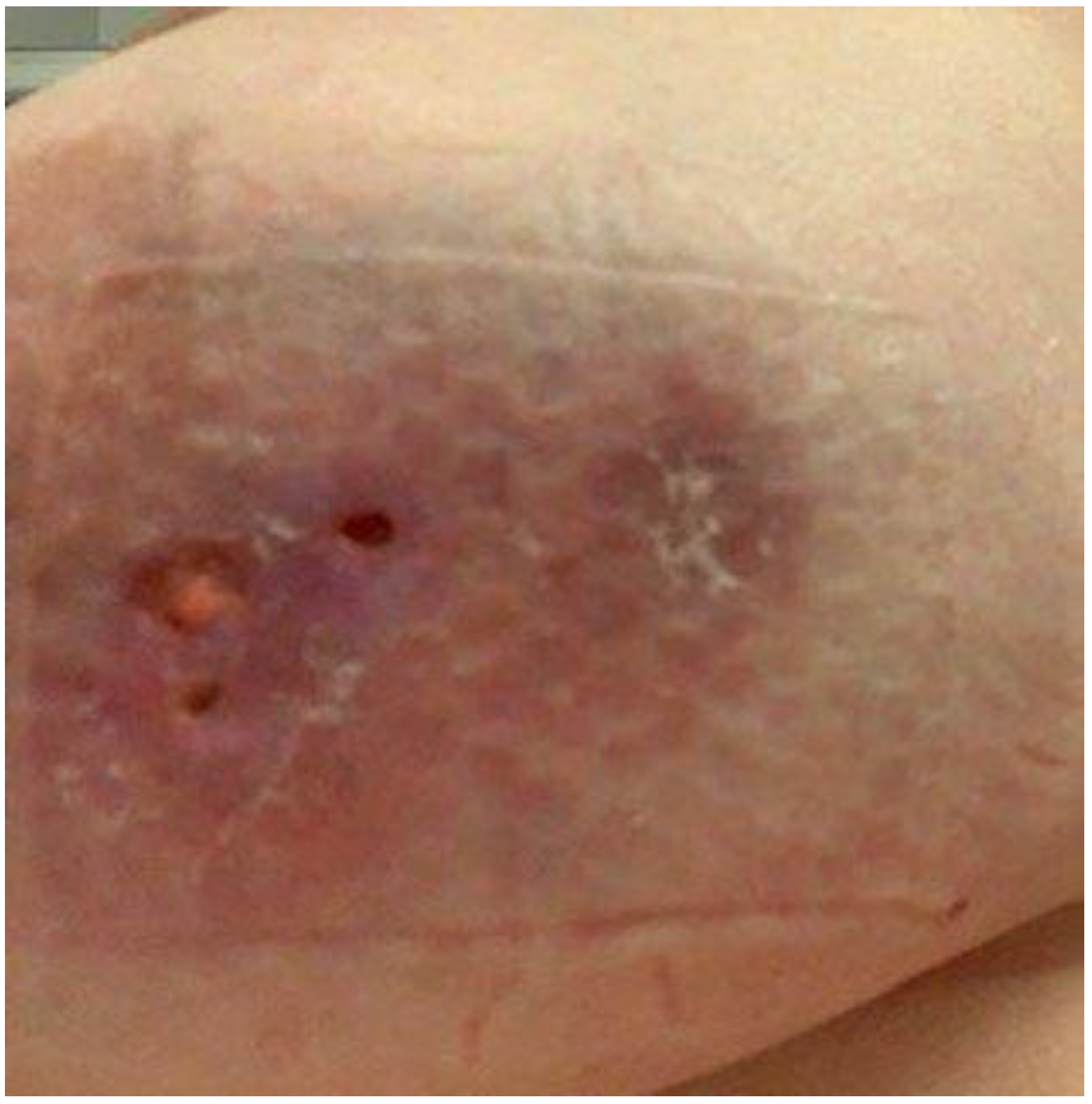
2. Case Report
2.1. Patient 1
2.2. Patient 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang, Y.; LeGolvan, M.; Chapin, K.; Mainiero, M. Cystic neutrophilic granulomatous mastitis with corynebacterium and staphylococcus mimicking breast carcinoma. Clin. Case Rep. 2018, 6, 2208–2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.; Lu, F.I.; Aftanas, P.; Tsang, K.K.; Mubareka, S.; Chan, A.; Kozak, R. Whole genome sequence of Corynebacterium kroppenstedtii isolated from a case of recurrent granulomatous mastitis. IDCases 2020, 23, e01034. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Turashvili, G. Cystic neutrophilic granulomatous mastitis: An update. J. Clin. Pathol. 2020, 73, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Maung, M.H.; Bethune, G.C.; Patriquin, G.; Barnes, P.J. Cystic neutrophilic granulomatous mastitis—A review of 12 consecutive cases. Histopathology 2020, 77, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Gautham, I.; Radford, D.M.; Kovacs, C.S.; Calhoun, B.C.; Procop, G.W.; Shepardson, L.B.; Dawson, A.E.; Downs-Kelly, E.P.; Zhang, G.X.; Al-Hilli, Z.; et al. Cystic neutrophilic granulomatous mastitis: The Cleveland Clinic experience with diagnosis and management. Breast J. 2019, 25, 80–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naik, M.A.; Korlimarla, A.; Shetty, S.T.; Fernandes, A.M.; Pai, S.A. Cystic Neutrophilic Granulomatous Mastitis: A Clinicopathological Study With 16s rRNA Sequencing for the Detection of Corynebacteria in Formalin-Fixed Paraffin-Embedded Tissue. Int. J. Surg. Pathol. 2020, 28, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.W.; Goodwin, K.; Vohra, P.; Amerson, E. Cystic Neutrophilic Granulomatous Mastitis Regression with the Tumor Necrosis Factor-α Inhibitor, Adalimumab. Eur. J. Breast Health 2022, 18, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Sangoi, A.R. “Thick Section” Gram Stain Yields Improved Detection of Organisms in Tissue Sections of Cystic Neutrophilic Granulomatous Mastitis. Am. J. Clin. Pathol. 2020, 153, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jorns, J.M. Cystic neutrophilic granulomatous mastitis: Corynebacterium species-associated infection with distinct histology. Clin. Microbiol. Infect. 2021, 27, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Ringsted, S.; Friedman, M. A rheumatologic approach to granulomatous mastitis: A case series and review of the literature. Int. J. Rheum. Dis. 2021, 24, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Lin, J.C.; Li, C.F.; Lee, Y.H. A successful case of etanercept used for idiopathic granulomatous mastitis. Breast J. 2019, 25, 343–345. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamat, S.; Schaffenburg, W.; Bongiorno, M. Cystic Neutrophilic Granulomatous Mastitis Treatment with Consecutive Dapsone and Adalimumab. Dermatopathology 2022, 9, 408-412. https://doi.org/10.3390/dermatopathology9040047
Kamat S, Schaffenburg W, Bongiorno M. Cystic Neutrophilic Granulomatous Mastitis Treatment with Consecutive Dapsone and Adalimumab. Dermatopathology. 2022; 9(4):408-412. https://doi.org/10.3390/dermatopathology9040047
Chicago/Turabian StyleKamat, Samir, William Schaffenburg, and Michelle Bongiorno. 2022. "Cystic Neutrophilic Granulomatous Mastitis Treatment with Consecutive Dapsone and Adalimumab" Dermatopathology 9, no. 4: 408-412. https://doi.org/10.3390/dermatopathology9040047
APA StyleKamat, S., Schaffenburg, W., & Bongiorno, M. (2022). Cystic Neutrophilic Granulomatous Mastitis Treatment with Consecutive Dapsone and Adalimumab. Dermatopathology, 9(4), 408-412. https://doi.org/10.3390/dermatopathology9040047





